Functionalization of Polyethyleneimine with Hollow Cyclotriveratrylene and Its Subsequent Supramolecular Interaction with Doxorubicin
Abstract
:1. Introduction
2. Methods and Results
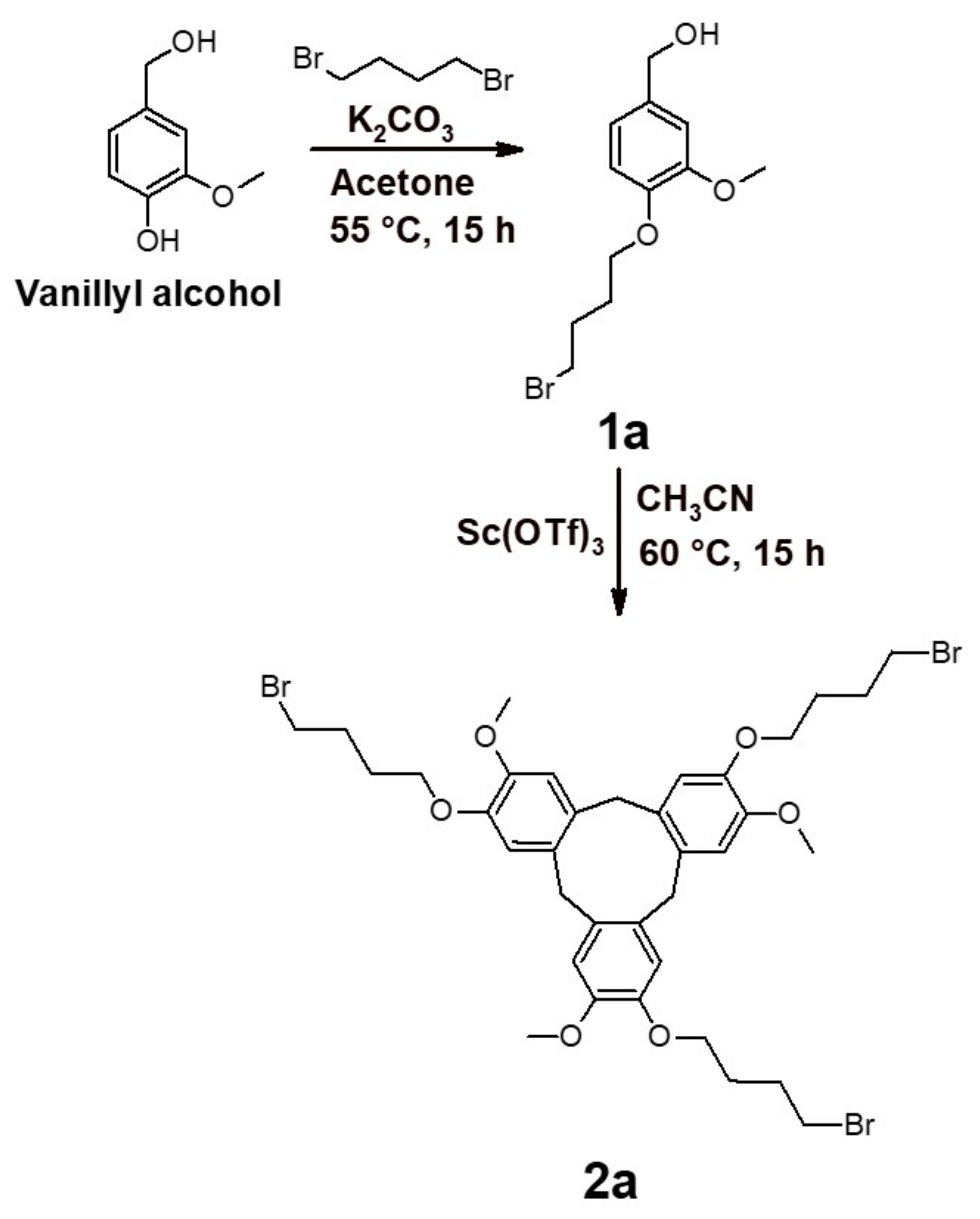
2.1. Synthesis of the Cross-Linker
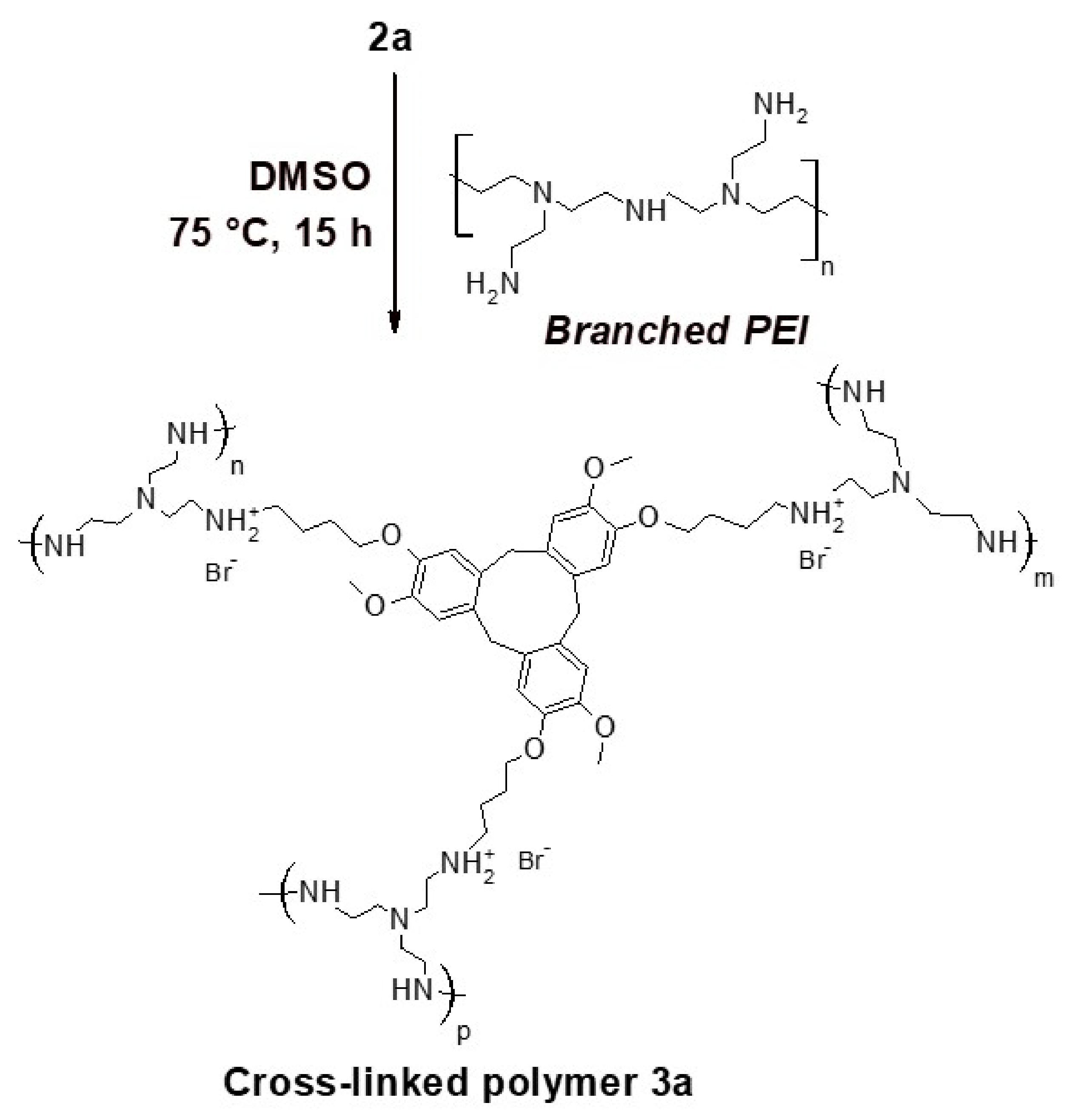
2.2. Preparation of the Cross-Linked Polymer
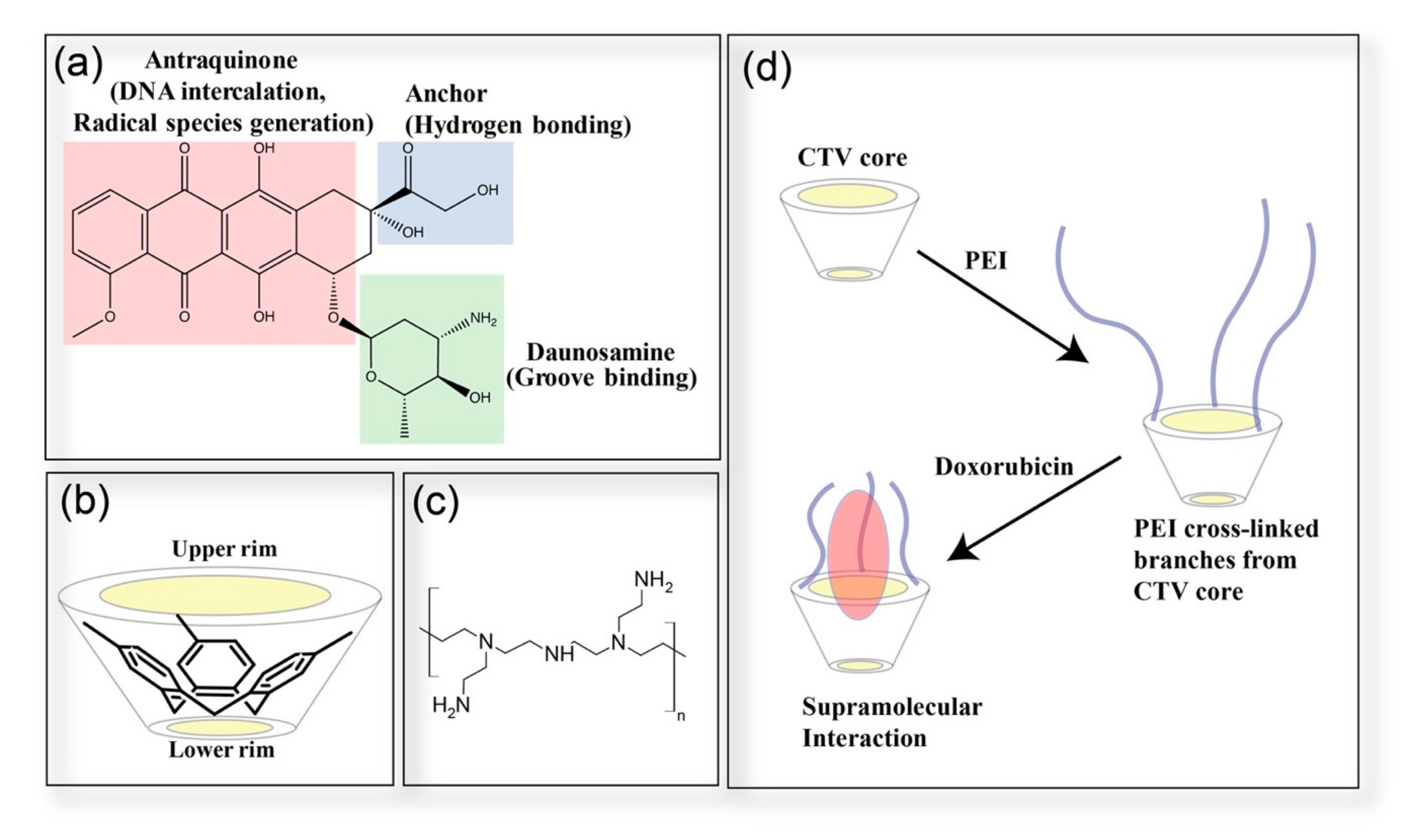
2.3. Coordination of Doxorubicin
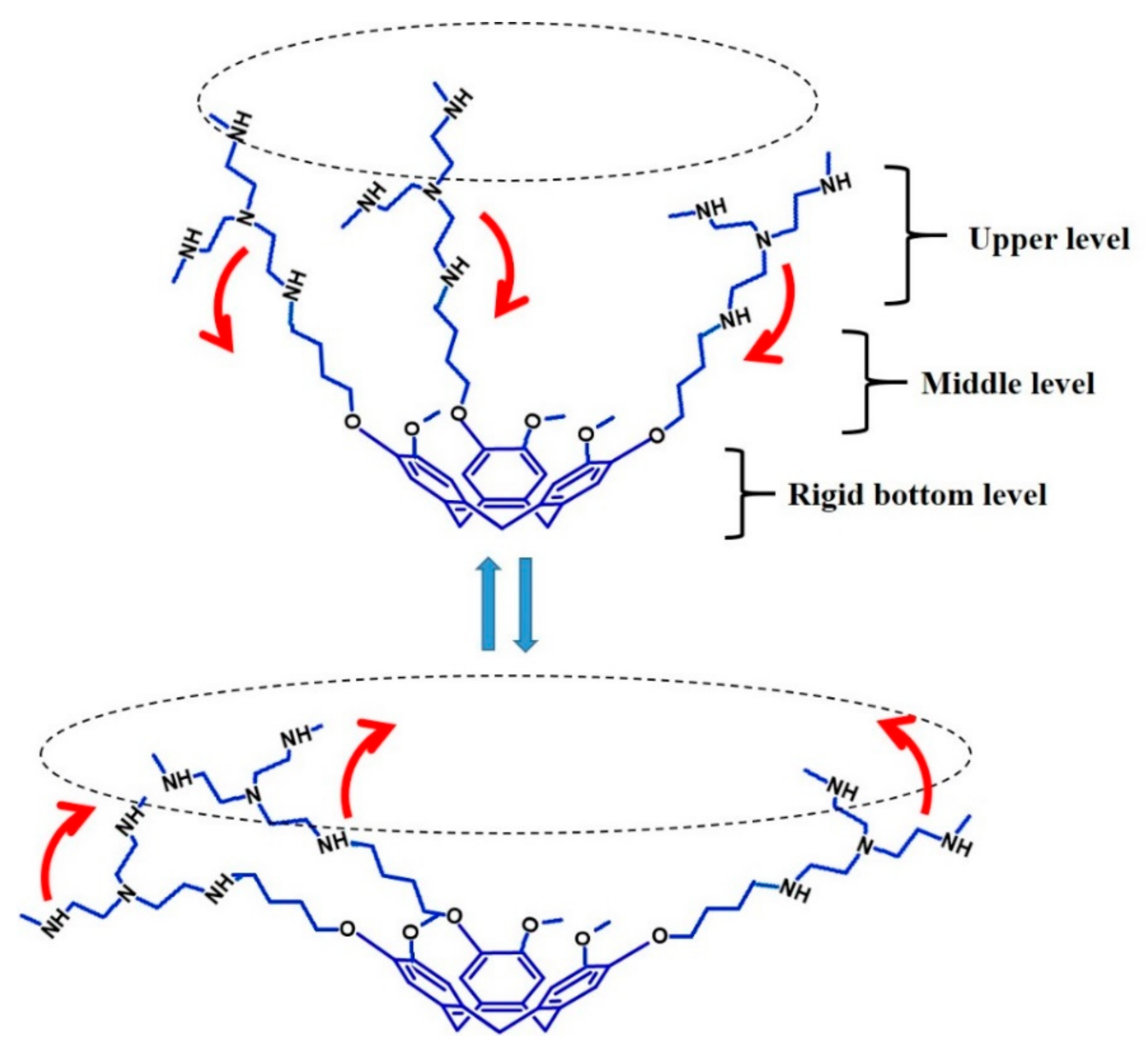
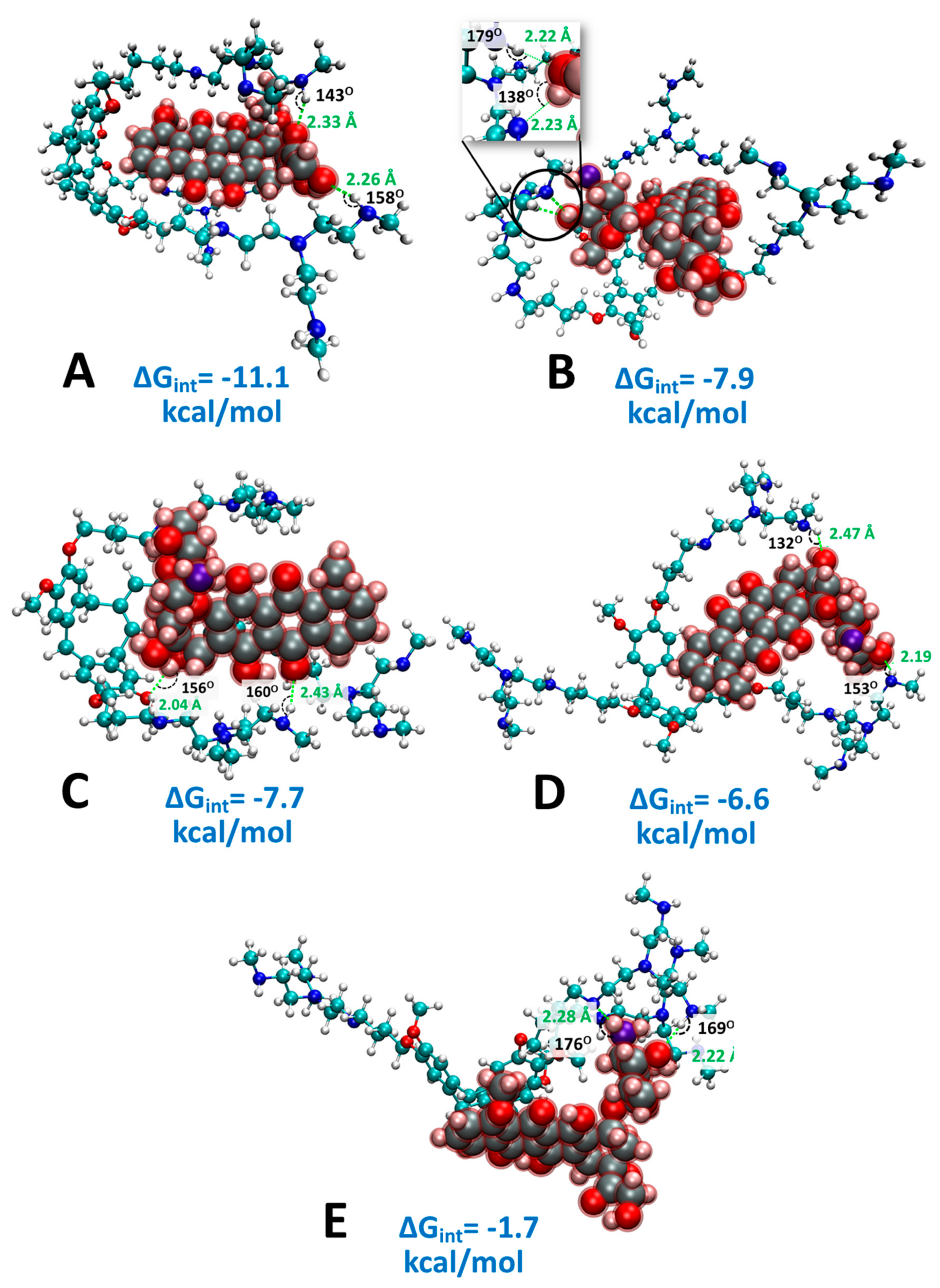
2.4. Computational Analysis of Supramolecular Assembly
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures
3.3. Computational Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guengerich, F.P. Mechanisms of Drug Toxicity and Relevance to Pharmaceutical Development. Drug Metab. Pharm. 2011, 26, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologie developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [Green Version]
- Mobaraki, M.; Faraji, A.; Zare, M.; Dolati, P.; Ataei, M.; Dehghan Manshadi, H.R. Molecular mechanisms of cardiotoxicity: A review on the major side-effects of doxorubicin. Indian J. Pharm. Sci. 2017, 79, 335–344. [Google Scholar] [CrossRef]
- Jawad, B.; Poudel, L.; Podgornik, R.; Steinmetz, N.F.; Ching, W.Y. Molecular mechanism and binding free energy of doxorubicin intercalation in DNA. Phys. Chem. Chem. Phys. 2019, 21, 3877–3893. [Google Scholar] [CrossRef]
- Borišev, I.; Mrdanovic, J.; Petrovic, D.; Seke, M.; Jović, D.; Srdenović, B.; Latinovic, N.; Djordjevic, A. Nanoformulations of doxorubicin: How far have we come and where do we go from here? Nanotechnology 2018, 29, 1–21. [Google Scholar] [CrossRef]
- De Angelis, A.; Urbanek, K.; Cappetta, D.; Piegari, E.; Ciuffreda, L.P.; Rivellino, A.; Russo, R.; Esposito, G.; Rossi, F.; Berrino, L. Doxorubicin cardiotoxicity and target cells: A broader perspective. Cardio-Oncology 2016, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kalyanaraman, B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree? Redox Biol. 2020, 29, 1–9. [Google Scholar] [CrossRef]
- Henninger, C.; Fritz, G. Statins in anthracycline-induced cardiotoxicity: Rac and Rho, and the heartbreakers. Cell Death Dis. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.M.; Dass, C.R. Review: Doxorubicin delivery systems based on chitosan for cancer therapy. J. Pharm. Pharmacol. 2009, 61, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lao, J.; Madani, J.; Puértolas, T.; Álvarez, M.; Hernández, A.; Pazo-Cid, R.; Artal, Á.; Antón Torres, A. Liposomal Doxorubicin in the Treatment of Breast Cancer Patients: A Review. J. Drug Deliv. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, N.; C Woodle, M.; Mixson, A.J. Advances in Delivery Systems for Doxorubicin. J. Nanomed. Nanotechnol. 2018, 09, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.B.; Sun, J.; Yang, P.; Li, B.; Gao, Y.; Li, Z. A Novel Doxorubicin Prodrug with GRP78 Recognition and Nucleus-Targeting Ability for Safe and Effective Cancer Therapy. Mol. Pharm. 2018, 15, 238–246. [Google Scholar] [CrossRef]
- Bao, Y.; Yin, M.; Hu, X.; Zhuang, X.; Sun, Y.; Guo, Y.; Tan, S.; Zhang, Z. A safe, simple and efficient doxorubicin prodrug hybrid micelle for overcoming tumor multidrug resistance and targeting delivery. J. Control. Release 2016, 235, 182–194. [Google Scholar] [CrossRef]
- Krasnovskaya, O.O.; Malinnikov, V.M.; Dashkova, N.S.; Gerasimov, V.M.; Grishina, I.V.; Kireev, I.I.; Lavrushkina, S.V.; Panchenko, P.A.; Zakharko, M.A.; Ignatov, P.A.; et al. Thiourea Modified Doxorubicin: A Perspective pH-Sensitive Prodrug. Bioconjug. Chem. 2019, 30, 741–750. [Google Scholar] [CrossRef]
- Moon, C.; Kwon, Y.M.; Lee, W.K.; Park, Y.J.; Yang, V.C. In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide. J. Control Release 2007, 124, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Ding, J.; Xiao, C.; Chen, J.; Zhuang, X.; Chen, X. Preclinical evaluation of antitumor activity of acid-sensitive PEGylated doxorubicin. ACS Appl. Mater. Interfaces 2014, 6, 21202–21214. [Google Scholar] [CrossRef]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef]
- Bonner, D.K.; Zhao, X.; Buss, H.; Langer, R.; Hammond, P.T. Crosslinked linear polyethylenimine enhances delivery of DNA to the cytoplasm. J. Control Release 2013, 167, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Karimi Zade, A.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussif, O.; LezoualC’H, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhang, X.; Zhang, X.; Yang, B.; Li, Z.; Zhang, Q.; Huang, Z.; Wei, Y. Fabrication of cross-linked fluorescent polymer nanoparticles and their cell imaging applications. J. Mater. Chem. C 2015, 3, 1854–1860. [Google Scholar] [CrossRef]
- Chen, S.; Jin, T. Poly-cross-linked PEI through aromatically conjugated imine linkages as a new class of pH-responsive nucleic acids packing cationic polymers. Front. Pharmacol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Amjad, M.W.; Amin, M.C.I.M.; Katas, H.; Butt, A.M. Doxorubicin-loaded cholic acid-polyethyleneimine micelles for targeted delivery of antitumor drugs: Synthesis, characterization, and evaluation of their in vitro cytotoxicity. Nanoscale Res. Lett. 2012, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.W.; Tong, S.W.; Qi, X.R. Comparative studies of polyethylenimine-doxorubicin conjugates with pH-sensitive and pH-insensitive linkers. J. Biomed. Mater. Res. Part A 2013, 101 A, 1336–1344. [Google Scholar] [CrossRef]
- Lindsey, A. The structure of cyclotriveratrylene (10,15-dihydro-2,3,7,8,12,13- hexamethoxy-5H-tribenzo[a,d,g]cyclononene) and related compounds. J. Chem. Soc. 1965, 1685–1692. [Google Scholar] [CrossRef]
- Hardie, M.J. Recent advances in the chemistry of cyclotriveratrylene. Chem. Soc. Rev. 2010, 39, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Sumby, C.J.; Fisher, J.; Prior, T.J.; Hardie, M.J. Tris(pyridylmethylamino)cyclotriguaiacylene cavitands: An investigation of the solution and solid-state behaviour of metallo-supramolecular cages and cavitand-based coordination polymers. Chem. A Eur. J. 2006, 12, 2945–2959. [Google Scholar] [CrossRef] [PubMed]
- Ronson, T.K.; Fisher, J.; Harding, L.P.; Hardie, M.J. Star-burst prisms with cyclotriveratrylene-type ligands: A [Pd 6L8]12+ stella octangular structure. Angew. Chemie. Int. Ed. 2007, 46, 9086–9088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Echegoyen, L. Selective anion sensing by a tris-amide CTV derivative: 1H NMR titration, self-assembled monolayers, and impedance spectroscopy. J. Am. Chem. Soc. 2005, 127, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Bardelang, D.; Camerel, F.; Ziessel, R.; Schmutz, M.; Hannon, M.J. New organogelators based on cyclotriveratrylene platforms bearing 2-dimethylacetal-5-carbonylpyridine fragments. J. Mater. Chem. 2008, 18, 489–494. [Google Scholar] [CrossRef]
- Westcott, A.; Sumby, C.J.; Walshaw, R.D.; Hardie, M.J. Metallo-gels and organo-gels with tripodal cyclotriveratrylene-type and 1,3,5-substituted benzene-type ligands. New J. Chem. 2009, 33, 902–912. [Google Scholar] [CrossRef]
- Little, M.A.; Halcrow, M.A.; Harding, L.P.; Hardie, M.J. Ag(I) organometallic coordination polymers and capsule with Tris-allyl cyclotriveratrylene derivatives. Inorg. Chem. 2010, 49, 9486–9496. [Google Scholar] [CrossRef]
- Cai, F.; Shen, J.S.; Wang, J.H.; Zhang, H.; Zhao, J.S.; Zeng, E.M.; Jiang, Y.B. Hydrogelators of cyclotriveratrylene derivatives. Org. Biomol. Chem. 2012, 10, 1418–1423. [Google Scholar] [CrossRef]
- Percec, V.; Imam, M.R.; Peterca, M.; Wilson, D.A.; Heiney, P.A. Self-assembly of dendritic crowns into chiral supramolecular spheres. J. Am. Chem. Soc. 2009, 131, 1294–1304. [Google Scholar] [CrossRef]
- Ahmad, R.; Hardie, M.J. Variable Ag(I) coordination modes in silver cobalt(III) bis(dicarbollide) supramolecular assemblies with cyclotriveratrylene host molecules. Cryst. Growth Des. 2003, 3, 493–499. [Google Scholar] [CrossRef]
- Maiti, D.; Woertink, J.S.; Ghiladi, R.A.; Solomon, E.I.; Karlin, K.D. Molecular oxygen and sulfur reactivity of a cyclotriveratrylene derived trinuclear copper(I) complex. Inorg. Chem. 2009, 48, 8342–8356. [Google Scholar] [CrossRef]
- Zhong, Z.; Ikeda, A.; Shinkai, S.; Sakamoto, S.; Yamaguchi, K. Creation of Novel Chiral Cryptophanes by a Self-Assembling Method Utilizing a Pyridyl − Pd (II) Interaction. Org. Lett. 2001, 3, 1085–1087. [Google Scholar] [CrossRef]
- Hardie, M.J.; Sumby, C.J. Interwoven 2-D Coordination Network Prepared from the Molecular Host Tris (isonicotinoyl) cyclotriguaiacylene and Silver (I) Cobalt (III) Bis (dicarbollide). Inorg. Chem. Commun. 2004, 43, 6872–6874. [Google Scholar] [CrossRef] [PubMed]
- Sumby, C.J.; Hardie, M.J. Disentangling Disorder in the Three-Dimensional Coordination Network of {Ag3[Tris(2-pyridylmethyl)cyclotriguaiacylene]2}(PF6)3. Cryst. Growth Des. 2005, 5, 1321–1324. [Google Scholar] [CrossRef]
- Konarev, D.V.; Khasanov, S.S.; Vorontsov, I.I.; Saito, G.; Antipin, Y. The formation of a single-bonded (C 70 2) 2 dimer in a new ionic multicomponent complex of cyclotriveratrylene. Chem. Commun. 2002, 2, 2548–2549. [Google Scholar] [CrossRef]
- Dumartin, M.-L.; Givelet, C.; Meyrand, P.; Bibal, B.; Gosse, I. A fluorescent cyclotriveratrylene: Synthesis, emission properties and acetylcholine recognition in water. Org. Biomol. Chem. 2009, 7, 2725–2728. [Google Scholar] [CrossRef] [PubMed]
- Huerta, E.; Isla, H.; Pérez, E.M.; Bo, C.; Martín, N.; Mendoza, J. De Tripodal exTTF-CTV hosts for fullerenes. J. Am. Chem. Soc. 2010, 132, 5351–5353. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, J.F.; Oswald, L.; Eckert, J.F.; Nicoud, J.F.; Armaroli, N. Complexation of fullerenes with dendritic cyclotriveratrylene derivatives. Tetrahedron Lett. 1999, 40, 5681–5684. [Google Scholar] [CrossRef]
- Steed, J.W.; Junk, P.C.; Atwood, J.L.; Barnes, M.J.; Raston, C.L.; Burkhalter, R.S. Ball-and-Socket Nanostructures - New Supramolecular Chemistry Based on Cyclotriveratrylene. J. Am. Chem. Soc. 1994, 116, 10346–10347. [Google Scholar] [CrossRef]
- Huerta, E.; Cequier, E.; de Mendoza, J. Preferential separation of fullerene- [84] from fullerene mixtures by encapsulation. Chem. Commun. 2007, 5016–5018. [Google Scholar] [CrossRef]
- Felder, D.; Heinrich, B.; Guillon, D.; Nicoud, J.-F.; Nierengarten, J.-F. A Liquid Crystalline Supramolecular Complex of C60 with a Cyclotriveratrylene Derivative. Chem. A Eur. J. 2000, 6, 3501–3507. [Google Scholar] [CrossRef]
- Atwood, J.L.; Barnes, M.J.; Gardiner, M.G.; Raston, C.L. Cyclotriveratrylene polarisation assisted aggregation of C60. Chem. Commun. 1996, 1449–1450. [Google Scholar] [CrossRef]
- Rio, Y. Water soluble supramolecular cyclotriveratrylene- [60] fullerene. Tetrahedron Lett. 2002, 43, 4321–4324. [Google Scholar] [CrossRef]
- Lekar, A.V.; Borisenko, S.N.; Vetrova, E.V.; Borisenko, R.N.; Borisenko, N.I. Mass Spectrometry Study of Non-Covalent Complexes of Bioflavonoids with Cyclotriveratrylene Synthesized in Subcritical Water. Am. J. Anal. Chem. 2013, 04, 464–468. [Google Scholar] [CrossRef] [Green Version]
- Dawn, A.; Chandra, H.; Ade-Browne, C.; Yadav, J.; Kumari, H. Multifaceted Supramolecular Interactions from C-Methylresorcin[4 ]arene Lead to an Enhancement in In Vitro Antibacterial Activity of Gatifloxacin. Chem. A Eur. J. 2017, 23, 18171–18179. [Google Scholar] [CrossRef]
- Riggle, B.A.; Wang, Y.; Dmochowski, I.J. A “Smart” 129Xe NMR biosensor for pH-dependent cell labeling. J. Am. Chem. Soc. 2015, 137, 5542–5548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Seward, G.K.; Hill, P.A.; Patton, B.; Dimitrov, I.E.; Kuzma, N.N.; Dmochowski, I.J. Designing 129Xe NMR biosensors for matrix metalloproteinase detection. J. Am. Chem. Soc. 2006, 128, 13274–13283. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chatelet, B.; Dufaud, V.; Hérault, D.; Jean, M.; Vanthuyne, N.; Mulatier, J.C.; Pitrat, D.; Guy, L.; Dutasta, J.P.; et al. Enantio- And Substrate-Selective Recognition of Chiral Neurotransmitters with C3-Symmetric Switchable Receptors. Org. Lett. 2020, 22, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Brotin, T.; Dutasta, J.P. Xe@cryptophane complexes with C2 symmetry: Synthesis and investigations by 129Xe NMR of the consequences of the size of the host cavity for xenon encapsulation. Eur. J. Org. Chem. 2003, 973–984. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Ma, P.X. Host-guest interaction mediated polymeric assemblies: Multifunctional nanoparticles for drug and gene delivery. ACS Nano 2010, 4, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; da Silva Moreira Thiré, R.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Rev. Mater. 2016, 21, 767–779. [Google Scholar]
- Wang, D.-Y.; Das, A.; Costa, F.R.; Leuteritz, A.; Wang, Y.-Z.; Wagenknecht, U.; Heinrich, G. Synthesis of organo cobalt-aluminum layered double hydroxide via a novel single-step self-assembling method and its use as flame retardant nanofiller in PP. Langmuir 2010, 26, 14162–14169. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Shah, J.S.; Misra, R.D.K. A new family of folate-decorated and carbon nanotube-mediated drug delivery system: Synthesis and drug delivery response. Adv. Drug Deliv. Rev. 2011, 63, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.F.; Zhuang, W.R.; Hu, X.; Xing, L.; Yu, R.Y.; Qiao, J.B.; He, Y.J.; Li, F.; Ling, D.; Jiang, H.L. A new strategy for hydrophobic drug delivery using a hydrophilic polymer equipped with stacking units. Chem. Commun. 2018, 54, 8218–8221. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, Y.; Peng, X.; Su, T.; Luo, S.; Cao, J.; Gu, Z.; He, B. A facile strategy to generate polymeric nanoparticles for synergistic chemo-photodynamic therapy. Chem. Commun. 2015, 51, 4271–4274. [Google Scholar] [CrossRef] [PubMed]
- Piorecka, K.; Stanczyk, W.; Florczak, M. NMR analysis of antitumor drugs: Doxorubicin, daunorubicin and their functionalized derivatives. Tetrahedron Lett. 2017, 58, 152–155. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Lu, X.; Lu, W.; Zhang, C.; Liang, W. Pegylated phospholipids-based self-assembly with water-soluble drugs. Pharm. Res. 2010, 27, 361–370. [Google Scholar] [CrossRef]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Siskos, M.; Gerothanassis, I.P. 1H-NMR as a structural and analytical tool of intra- and intermolecular hydrogen bonds of phenol-containing natural products and model compounds. Molecules 2014, 19, 13643–13682. [Google Scholar] [CrossRef]
- MacHatova, Z.; Barbieriková, Z.; Poliak, P.; Jančovičová, V.; Lukeš, V.; Brezová, V. Study of natural anthraquinone colorants by EPR and UV/vis spectroscopy. Dye Pigment. 2016, 132, 79–93. [Google Scholar] [CrossRef]
- Fernandes, T.S.; Santos, E.C.S.; Madriaga, V.G.C.; Bessa, I.A.A.; Nascimento, V.; Garcia, F.; Ronconi, C.M. A Self-Assembled AMF-Responsive Nanoplatform Based on Pillar[5]arene and Superparamagnetic Nanoparticles for Controlled Release of Doxorubicin. J. Braz. Chem. Soc. 2019, 30, 2452–2463. [Google Scholar]
- Li, Y.; Tong, Y.; Cao, R.; Tian, Z.; Yang, B.; Yang, P. In vivo enhancement of anticancer therapy using bare or chemotherapeutic drug-bearing nanodiamond particles. Int. J. Nanomed. 2014, 9, 1065–1082. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C01 2016. Available online: http://gaussian.com/gaussian16/ (accessed on 23 September 2020).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Halgren, T. Merck Molecular Force Field. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Density functional theory with London dispersion corrections. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, H.; Grimme, S. A geometrical correction for the inter- and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems. J. Chem. Phys. 2012, 136, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Kruse, H. Website Ttle: gCP-D3 Webservice. Available online: http://wwwtc.thch.uni-bonn.de/ (accessed on 23 September 2020).
- Humphrey, W.; Dalke, A.; Schulten, K. Sartorius products. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
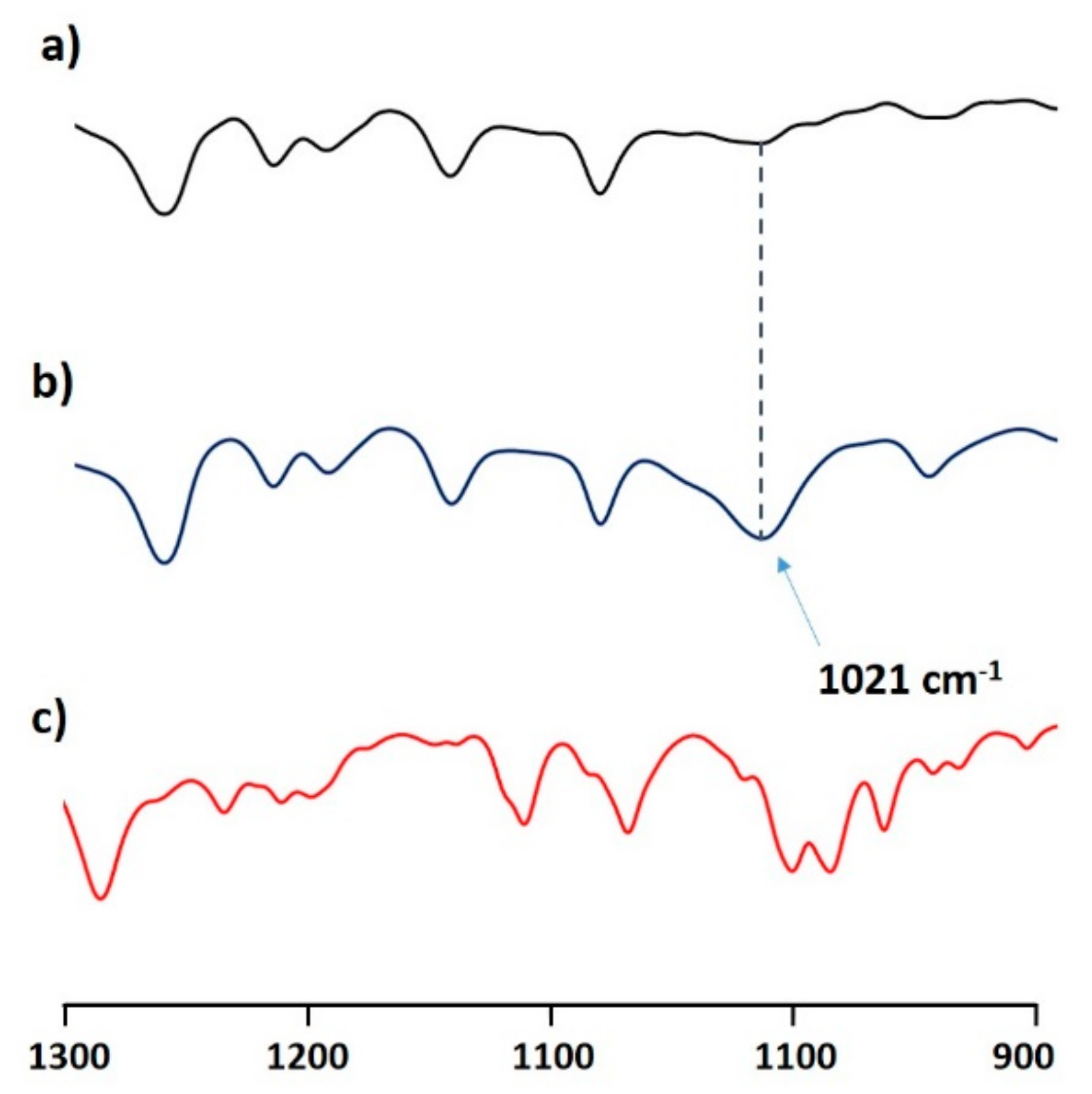
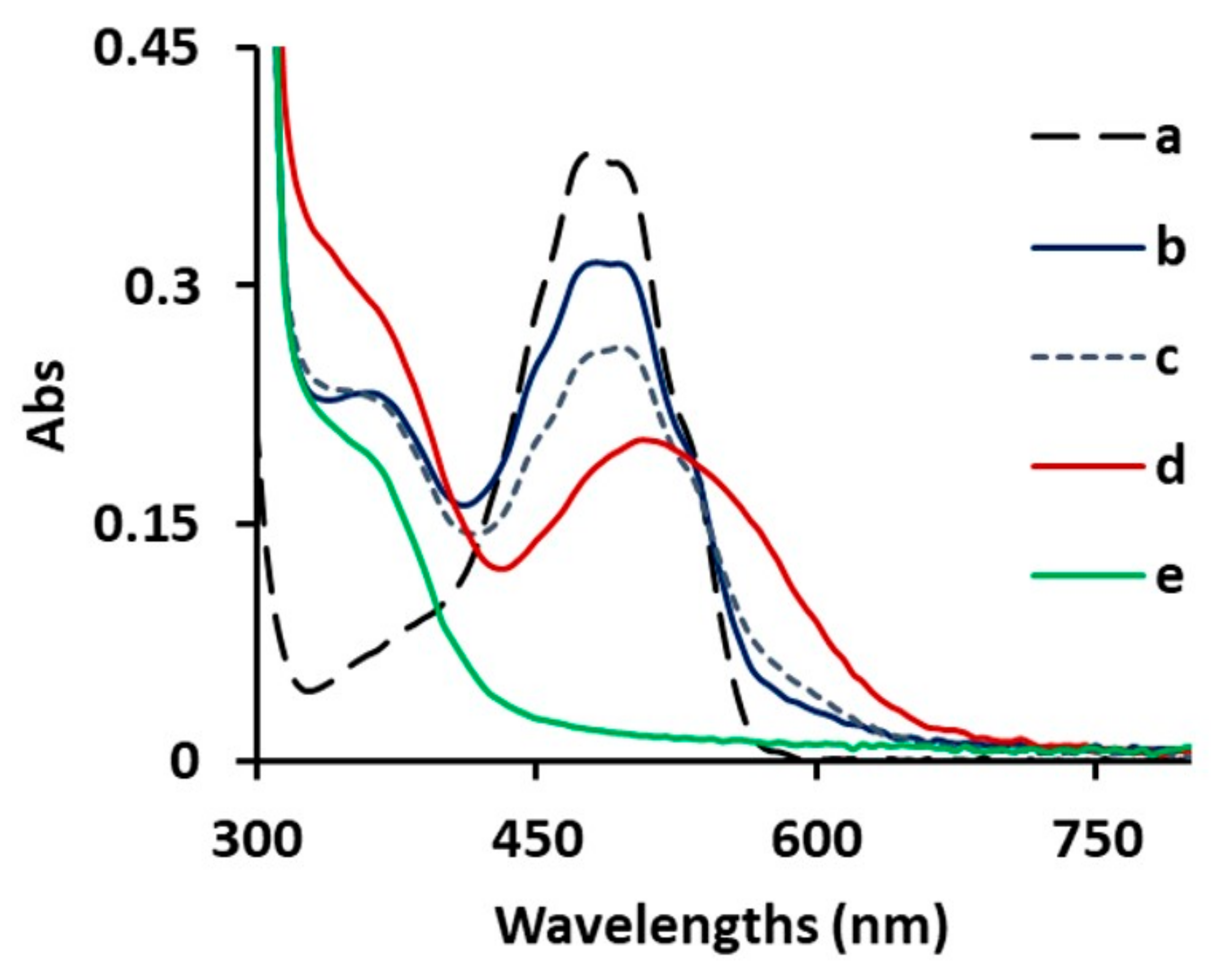
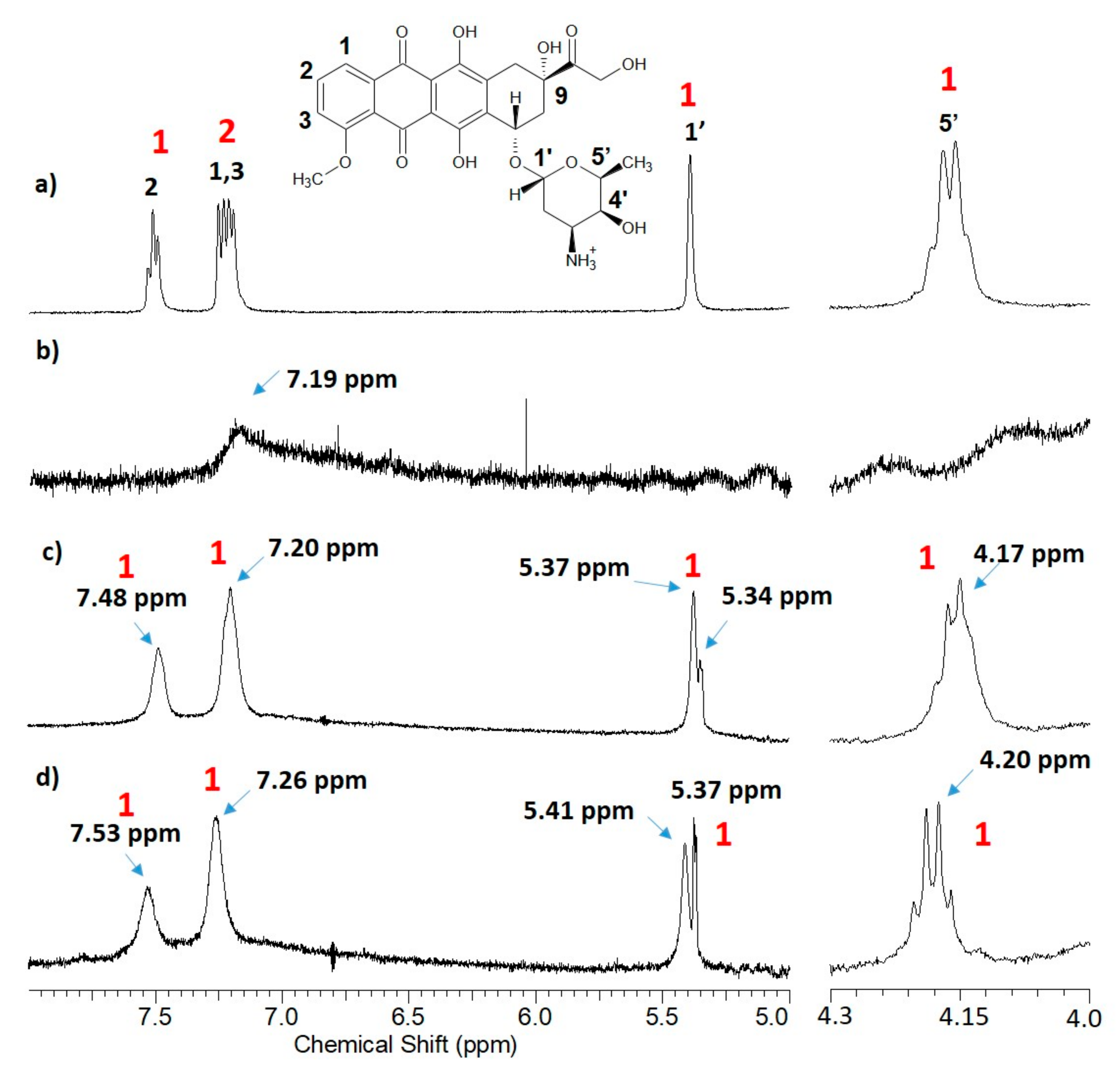

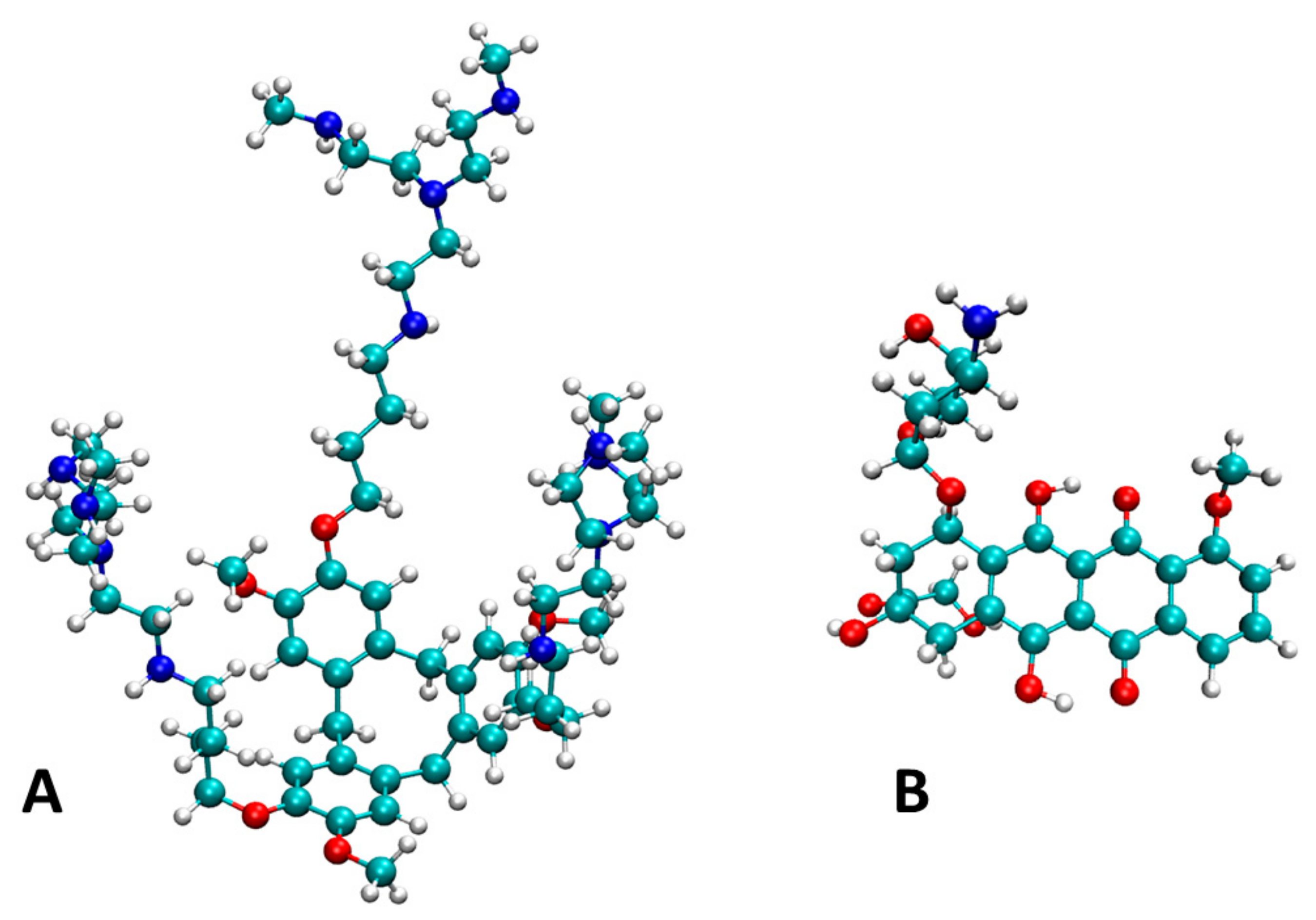
| mg of Titrant 1 (Solvent) | λ1, λ2 (nm) | ε1, ε2, (M−1 cm−1) |
|---|---|---|
| 0 mg (MeOH) | 475, 495 | 18,560, 17,460 |
| 5 mg 2 of 3a (MeOH) | 475, 495 | 10,860, 10,840 |
| 3 mg 3 (of 3a (MeOH aged 20 days) | 500, 535 | 13,060, 13,330 |
| 3 mg 3 of PEI (MeOH) | 480, 495 | 17,400, 17,460 |
| 0 mg (DMSO) | 480 | 9260 |
| 3 mg 3 of 3a (DMSO) | 485 | 10,200 |
| 3 mg 3 of 3a (DMSO aged 20 days) | 485 | 10,200 |
| 3 mg 4 of PEI (DMSO) | 500, 595 | 7330, 3420 |
| 0 mg of (H2O) | 480 | 13,200 |
| 3 mg 3 of 3a (H2O) | 485, 495 | 10,860, 10,850 |
| 3 mg 3 of 3a (H2O aged 20 days) | 510 | 7010 |
| 3 mg 4 of PEI (H2O) | 550, 590 | 10,300, 9100 |
| Protons 2 | (MeOD) | +3a (MeOD) | +PEI (MeOD) | (DMSO) | +3a (DMSO) | (D2O) | +3a (D2O) | +3a aged (D2O) |
|---|---|---|---|---|---|---|---|---|
| -H1 | 7.99 | 7.97 | 7.73 | 7.85 | 7.89 | 7.22 | 7.20 | 7.26 |
| -H2 | 7.86 | 7.83 | 7.46 | 7.85 | 7.89 | 7.50 | 7.48 | 7.53 |
| -H3 | 7.60 | 7.57 | 7.46 | 7.61 | 7.65 | 7.22 | 7.20 | 7.26 |
| -OH 9,4 | 5.46 | 5.43 | 5.35 | 5.46 | 5.43 | - | - | - |
| -H1′ | 5.12 | 5.12 | 4.98 | 5.27 | 5.27 | 5.39 | 5.37 | 5.41 |
| -H5′ | 4.29 | 4.25 | 4.18 | 4.19 | 4.16 | 4.18 | 4.18 | 4.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluccini, C.; Ng, Y.M.; Reyes, Y.I.A.; Chen, H.-Y.T.; Khung, Y.L. Functionalization of Polyethyleneimine with Hollow Cyclotriveratrylene and Its Subsequent Supramolecular Interaction with Doxorubicin. Molecules 2020, 25, 5455. https://doi.org/10.3390/molecules25225455
Coluccini C, Ng YM, Reyes YIA, Chen H-YT, Khung YL. Functionalization of Polyethyleneimine with Hollow Cyclotriveratrylene and Its Subsequent Supramolecular Interaction with Doxorubicin. Molecules. 2020; 25(22):5455. https://doi.org/10.3390/molecules25225455
Chicago/Turabian StyleColuccini, Carmine, Yoke Mooi Ng, Yves Ira A. Reyes, Hsin-Yi Tiffany Chen, and Yit Lung Khung. 2020. "Functionalization of Polyethyleneimine with Hollow Cyclotriveratrylene and Its Subsequent Supramolecular Interaction with Doxorubicin" Molecules 25, no. 22: 5455. https://doi.org/10.3390/molecules25225455
APA StyleColuccini, C., Ng, Y. M., Reyes, Y. I. A., Chen, H.-Y. T., & Khung, Y. L. (2020). Functionalization of Polyethyleneimine with Hollow Cyclotriveratrylene and Its Subsequent Supramolecular Interaction with Doxorubicin. Molecules, 25(22), 5455. https://doi.org/10.3390/molecules25225455








