Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development
Abstract
:1. Introduction
2. Pre-Clinical Evidence of Flavonoids for Pain Control
2.1. Flavonols
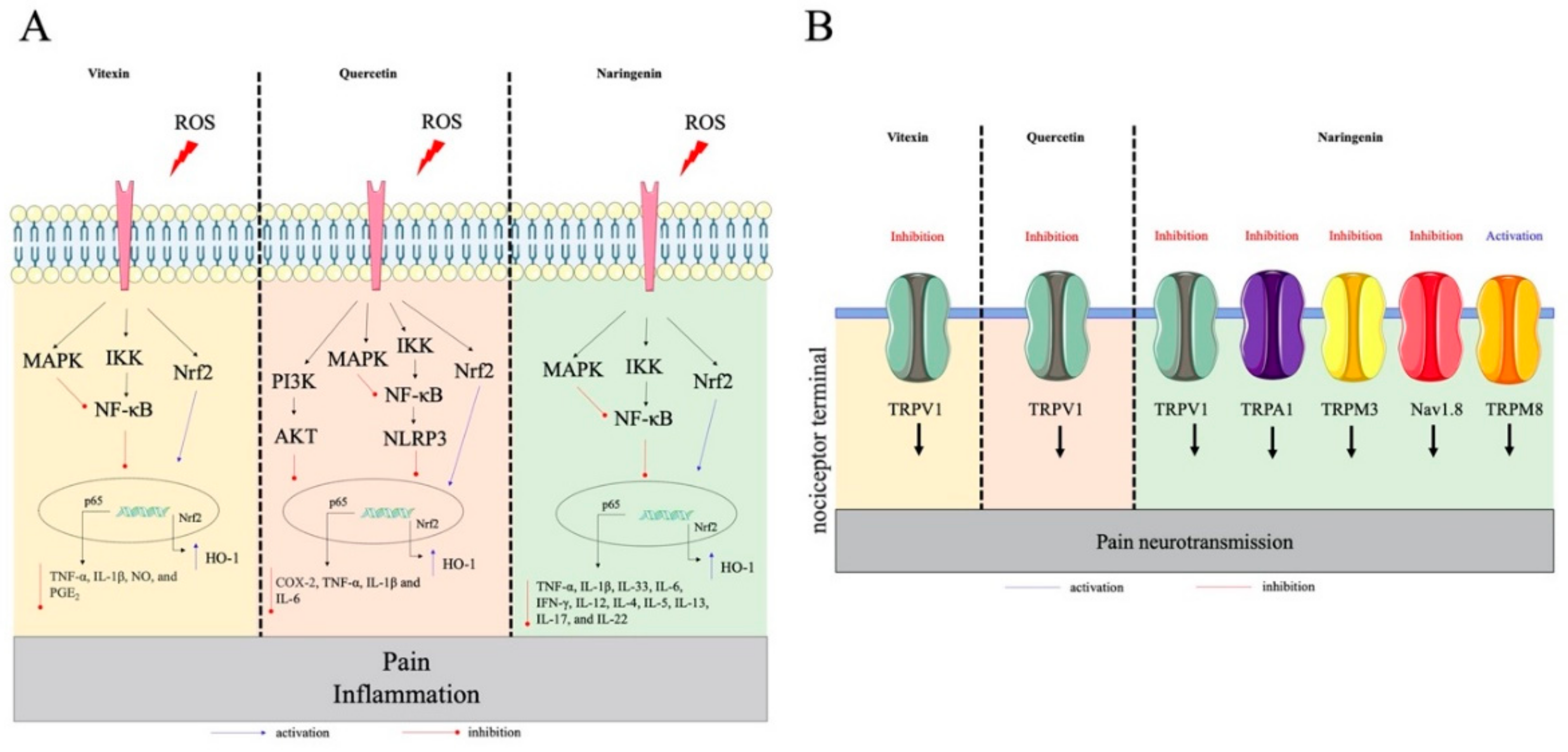
2.2. Flavones
2.3. Flavanones
2.4. Chalcone
2.5. Flavanols, Flavan-3-ols or Catechins
3. Structure-Activity Relationship (SAR)
4. Clinical Studies and Safety
4.1. Hesperidin
4.2. Catechins
4.3. Quercetin
4.4. Apigenin
4.5. Flavonoid-Based Compounds
5. Development of Pharmaceutical Formulations Containing Flavonoids
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev Immunol 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kang, B.S.; Lee, H.L.; Son, S.J.; Hwang, S.H.; Kim, D.S.; Park, J.S.; Cho, H.J. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci. 2004, 19, 3375–3381. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct Target. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, G.R.; Cunha, T.M.; Silva, R.L.; Lotufo, C.M.; Verri, W.A., Jr.; Funez, M.I.; Villarreal, C.F.; Talbot, J.; Sousa, L.P.; Parada, C.A.; et al. Involvement of nuclear factor κ B in the maintenance of persistent inflammatory hypernociception. Pharm. Biochem. Behav. 2015, 134, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraz, C.R.; Calixto-Campos, C.; Manchope, M.F.; Casagrande, R.; Clissa, P.B.; Baldo, C.; Verri, W.A., Jr. Jararhagin-induced mechanical hyperalgesia depends on TNF-alpha, IL-1beta and NFκB in mice. Toxicon 2015, 103, 119–128. [Google Scholar] [CrossRef]
- Fattori, V.; Amaral, F.A.; Verri, W.A., Jr. Neutrophils and arthritis: Role in disease and pharmacological perspectives. Pharm. Res. 2016, 112, 84–98. [Google Scholar] [CrossRef]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef]
- Woolf, C.J. What is this thing called pain? J. Clin. Invest. 2010, 120, 3742–3744. [Google Scholar] [CrossRef]
- Woolf, C.J.; Salter, M.W. Neuronal plasticity: Increasing the gain in pain. Science 2000, 288, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattori, V.; Borghi, S.M.; Rossaneis, A.C.; Bertozzi, M.M.; Cunha, T.M.; Verri, W.A., Jr. Neuroimmune Regulation of Pain and Inflammation: Targeting Glial Cells and Nociceptor Sensory Neurons Interaction. In Frontiers in CNS Drug Discovery; Atta-ur, R., Choudhary, M.I., Eds.; Bentham: New York, NY, USA, 2017; Volume 3, pp. 146–200. [Google Scholar]
- Zarpelon, A.C.; Rodrigues, F.C.; Lopes, A.H.; Souza, G.R.; Carvalho, T.T.; Pinto, L.G.; Xu, D.; Ferreira, S.H.; Alves-Filho, J.C.; McInnes, I.B.; et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. Faseb J. 2016, 30, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Borghi, S.M.; Rossaneis, A.C.; Casagrande, R.; Verri, W.A., Jr. The specialized pro-resolving lipid mediator Maresin-1 reduces inflammatory pain with a long-lasting analgesic effect. Br. J. Pharm. 2019, 176, 1728–1744. [Google Scholar] [CrossRef]
- Rayar, A.M.; Lagarde, N.; Ferroud, C.; Zagury, J.F.; Montes, M.; Sylla-Iyarreta Veitia, M. Update on COX-2 selective inhibitors: Chemical classification, side effects and their use in cancers and neuronal diseases. Curr. Top. Med. Chem. 2017, 17, 2935–2956. [Google Scholar] [CrossRef]
- Fattori, V.; Borghi, S.M.; Guazelli, C.F.; Giroldo, A.C.; Crespigio, J.; Bussmann, A.J.; Coelho-Silva, L.; Ludwig, N.G.; Mazzuco, T.L.; Casagrande, R.; et al. Vinpocetine reduces diclofenac-induced acute kidney injury through inhibition of oxidative stress, apoptosis, cytokine production, and NF-κB activation in mice. Pharmacol. Res. 2017, 120, 10–22. [Google Scholar] [CrossRef]
- Ungprasert, P.; Srivali, N.; Thongprayoon, C. Nonsteroidal anti-inflammatory drugs and risk of incident heart failure: A systematic review and meta-analysis of observational studies. Clin. Cardiol. 2016, 39, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Marcondes-Alves, L.; Fattori, V.; Borghi, S.M.; Lourenco-Gonzalez, Y.; Bussmann, A.J.C.; Hirooka, E.Y.; Casagrande, R.; Verri, W.A., Jr.; Arakawa, N.S. Kaurenoic acid extracted from Sphagneticola trilobata reduces acetaminophen-induced hepatotoxicity through inhibition of oxidative stress and pro-inflammatory cytokine production in mice. Nat. Prod. Res. 2019, 33, 921–924. [Google Scholar] [CrossRef]
- Larsen, F.S.; Wendon, J. Understanding paracetamol-induced liver failure. Intensive Care Med. 2014, 40, 888–890. [Google Scholar] [CrossRef]
- Karp, J.F.; Shega, J.W.; Morone, N.E.; Weiner, D.K. Advances in understanding the mechanisms and management of persistent pain in older adults. Br. J. Anaesth. 2008, 101, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutz, S.G.; Robinson-Papp, J. HIV-related neuropathy: Current perspectives. Hiv Aids (Auckl) 2013, 5, 243–251. [Google Scholar]
- Grace, P.M.; Maier, S.F.; Watkins, L.R. Opioid-induced central immune signaling: Implications for opioid analgesia. Headache 2015, 55, 475–489. [Google Scholar] [CrossRef] [Green Version]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Rice, K.C.; Maier, S.F.; Watkins, L.R. Protraction of neuropathic pain by morphine is mediated by spinal damage associated molecular patterns (DAMPs) in male rats. Brain Behav. Immun. 2018, 72, 45–50. [Google Scholar] [CrossRef]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Urban, D.J.; Wang, X.; Baratta, M.V.; Fabisiak, T.J.; Anderson, N.D.; Cheng, K.; Greene, L.I.; et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 2016, 113, E3441–E3450. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Chu, H.; Jiang, Y.; Yuan, L. Morphine enhances IL-1beta release through toll-like receptor 4-mediated endocytic pathway in microglia. Purinergic Signal. 2016, 12, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poetker, D.M.; Reh, D.D. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin. N. Am. 2010, 43, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Slordal, L.; Spigset, O. Heart Failure Induced by Non-Cardiac Drugs. Drug Saf. 2006, 29, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Orlicka, K.; Barnes, E.; Culver, E.L. Prevention of infection caused by immunosuppressive drugs in gastroenterology. Adv. Chronic Dis. 2013, 4, 167–185. [Google Scholar] [CrossRef] [Green Version]
- Cabral, V.P.; Andrade, C.A.; Passos, S.R.; Martins, M.F.; Hokerberg, Y.H. Severe infection in patients with rheumatoid arthritis taking anakinra, rituximab, or abatacept: A systematic review of observational studies. Rev. Bras. Reum. Engl. Ed. 2016, 56, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Plein, L.M.; Rittner, H.L. Opioids and the immune system - friend or foe. Br. J. Pharm. 2018, 175, 2717–2725. [Google Scholar] [CrossRef]
- Borghi, S.M.; Mizokami, S.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Crespigio, J.; Clemente-Napimoga, J.T.; Napimoga, M.H.; Pitol, D.L.; Issa, J.P.M.; Fukada, S.Y.; et al. The flavonoid quercetin inhibits titanium dioxide (TiO2)-induced chronic arthritis in mice. J. Nutr. Biochem. 2018, 53, 81–95. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Vicentini, F.T.; Baracat, M.M.; Georgetti, S.R.; Cardoso, R.D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Fonseca, M.J.; Casagrande, R. Flavonoids as Anti-Inflammatory and Analgesic Drugs: Mechanisms of Action and Perspectives in the Development of Pharmaceutical Forms. In Studies in Natural Products Chemistry, 1st ed.; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 36, pp. 297–330. [Google Scholar]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of inflammatory mediators with pain perception. Biomed. Pharm. 2017, 96, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharm. 2016, 771, 84–92. [Google Scholar] [CrossRef]
- Carvalho, T.T.; Mizokami, S.S.; Ferraz, C.R.; Manchope, M.F.; Borghi, S.M.; Fattori, V.; Calixto-Campos, C.; Camilios-Neto, D.; Casagrande, R.; Verri, W.A., Jr. The granulopoietic cytokine granulocyte colony-stimulating factor (G-CSF) induces pain: Analgesia by rutin. Inflammopharmacology 2019, 27, 1285–1296. [Google Scholar] [CrossRef]
- Lamoke, F.; Labazi, M.; Montemari, A.; Parisi, G.; Varano, M.; Bartoli, M. Trans-Chalcone prevents VEGF expression and retinal neovascularization in the ischemic retina. Exp. Eye Res. 2011, 93, 350–354. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Fattori, V.; Zaninelli, T.H.; Badaro-Garcia, S.; Borghi, S.M.; Carvalho, T.T.; Alves-Filho, J.C.; Cunha, T.M.; et al. Trans-Chalcone Attenuates Pain and Inflammation in Experimental Acute Gout Arthritis in Mice. Front. Pharm. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Qi, W.; Lin, C.; Fan, K.; Chen, Z.; Liu, L.; Feng, X.; Zhang, H.; Shao, Y.; Fang, H.; Zhao, C.; et al. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem Biol Interact. 2019, 306, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Vaibhav, K.; Ahmed, M.E.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M.M.; Islam, F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J. Neurol. Sci. 2015, 348, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lin, J.K. (−)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-κB. Mol. Pharm. 1997, 52, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Cardenas, H.; Arango, D.; Nicholas, C.; Duarte, S.; Nuovo, G.J.; He, W.; Voss, O.H.; Gonzalez-Mejia, M.E.; Guttridge, D.C.; Grotewold, E.; et al. Dietary Apigenin Exerts Immune-Regulatory Activity in Vivo by Reducing NF-κB Activity, Halting Leukocyte Infiltration and Restoring Normal Metabolic Function. Int. J. Mol. Sci. 2016, 17, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, M.; Rehman, M.U.; Lateef, A.; Khan, R.; Khan, A.Q.; Qamar, W.; Ali, F.; O’Hamiza, O.; Sultana, S. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-alpha and NF-κB activation. Alcohol 2013, 47, 131–139. [Google Scholar] [CrossRef]
- Rasquel-Oliveira, F.S.; Manchope, M.F.; Staurengo-Ferrari, L.; Ferraz, C.R.; Santos, T.S.; Zaninelli, T.H.; Fattori, V.; Antero, N.A.; Badaro-Garcia, S.; Freitas, A.; et al. Hesperidin methyl chalcone interacts with NFκB Ser276 and inhibits zymosan-induced joint pain and inflammation, and RAW 264.7 macrophage activation. Inflammopharmacology 2020. accepted. [Google Scholar]
- Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Staurengo-Ferrari, L.; Fattori, V.; Amaral, F.A.; Teixeira, M.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; et al. Hesperidin Methylchalcone Suppresses Experimental Gout Arthritis in Mice by Inhibiting NF-κB Activation. J. Agric. Food Chem. 2018, 66, 6269–6280. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Pala, D.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Topical formulation containing hesperidin methyl chalcone inhibits skin oxidative stress and inflammation induced by ultraviolet B irradiation. Photochem. Photobiol Sci. 2016, 15, 554–563. [Google Scholar] [CrossRef]
- Jiang, J.; Jia, Y.; Lu, X.; Zhang, T.; Zhao, K.; Fu, Z.; Pang, C.; Qian, Y. Vitexin suppresses RANKL-induced osteoclastogenesis and prevents lipopolysaccharide (LPS)-induced osteolysis. J. Cell Physiol. 2019, 234, 17549–17560. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, T.; Liu, J.; Gu, L. Vitexin attenuates lipopolysaccharide-induced acute lung injury by controlling the Nrf2 pathway. PLoS ONE 2018, 13, e0196405. [Google Scholar] [CrossRef]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Antiinflammatory effect of quercetin and galangin in LPSstimulated RAW264.7 macrophages and DNCBinduced atopic dermatitis animal models. Int J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [PubMed]
- Guazelli, C.F.S.; Staurengo-Ferrari, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Ruiz-Miyazawa, K.W.; Vicentini, F.; Vignoli, J.A.; Camilios-Neto, D.; Georgetti, S.R.; Baracat, M.M.; et al. Quercetin attenuates zymosan-induced arthritis in mice. Biomed. Pharm. 2018, 102, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. J. Derm. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Harter, D.; Desai, D.; Amin, S.; Murali, R.; Jhanwar-Uniyal, M. Quercetin inhibits the akt pathway, leading to suppression of survival and induction of apoptosis in cancer cells. Cancer Res. 2005, 65, 536. [Google Scholar]
- Domiciano, T.P.; Wakita, D.; Jones, H.D.; Crother, T.R.; Verri, W.A., Jr.; Arditi, M.; Shimada, K. Quercetin Inhibits Inflammasome Activation by Interfering with ASC Oligomerization and Prevents Interleukin-1 Mediated Mouse Vasculitis. Sci Rep. 2017, 7, 41539. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A., Jr. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Bussmann, A.J.C.; Borghi, S.M.; Zaninelli, T.H.; Dos Santos, T.S.; Guazelli, C.F.S.; Fattori, V.; Domiciano, T.P.; Pinho-Ribeiro, F.A.; Ruiz-Miyazawa, K.W.; Casella, A.M.B.; et al. The citrus flavanone naringenin attenuates zymosan-induced mouse joint inflammation: Induction of Nrf2 expression in recruited CD45(+) hematopoietic cells. Inflammopharmacology 2019, 27, 1229–1242. [Google Scholar] [CrossRef]
- Manchope, M.F.; Artero, N.A.; Fattori, V.; Mizokami, S.S.; Pitol, D.L.; Issa, J.P.M.; Fukada, S.Y.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; et al. Naringenin mitigates titanium dioxide (TiO2)-induced chronic arthritis in mice: Role of oxidative stress, cytokines, and NFκB. Inflamm Res. 2018, 67, 997–1012. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.; Vicentini, F.T.; Vignoli, J.A.; Baracat, M.M.; et al. Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced Skin Inflammation and Oxidative Stress in Mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A., Jr. Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines, Nrf-2 and the NO-cGMP-PKG-KATPChannel Signaling Pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Gao, W.; Zan, Y.; Wang, Z.J.; Hu, X.Y.; Huang, F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCepsilon-dependent activation of TRPV1. Acta Pharm. Sin. 2016, 37, 1166–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straub, I.; Mohr, F.; Stab, J.; Konrad, M.; Philipp, S.E.; Oberwinkler, J.; Schaefer, M. Citrus fruit and fabacea secondary metabolites potently and selectively block TRPM3. Br. J. Pharmacol. 2013, 168, 1835–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Cai, S.; Moutal, A.; Yu, J.; Gomez, K.; Madura, C.L.; Shan, Z.; Pham, N.Y.N.; Serafini, M.J.; Dorame, A.; et al. The Natural Flavonoid Naringenin Elicits Analgesia through Inhibition of NaV1.8 Voltage-Gated Sodium Channels. ACS Chem. Neurosci. 2019, 10, 4834–4846. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Silva, D.B.; Prado, L.; Canabrava, H.A.N.; Bispo-da-Silva, L.B. Antidiarrhoeic effect and dereplication of the aqueous extract of Annona crassiflora (Annonaceae). Nat. Prod. Res. 2019, 33, 563–567. [Google Scholar] [CrossRef]
- Guazelli, C.F.; Fattori, V.; Colombo, B.B.; Georgetti, S.R.; Vicentini, F.T.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J. Nat. Prod. 2013, 76, 200–208. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Clemente-Napimoga, J.T.; Macedo, C.G.; Freitas, F.F.; Stipp, R.N.; Pinho-Ribeiro, F.A.; Casagrande, R.; Verri, W.A., Jr. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J. Nat. Prod. 2013, 76, 2316–2321. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Correa, M.P.; Carvalho, T.T.; Zarpelon, A.C.; Hohmann, M.S.; Rossaneis, A.C.; Coelho-Silva, L.; Pavanelli, W.R.; Pinge-Filho, P.; Crespigio, J.; et al. Quercetin Reduces Ehrlich Tumor-Induced Cancer Pain in Mice. Anal. Cell Pathol. 2015, 2015, 285708. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B 2006, 84, 21–27. [Google Scholar] [CrossRef]
- Valerio, D.A.; Georgetti, S.R.; Magro, D.A.; Casagrande, R.; Cunha, T.M.; Vicentini, F.T.; Vieira, S.M.; Fonseca, M.J.; Ferreira, S.H.; Cunha, F.Q.; et al. Quercetin reduces inflammatory pain: Inhibition of oxidative stress and cytokine production. J. Nat. Prod. 2009, 72, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Maioli, N.A.; Zarpelon, A.C.; Mizokami, S.S.; Calixto-Campos, C.; Guazelli, C.F.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Carvalho, T.T.; Manchope, M.F.; Ferraz, C.R.; et al. The superoxide anion donor, potassium superoxide, induces pain and inflammation in mice through production of reactive oxygen species and cyclooxygenase-2. Braz. J. Med. Biol Res. 2015, 48, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Souto, F.O.; Zarpelon, A.C.; Staurengo-Ferrari, L.; Fattori, V.; Casagrande, R.; Fonseca, M.J.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A., Jr. Quercetin reduces neutrophil recruitment induced by CXCL8, LTB4, and fMLP: Inhibition of actin polymerization. J. Nat. Prod. 2011, 74, 113–118. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.; Ha, J.H.; Lee, M.G.; Kim, J.E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Katsarou-Katsari, A.; Antoniou, C.; Theoharides, T.C. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE 2012, 7, e33805. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, C.H.; Yen, G.C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Boomgaarden, I.; Egert, S.; Rimbach, G.; Wolffram, S.; Muller, M.J.; Doring, F. Quercetin supplementation and its effect on human monocyte gene expression profiles in vivo. Br. J. Nutr. 2010, 104, 336–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.Y.; Yu, Y.L.; Cheng, W.C.; OuYang, C.N.; Fu, E.; Chu, C.L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Wang, W.; Wang, D.; Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 2017, 61, 1700031. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, W. Anti-Inflammatory Effect of Quercetin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen, M.; Nieminen, R.; Asmawi, M.Z.; Vuorela, P.; Vapaatalo, H.; Moilanen, E. Effects of flavonoids on prostaglandin E2 production and on COX-2 and mPGES-1 expressions in activated macrophages. Planta Med. 2011, 77, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- Lara-Guzman, O.J.; Tabares-Guevara, J.H.; Leon-Varela, Y.M.; Alvarez, R.M.; Roldan, M.; Sierra, J.A.; Londono-Londono, J.A.; Ramirez-Pineda, J.R. Proatherogenic macrophage activities are targeted by the flavonoid quercetin. J. Pharm. Exp. 2012, 343, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhang, X.; Zhang, L.; Bian, H.X.; Xu, N.; Bao, B.; Liu, J. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: A mechanism including AMPKalpha1/SIRT1. J. Lipid Res. 2014, 55, 363–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghi, S.M.; Pinho-Ribeiro, F.A.; Fattori, V.; Bussmann, A.J.; Vignoli, J.A.; Camilios-Neto, D.; Casagrande, R.; Verri, W.A., Jr. Quercetin Inhibits Peripheral and Spinal Cord Nociceptive Mechanisms to Reduce Intense Acute Swimming-Induced Muscle Pain in Mice. PLoS ONE 2016, 11, e0162267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carcole, M.; Castany, S.; Leanez, S.; Pol, O. Treatment with a heme oxygenase 1 inducer enhances the antinociceptive effects of micro-opioid, delta-opioid, and cannabinoid 2 receptors during inflammatory pain. J. Pharm. Exp. 2014, 351, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A., Jr.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl Acad Sci. USA 2010, 107, 4442–4447. [Google Scholar] [CrossRef] [Green Version]
- Sachs, D.; Cunha, F.Q.; Ferreira, S.H. Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 3680–3685. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.H.; Qu, J.; Shen, X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef]
- Wu, C.H.; Lin, M.C.; Wang, H.C.; Yang, M.Y.; Jou, M.J.; Wang, C.J. Rutin inhibits oleic acid induced lipid accumulation via reducing lipogenesis and oxidative stress in hepatocarcinoma cells. J. Food Sci. 2011, 76, T65–T72. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Invest. 2014, 37, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Nadella, V.; Ranjan, R.; Senthilkumaran, B.; Qadri, S.; Pothani, S.; Singh, A.K.; Gupta, M.L.; Prakash, H. Podophyllotoxin and Rutin Modulate M1 (iNOS+) Macrophages and Mitigate Lethal Radiation (LR) Induced Inflammatory Responses in Mice. Front. Immunol. 2019, 10, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikfarjam, B.A.; Adineh, M.; Hajiali, F.; Nassiri-Asl, M. Treatment with Rutin - A Therapeutic Strategy for Neutrophil-Mediated Inflammatory and Autoimmune Diseases: - Anti-inflammatory Effects of Rutin on Neutrophils. J. Pharmacopunct. 2017, 20, 52–56. [Google Scholar]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Barrier protective effects of rutin in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 3048–3055. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.Y.; Zhu, X.J.; Zhang, Y.N.; Zhou, F.F.; Yang, X.F. Protective effects of apigenin on LPS-induced endometritis via activating Nrf2 signaling pathway. Microb. Pathog. 2018, 123, 139–143. [Google Scholar] [CrossRef]
- Patil, R.H.; Babu, R.L.; Naveen Kumar, M.; Kiran Kumar, K.M.; Hegde, S.M.; Nagesh, R.; Ramesh, G.T.; Sharma, S.C. Anti-Inflammatory Effect of Apigenin on LPS-Induced Pro-Inflammatory Mediators and AP-1 Factors in Human Lung Epithelial Cells. Inflammation 2016, 39, 138–147. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Huang, Y.; Chen, J.; Fu, H.; Yang, N.; et al. Activation of PPARgamma by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Lucas, C.D.; Allen, K.C.; Dorward, D.A.; Hoodless, L.J.; Melrose, L.A.; Marwick, J.A.; Tucker, C.S.; Haslett, C.; Duffin, R.; Rossi, A.G. Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB J. 2012, 27, 1084–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Han, Y.; Zhou, Q.; Jie, H.; He, Y.; Han, J.; He, J.; Jiang, Y.; Sun, E. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J. Cell. Mol. Med. 2016, 20, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharm. Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Lee, J.H.; Kwon, D.Y. Apigenin inhibits release of inflammatory mediators by blocking the NF-κB activation pathways in the HMC-1 cells. Immunopharmacol. Immunotoxicol. 2011, 33, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Rafat Husain, S.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Van Acker, S.A.; van Balen, G.P.; van den Berg, D.J.; Bast, A.; van der Vijgh, W.J. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem Pharm. 1998, 56, 935–943. [Google Scholar] [CrossRef]
- Ganjare, A.B.; Nirmal, S.A.; Patil, A.N. Use of apigenin from Cordia dichotoma in the treatment of colitis. Fitoterapia 2011, 82, 1052–1056. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin alleviates interleukin-1beta-induced inflammatory responses in chondrocytes from osteoarthritis patients: Involvement of HIF-1alpha pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef]
- Kim, H.J.; Nam, Y.R.; Kim, E.J.; Nam, J.H.; Kim, W.K. Spirodela polyrhiza and its Chemical Constituent Vitexin Exert Anti-Allergic Effect via ORAI1 Channel Inhibition. Am. J. Chin. Med. 2018, 46, 1243–1261. [Google Scholar] [CrossRef]
- Nikfarjam, B.A.; Hajiali, F.; Adineh, M.; Nassiri-Asl, M. Anti-inflammatory Effects of Quercetin and Vitexin on Activated Human Peripheral Blood Neutrophils: - The effects of quercetin and vitexin on human neutrophils. J. Pharmacopunct. 2017, 20, 127–131. [Google Scholar]
- Rosa, S.I.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, B.; Shan, S.; Zhao, X. Neuroprotective effects of vitexin against isoflurane-induced neurotoxicity by targeting the TRPV1 and NR2B signaling pathways. Mol. Med. Rep. 2016, 14, 5607–5613. [Google Scholar] [CrossRef] [PubMed]
- Demir Ozkay, U.; Can, O.D. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharm. Biochem. Behav. 2013, 109, 23–30. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dholakiya, S.L.; Benzeroual, K.E. Protective effect of diosmin on LPS-induced apoptosis in PC12 cells and inhibition of TNF-alpha expression. Toxicol. In Vitro 2011, 25, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, M.M.; Rossaneis, A.C.; Fattori, V.; Longhi-Balbinot, D.T.; Freitas, A.; Cunha, F.Q.; Alves-Filho, J.C.; Cunha, T.M.; Casagrande, R.; Verri, W.A., Jr. Diosmin reduces chronic constriction injury-induced neuropathic pain in mice. Chem. Biol. Interact. 2017, 273, 180–189. [Google Scholar] [CrossRef]
- Carballo-Villalobos, A.I.; Gonzalez-Trujano, M.E.; Pellicer, F.; Alvarado-Vasquez, N.; Lopez-Munoz, F.J. Central and peripheral anti-hyperalgesic effects of diosmin in a neuropathic pain model in rats. Biomed. Pharm. 2018, 97, 310–320. [Google Scholar] [CrossRef]
- Berkoz, M. Diosmin suppresses the proinflammatory mediators in lipopolysaccharide-induced RAW264.7 macrophages via NF-κB and MAPKs signal pathways. Gen. Physiol. Biophys. 2019, 38, 315–324. [Google Scholar] [CrossRef]
- Crespo, M.E.; Galvez, J.; Cruz, T.; Ocete, M.A.; Zarzuelo, A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999, 65, 651–653. [Google Scholar] [CrossRef]
- Shalkami, A.S.; Hassan, M.; Bakr, A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2018, 37, 78–86. [Google Scholar] [CrossRef]
- Lee, C.H.; Jeong, T.S.; Choi, Y.K.; Hyun, B.H.; Oh, G.T.; Kim, E.H.; Kim, J.R.; Han, J.I.; Bok, S.H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Mizokami, S.S.; Borghi, S.M.; Bordignon, J.; Silva, R.L.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Casagrande, R.; et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J. Nutr. Biochem. 2016, 33, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Al-Rejaie, S.S.; Aleisa, A.M.; Abuohashish, H.M.; Parmar, M.Y.; Ola, M.S.; Al-Hosaini, A.A.; Ahmed, M.M. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol. Res. 2015, 37, 924–933. [Google Scholar] [CrossRef]
- Oguido, A.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Crespigio, J.; Domiciano, T.P.; Verri, W.A., Jr.; Casella, A.M.B. Naringenin Eye Drops Inhibit Corneal Neovascularization by Anti-Inflammatory and Antioxidant Mechanisms. Invest. Ophthalmol. Vis. Sci. 2017, 58, 5764–5776. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wei, Y.Z.; Wang, G.Q.; Li, D.D.; Shi, J.S.; Zhang, F. Targeting MAPK Pathways by Naringenin Modulates Microglia M1/M2 Polarization in Lipopolysaccharide-Stimulated Cultures. Front. Cell. Neurosci. 2018, 12, 531. [Google Scholar] [CrossRef] [Green Version]
- Bodet, C.; La, V.D.; Epifano, F.; Grenier, D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J. Periodontal. Res. 2008, 43, 400–407. [Google Scholar] [CrossRef]
- Nishimura Fde, C.; de Almeida, A.C.; Ratti, B.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Ximenes, V.F.; Silva Sde, O. Antioxidant effects of quercetin and naringenin are associated with impaired neutrophil microbicidal activity. Evid. Based Complement. Altern. Med. 2013, 2013, 795916. [Google Scholar] [CrossRef]
- Li, Y.R.; Chen, D.Y.; Chu, C.L.; Li, S.; Chen, Y.K.; Wu, C.L.; Lin, C.C. Naringenin inhibits dendritic cell maturation and has therapeutic effects in a murine model of collagen-induced arthritis. J. Nutr. Biochem. 2015, 26, 1467–1478. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Molecules 2012, 17, 12868–12881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballo-Villalobos, A.I.; Gonzalez-Trujano, M.E.; Alvarado-Vazquez, N.; Lopez-Munoz, F.J. Pro-inflammatory cytokines involvement in the hesperidin antihyperalgesic effects at peripheral and central levels in a neuropathic pain model. Inflammopharmacology 2017, 25, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Monforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Trovato, A.; Tripodo, M.M. Biological effects of hesperidin, a citrus flavonoid. (Note I): Antiinflammatory and analgesic activity. Farmaco 1994, 40, 709–712. [Google Scholar] [PubMed]
- Visnagri, A.; Kandhare, A.D.; Chakravarty, S.; Ghosh, P.; Bodhankar, S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014, 52, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Tirkey, N.; Chopra, K. Beneficial effect of hesperidin on lipopolysaccharide-induced hepatotoxicity. Toxicology 2006, 226, 152–160. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, S.H.; Park, S.H.; Park, E.K.; Kim, D.H. Antiallergic activity of hesperidin is activated by intestinal microflora. Pharmacology 2004, 71, 174–180. [Google Scholar] [CrossRef]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Zielinska-Przyjemska, M.; Ignatowicz, E. Citrus fruit flavonoids influence on neutrophil apoptosis and oxidative metabolism. Phytother. Res. 2008, 22, 1557–1562. [Google Scholar] [CrossRef]
- Choi, I.Y.; Kim, S.J.; Jeong, H.J.; Park, S.H.; Song, Y.S.; Lee, J.H.; Kang, T.H.; Park, J.H.; Hwang, G.S.; Lee, E.J.; et al. Hesperidin inhibits expression of hypoxia inducible factor-1 alpha and inflammatory cytokine production from mast cells. Mol. Cell Biochem. 2007, 305, 153–161. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomas-Barberan, F.A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Hesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damage. J. Photochem. Photobiol. B 2015, 148, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Hohmann, M.S.; Borghi, S.M.; Zarpelon, A.C.; Guazelli, C.F.; Manchope, M.F.; Casagrande, R.; Verri, W.A., Jr. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: Role of TRPV1, oxidative stress, cytokines and NF-κB. Chem. Biol. Interact. 2015, 228, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Sidhu, S.; Chopra, K.; Khan, M.U. Hepatoprotective effect of trans-Chalcone on experimentally induced hepatic injury in rats: Inhibition of hepatic inflammation and fibrosis. Can. J. Physiol. Pharm. 2016, 94, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Mola, M.; Homs, J.; Alvarez-Perez, B.; Puig, T.; Reina, F.; Verdu, E.; Boadas-Vaello, P. (−)-Epigallocatechin-3-Gallate Antihyperalgesic Effect Associates With Reduced CX3CL1 Chemokine Expression in Spinal Cord. Phytother. Res. 2017, 31, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef]
- Yoneyama, S.; Kawai, K.; Tsuno, N.H.; Okaji, Y.; Asakage, M.; Tsuchiya, T.; Yamada, J.; Sunami, E.; Osada, T.; Kitayama, J.; et al. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J. Allergy Clin. Immunol. 2008, 121, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Suzuki, Y.; Ra, C. Epigallocatechin-3-gallate inhibits mast cell degranulation, leukotriene C4 secretion, and calcium influx via mitochondrial calcium dysfunction. Free Radic Biol Med. 2010, 49, 632–640. [Google Scholar] [CrossRef]
- Takano, K.; Nakaima, K.; Nitta, M.; Shibata, F.; Nakagawa, H. Inhibitory effect of (-)-epigallocatechin 3-gallate, a polyphenol of green tea, on neutrophil chemotaxis in vitro and in vivo. J. Agric. Food Chem. 2004, 52, 4571–4576. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. 2014, 16, 508. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, X. Epigallocatechin-3-gallate attenuates bone cancer pain involving decreasing spinal Tumor Necrosis Factor-alpha expression in a mouse model. Int. Immunopharmacol. 2015, 29, 818–823. [Google Scholar] [CrossRef]
- Alvarez-Perez, B.; Homs, J.; Bosch-Mola, M.; Puig, T.; Reina, F.; Verdu, E.; Boadas-Vaello, P. Epigallocatechin-3-gallate treatment reduces thermal hyperalgesia after spinal cord injury by down-regulating RhoA expression in mice. Eur. J. Pain 2016, 20, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [PubMed]
- Okawa, M.; Kinjo, J.; Nohara, T.; Ono, M. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol. Pharm. Bull. 2001, 24, 1202–1205. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Chow, M.P.; Huang, W.C.; Lin, Y.C.; Chang, Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: Structure-activity relationships. Mol. Pharm. 2004, 66, 683–693. [Google Scholar]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Fattori, V.; Bussmann, A.J.C.; Bottura, C.; Fonseca, M.J.V.; Vignoli, J.A.; Baracat, M.M.; et al. trans-Chalcone, a flavonoid precursor, inhibits UV-induced skin inflammation and oxidative stress in mice by targeting NADPH oxidase and cytokine production. Photochem. Photobiol. Sci. 2017, 16, 1162–1173. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Mascolo, N.; Pinto, A.; Capasso, F. Flavonoids, leucocyte migration and eicosanoids. J. Pharm. Pharm. 1988, 40, 293–295. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juarez, A.O.; de la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharm. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Fattori, V.; Zarpelon, A.C.; Borghi, S.M.; Staurengo-Ferrari, L.; Carvalho, T.T.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M.; Casagrande, R.; et al. Pyrrolidine dithiocarbamate inhibits superoxide anion-induced pain and inflammation in the paw skin and spinal cord by targeting NF-κB and oxidative stress. Inflammopharmacology 2016, 24, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Yamacita-Borin, F.Y.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Fattori, V.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M.; Casagrande, R.; Verri, W.A., Jr. Superoxide anion-induced pain and inflammation depends on TNFalpha/TNFR1 signaling in mice. Neurosci. Lett. 2015, 605, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndengele, M.M.; Cuzzocrea, S.; Esposito, E.; Mazzon, E.; Di Paola, R.; Matuschak, G.M.; Salvemini, D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. Faseb J. 2008, 22, 3154–3164. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.W.; Zhu, Z.Q.; Hu, T.X.; Zhu, D.Y. Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects. Acta Pharm. Sin. 2002, 23, 667–672. [Google Scholar]
- Cos, P.; Calomme, M.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure-Activity Relationship of Flavonoids as Antioxidant and Pro-Oxidant Compounds. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 22, pp. 307–341. [Google Scholar]
- Staurengo-Ferrari, L.; Badaro-Garcia, S.; Hohmann, M.S.N.; Manchope, M.F.; Zaninelli, T.H.; Casagrande, R.; Verri, W.A., Jr. Contribution of Nrf2 Modulation to the Mechanism of Action of Analgesic and Anti-inflammatory Drugs in Pre-clinical and Clinical Stages. Front. Pharm. 2018, 9, 1536. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-R.; Li, G.-H.; Zhou, M.-X.; Xiang, L.; Ren, D.-M.; Lou, H.-X.; Wang, X.-N.; Shen, T. Discovery of natural flavonoids as activators of Nrf2-mediated defense system: Structure-activity relationship and inhibition of intracellular oxidative insults. Bioorg. Med. Chem. 2018, 26, 5140–5150. [Google Scholar] [CrossRef]
- Shin, S.Y.; Woo, Y.; Hyun, J.; Yong, Y.; Koh, D.; Lee, Y.H.; Lim, Y. Relationship between the structures of flavonoids and their NF-κB-dependent transcriptional activities. Bioorg. Med. Chem. Lett. 2011, 21, 6036–6041. [Google Scholar] [CrossRef]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J. Immunol 2007, 179, 7121–7127. [Google Scholar] [CrossRef] [Green Version]
- Ziyan, L.; Yongmei, Z.; Nan, Z.; Ning, T.; Baolin, L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 2007, 73, 221–226. [Google Scholar] [CrossRef]
- Lodhi, S.; Vadnere, G.P.; Patil, K.D.; Patil, T.P. Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and pro-inflammatory cytokines in rats. J. Tradit. Complement. Med. 2020, 10, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Tome, S.M.; Silva, A.M.; Laufer, S.; Lima, J.L.; Fernandes, E. Flavonoids inhibit COX-1 and COX-2 enzymes and cytokine/chemokine production in human whole blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, P.; Gadhwal, M.; Joshi, U.; Shetgiri, P.; Pharmacy, P. Mumbai, Modeling of COX-2 inhibotory activity of flavonoids. Int. J. Pharm. Pharm. Sci. 2011, 3, 33–40. [Google Scholar]
- Hohmann, M.S.; Cardoso, R.D.; Fattori, V.; Arakawa, N.S.; Tomaz, J.C.; Lopes, N.P.; Casagrande, R.; Verri, W.A., Jr. Hypericum perforatum Reduces Paracetamol-Induced Hepatotoxicity and Lethality in Mice by Modulating Inflammation and Oxidative Stress. Phytother Res. 2015, 29, 1097–1101. [Google Scholar] [CrossRef]
- Shanmugam, S.; Thangaraj, P.; Lima, B.D.S.; Chandran, R.; de Souza Araujo, A.A.; Narain, N.; Serafini, M.R.; Junior, L.J.Q. Effects of luteolin and quercetin 3-beta-d-glucoside identified from Passiflora subpeltata leaves against acetaminophen induced hepatotoxicity in rats. Biomed. Pharm. 2016, 83, 1278–1285. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Min, D.S.; Park, H.; Kim, H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol Appl. Pharm. 2018, 355, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Miyazawa, K.W.; Borghi, S.M.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Fattori, V.; Fernandes, G.S.A.; Casella, A.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; et al. The citrus flavanone naringenin reduces gout-induced joint pain and inflammation in mice by inhibiting the activation of NFκB and macrophage release of IL-1β. J. Funct. Foods 2018, 48, 106–116. [Google Scholar] [CrossRef]
- Lu, H.; Yao, H.; Zou, R.; Chen, X.; Xu, H. Galangin Suppresses Renal Inflammation via the Inhibition of NF-κB, PI3K/AKT and NLRP3 in Uric Acid Treated NRK-52E Tubular Epithelial Cells. Biomed. Res. Int. 2019, 2019, 3018357. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Schabbauer, G.; Tencati, M.; Pedersen, B.; Pawlinski, R.; Mackman, N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arter. Thromb Vasc. Biol. 2004, 24, 1963–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Viegas Jr, C.; Bolzani, V.D.S.; Barreiro, E.J. Os produtos naturais e a química medicinal moderna. Química Nova 2006, 29, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural Products as Sources of New Drugs over the Period 1981−2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Cassidy, A.; O’Reilly, É.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, Flavonoid Subclasses and Breast Cancer Risk: A Meta-Analysis of Epidemiologic Studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.-A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef] [PubMed]
- Cospite, M. Double-blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology 1994, 45, 566–573. [Google Scholar] [PubMed]
- Godeberge, P. Daflon 500 mg in the treatment of hemorrhoidal disease: A demonstrated efficacy in comparison with placebo. Angiology 1994, 45, 574–578. [Google Scholar] [PubMed]
- Meshikhes, A.-W.N. Efficacy of Daflon in the treatment of hemorrhoids. Saudi Med. J. 2002, 23, 1496–1498. [Google Scholar]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef]
- Zargaran, A.; Borhani-Haghighi, A.; Salehi-Marzijarani, M.; Faridi, P.; Daneshamouz, S.; Azadi, A.; Sadeghpour, H.; Sakhteman, A.; Mohagheghzadeh, A. Evaluation of the effect of topical chamomile (Matricaria chamomilla L.) oleogel as pain relief in migraine without aura: A randomized, double-blind, placebo-controlled, crossover study. Neurol. Sci. 2018, 39, 1345–1353. [Google Scholar] [CrossRef]
- Shavandi, M.; Moini, A.; Shakiba, Y.; Mashkorinia, A.; Dehghani, M.; Asar, S.; Kiani, A. Silymarin (Livergol®) Decreases Disease Activity Score in Patients with Rheumatoid Arthritis: A Non-randomized Single-arm Clinical Trial. Iran. J. Allergyasthmaand Immunol. 2017, 16, 99–106. [Google Scholar]
- Feragalli, B.; Dugall, M.; Luzzi, R.; Ledda, A.; Hosoi, M.; Belcaro, G.; Cesarone, M.R. Pycnogenol®: Supplementary management of symptomatic osteoarthritis with a patch. An observational registry study. Minerva Endocrinol. 2018, 44, 97–101. [Google Scholar] [CrossRef]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtaššák, J.; Ďuraćková, Z.; Lisý, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Farid, R.; Mirfeizi, Z.; Mirheidari, M.; Rezaieyazdi, Z.; Mansouri, H.; Esmaelli, H.; Zibadi, S.; Rohdewald, P.; Watson, R.R. Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee osteoarthritis. Nutr. Res. 2007, 27, 692–697. [Google Scholar] [CrossRef]
- Awan, F.T.; Jones, J.A.; Maddocks, K.; Poi, M.; Grever, M.R.; Johnson, A.; Byrd, J.C.; Andritsos, L.A. A phase 1 clinical trial of flavopiridol consolidation in chronic lymphocytic leukemia patients following chemoimmunotherapy. Ann. Hematol. 2016, 95, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverria, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sanchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [Green Version]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Kurowska, E.M.; Spence, J.D.; Jordan, J.; Wetmore, S.; Freeman, D.J.; Piché, L.A.; Serratore, P. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am. J. Clin. Nutr. 2000, 72, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Miwa, Y.; Yamada, M.; Sunayama, T.; Mitsuzumi, H.; Tsuzaki, Y.; Chaen, H.; Mishima, Y.; Kibata, M. Effects of Glucosyl Hesperidin on Serum Lipids in Hyperlipidemic Subjects: Preferential Reduction in Elevated Serum Triglyceride Level. J. Nutr. Sci. Vitaminol. 2004, 50, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Amato, C. Advantage of a micronized flavonoidic fraction (Daflon 500 mg) in comparison with a nonmicronized diosmin. Angiology 1994, 45, 531–536. [Google Scholar]
- Laurent, R.; Gilly, R.; Frileux, C. Clinical evaluation of a venotropic drug in man. Example of Daflon 500 mg. Int. Angiol. 1988, 7, 39–43. [Google Scholar]
- Geroulakos, G.; Nicolaides, A.N. Controlled studies of Daflon 500 mg in chronic venous insufficiency. Angiology 1994, 45, 549–553. [Google Scholar]
- Kusunoki, H.; Haruma, K.; Hata, J.; Ishii, M.; Kamada, T.; Yamashita, N.; Honda, K.; Inoue, K.; Imamura, H.; Manabe, N.; et al. Efficacy of Rikkunshito, a Traditional Japanese Medicine (Kampo), in Treating Functional Dyspepsia. Intern. Med. 2010, 49, 2195–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, M.; Matsumura, T.; Tsuchiya, N.; Sadakane, C.; Inami, R.; Suzuki, T.; Yoshikawa, M.; Imazeki, F.; Yokosuka, O. Rikkunshito Improves the Symptoms in Patients with Functional Dyspepsia, Accompanied by an Increase in the Level of Plasma Ghrelin. Hepatogastroenterology 2012, 59, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Asakawa, A.; Uezono, Y.; Minami, K.; Yamaguchi, T.; Niijima, A.; Yada, T.; Maejima, Y.; Sedbazar, U.; Sakai, T.; et al. Potentiation of ghrelin signaling attenuates cancer anorexia–cachexia and prolongs survival. Transl. Psychiatry 2011, 1, e23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, T.; Yanai, M.; Ando, H.; Toyomasu, Y.; Ogawa, A.; Morita, H.; Ogata, K.; Mochiki, E.; Asao, T.; Kuwano, H. Rikkunshito, a traditional Japanese medicine, suppresses cisplatin-induced anorexia in humans. Clin. Exp. Gastroenterol. 2011, 4, 291. [Google Scholar] [CrossRef] [Green Version]
- Takiguchi, S.; Hiura, Y.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Miyata, H.; Mori, M.; Hosoda, H.; Kangawa, K.; et al. Effect of rikkunshito, a Japanese herbal medicine, on gastrointestinal symptoms and ghrelin levels in gastric cancer patients after gastrectomy. Gastric Cancer 2013, 16, 167–174. [Google Scholar] [CrossRef]
- Boyle, P.; Diehm, C.; Robertson, C. Meta-analysis of clinical trials of Cyclo 3 Fort in the treatment of chronic venous insufficiency. Int. Angiol. 2003, 22, 250. [Google Scholar]
- Kakkos, S.K.; Allaert, F.A. Efficacy of Ruscus extract, HMC and vitamin C, constituents of Cyclo 3 fort®, on improving individual venous symptoms and edema: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int. Angiol. 2017, 36, 93–106. [Google Scholar]
- Matsui, T. Condensed catechins and their potential health-benefits. Eur. J. Pharmacol. 2015, 765, 495–502. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Hu, T.; Han, Y.; Huang, W.; Yuan, H.-B.; Zhang, Y.-T.; Du, Y.; Jiang, Y.-W. Preventive Effects of Catechins on Cardiovascular Disease. Molecules 2016, 21, 1759. [Google Scholar] [CrossRef] [Green Version]
- Mandel, S.; Weinreb, O.; Amit, T.; Youdim, M.B.H. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: Implications for neurodegenerative diseases. J. Neurochem. 2004, 88, 1555–1569. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Liu, D.; Liu, D. Green tea catechins and cardiovascular health: An update. Curr. Med. Chem. 2008, 15, 1840–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-of-Principle Study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, L.; Xie, L.P.; Lee, A.H.; Binns, C.W. Protective effect of green tea against prostate cancer: A case-control study in southeast China. Int. J. Cancer 2004, 108, 130–135. [Google Scholar] [CrossRef]
- Kurahashi, N.; Sasazuki, S.; Iwasaki, M.; Inoue, M.; Tsugane, S.; Group, J.S. Green Tea Consumption and Prostate Cancer Risk in Japanese Men: A Prospective Study. Am. J. Epidemiol. 2007, 167, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Brausi, M.; Rizzi, F.; Bettuzzi, S. Chemoprevention of Human Prostate Cancer by Green Tea Catechins: Two Years Later. A Follow-up Update. Eur. Urol. 2008, 54, 472–473. [Google Scholar] [CrossRef]
- Bonner, M.R.; Rothman, N.; Mumford, J.L.; He, X.; Shen, M.; Welch, R.; Yeager, M.; Chanock, S.; Caporaso, N.; Lan, Q. Green tea consumption, genetic susceptibility, PAH-rich smoky coal, and the risk of lung cancer. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 582, 53–60. [Google Scholar] [CrossRef]
- Ohno, Y.; Wakai, K.; Genka, K.; Ohmine, K.; Kawamura, T.; Tamakoshi, A.; Aoki, R.; Senda, M.; Hayashi, Y.; Nagao, K.; et al. Tea Consumption and Lung Cancer Risk: A Case-Control Study in Okinawa, Japan. Jpn. J. Cancer Res. 1995, 86, 1027–1034. [Google Scholar] [CrossRef]
- Yang, C.S.; Lambert, J.D.; Ju, J.; Lu, G.; Sang, S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007, 224, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A Catechin-rich Beverage Improves Obesity and Blood Glucose Control in Patients With Type 2 Diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.-L.; Chyu, M.-C. Tea flavonoids for bone health: From animals to humans. J. Investig. Med. 2016, 64, 1151–1157. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Serban, M.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Hear. Assoc. 2016, 5, e002713. [Google Scholar]
- Mohammadi-Sartang, M.; Mazloom, Z.; Sherafatmanesh, S.; Ghorbani, M.; Firoozi, D. Effects of supplementation with quercetin on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 1033–1039. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. DIETARY FLAVONOIDS: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Ross, S.M. Generalized Anxiety Disorder (GAD). Holist. Nurs. Pract. 2013, 27, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Youn, J.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.-S.; Kwon, S.; Youn, H.J.; et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int. J. Mol. Med. 2016, 38, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Hoensch, H.; Groh, B.; Edler, L.; Kirch, W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. 2008, 14, 2187–2193. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, Á.; Esquivel-Soto, J.; Esquivel-Chirino, C.; González-Rubio, M.G.-L.Y.; Gayosso-De-Lucio, J.A.; Morales-González, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Fan, Y.; Yan, Q.; Fan, X.; Wu, B.; Han, Y.; Zhang, Y.; Chen, Y.; Zhang, H.; Niu, J. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine 2017, 96, e9061. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; Di Renzo, A.; Stuard, S.; Dugall, M.; et al. Variations in C-reactive protein, plasma free radicals and fibrinogen values in patients with osteoarthritis treated with Pycnogenol®. Redox Rep. 2008, 13, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Zeidner, J.F.; Karp, J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 2015, 39, 1312–1318. [Google Scholar] [CrossRef]
- Pipingas, A.; Silberstein, R.B.; Vitetta, L.; Rooy, C.V.; Harris, E.V.; Young, J.M.; Frampton, C.M.; Sali, A.; Nastasi, J. Improved cognitive performance after dietary supplementation with a Pinus radiata bark extract Formulation. Phytother. Res. 2008, 22, 1168–1174. [Google Scholar] [CrossRef]
- Theadom, A.; Mahon, S.; Barker-Collo, S.; McPherson, K.; Rush, E.; Vandal, A.C.; Feigin, V.L. Enzogenol for cognitive functioning in traumatic brain injury: A pilot placebo-controlled RCT. Eur. J. Neurol. 2013, 20, 1135–1144. [Google Scholar] [CrossRef]
- Shand, B.; Strey, C.; Scott, R.; Morrison, Z.; Gieseg, S. Pilot study on the clinical effects of dietary supplementation With Enzogenol®, a flavonoid extract of pine bark and vitamin C. Phytother. Res. 2003, 17, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Christie, S.; Walker, A.F.; Hicks, S.M.; Abeyasekera, S. Flavonoid supplement improves leg health and reduces fluid retention in pre-menopausal women in a double-blind, placebo-controlled study. Phytomedicine 2004, 11, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egert, S.; Rimbach, G. Which Sources of Flavonoids: Complex Diets or Dietary Supplements? Adv. Nutr. 2011, 2, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, H.J.S.; Scotto, K.W. Use of flavonoids to treat multidrug resistant cancer cells. U.S. Patent 5,336,685, 9 August 1994. [Google Scholar]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Lauro, M.R.; Torre, M.L.; Maggi, L.; De Simone, F.; Conte, U.; Aquino, R.P. Fast- and slow-release tablets for oral administration of flavonoids: Rutin and quercetin. Drug Dev. Ind. Pharm. 2002, 28, 371–379. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Vale, D.L.; Steffen, V.S.; Vicentini, F.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Trans-chalcone added in topical formulation inhibits skin inflammation and oxidative stress in a model of ultraviolet B radiation skin damage in hairless mice. J. Photochem. Photobiol B 2017, 171, 139–146. [Google Scholar] [CrossRef]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharm. 2019, 112, 108622. [Google Scholar] [CrossRef]
- Muller, R.H.; Schmidt, S.; Buttle, I.; Akkar, A.; Schmitt, J.; Bromer, S. SolEmuls-novel technology for the formulation of i.v. emulsions with poorly soluble drugs. Int. J. Pharm. 2004, 269, 293–302. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, D.C.; Mizokami, S.S.; Sfeir, N.; Georgetti, S.R.; Urbano, A.; Casagrande, R.; Verri, W.A.; Baracat, M.M. Preclinical Evaluation of Rutin-Loaded Microparticles with an Enhanced Analgesic Effect. ACS Omega 2019, 4, 1221–1227. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Abadi, R.E.N.; Kazemipour, N.; Pahlevanneshan, Z.; Beheshti, S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci. Rep. 2019, 9, 6876. [Google Scholar] [CrossRef] [PubMed]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and Evaluation of Naringenin Nanosuspensions for Bioavailability Enhancement. AAPS Pharmscitech. 2017, 18, 3151–3162. [Google Scholar] [CrossRef]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and alpha-glucosidase inhibition activities. Mater. Sci Eng. C Mater. Biol. Appl. 2016, 68, 594–602. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef]
- Sharma, S.; Narang, J.K.; Ali, J.; Baboota, S. Synergistic antioxidant action of vitamin E and rutin SNEDDS in ameliorating oxidative stress in a Parkinson’s disease model. Nanotechnology 2016, 27, 375101. [Google Scholar] [CrossRef]
- Macedo, A.S.; Quelhas, S.; Silva, A.M.; Souto, E.B. Nanoemulsions for delivery of flavonoids: Formulation and in vitro release of rutin as model drug. Pharm. Dev. Technol. 2014, 19, 677–680. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- Singh, D.; Rawat, M.S.; Semalty, A.; Semalty, M. Quercetin-phospholipid complex: An amorphous pharmaceutical system in herbal drug delivery. Curr. Drug Discov. Technol. 2012, 9, 17–24. [Google Scholar] [CrossRef]
- Singh, D.; Rawat, M.S.; Semalty, A.; Semalty, M. Rutin-phospholipid complex: An innovative technique in novel drug delivery system- NDDS. Curr. Drug Deliv. 2012, 9, 305–314. [Google Scholar] [CrossRef]
- Yang, L.J.; Ma, S.X.; Zhou, S.Y.; Chen, W.; Yuan, M.W.; Yin, Y.Q.; Yang, X.D. Preparation and characterization of inclusion complexes of naringenin with beta-cyclodextrin or its derivative. Carbohydr. Polym. 2013, 98, 861–869. [Google Scholar] [CrossRef]
- Patra, A.; Satpathy, S.; Shenoy, A.K.; Bush, J.A.; Kazi, M.; Hussain, M.D. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int. J. Nanomed. 2018, 13, 2869–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Wei, P. Studies on the antioxidant effect of rutin in the microenvironment of cationic micelles. Microchim. Acta 2008, 161, 233–239. [Google Scholar] [CrossRef]
- Li, B.; Liu, H.; Amin, M.; Wegiel, L.A.; Taylor, L.S.; Edgar, K.J. Enhancement of naringenin solution concentration by solid dispersion in cellulose derivative matrices. Cellulose 2013, 20, 2137–2149. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef]




| Flavonoids Groups | Flavonoid | Cell Line | Effects | Refs | |
|---|---|---|---|---|---|
| Flavonols | Quercetin | macrophages | RAW 264.7 | Reduce TNF-α, IL-1β and IL-6 production | [75] |
| BMDM | Inhibit ASC speck formation and ASC oligomerization | [57] | |||
| BMDM | Modulate M1 and M2 | [85] | |||
| RAW 264.7 | [83] | ||||
| J 774 | [84] | ||||
| neutrophils | Human neutrophils | Modulate actin polymerization | [76] | ||
| dendritic cell | BMDC | Activation and Maturation | [81,82] | ||
| mast cells | HMC-1 | Reduce TNF-α, IL-1β, IL-8 and IL-6 production | [77] | ||
| hCBMCs | Reduce histamine, leukotrienes and PGD2 | [78] | |||
| monocytes | Human THP-1 monocytic cells THP-1 | Reduce TNF-α, and IL-1β production | [79,80] | ||
| Rutin | macrophages | RAW 264.7 | promote M2 polarization | [95] | |
| CD11b+ primary macrophages | |||||
| neutrophils | Human peripheral blood neutrophils | Reduce NO and TNF-α production | [96] | ||
| mast cells | HMC-1 | Reduce TNF-α, IL-1β, IL-8 and IL-6 production | [77] | ||
| monocytes | Human THP-1 | Inhibit adhesion | [97] | ||
| Flavones | Apigenin | macrophages | ANA-1 | Modulate macrophages polarization | [102] |
| RAW264.7 | |||||
| RAW 264.7 | Reduce NO production and COX-2 expression | [105] | |||
| neutrophils | Human peripheral blood neutrophils | Down-regulation of Mcl-1 | [103] | ||
| dendritic cell | BMDC | Inhibit maturation and migration | [35,104] | ||
| mast cells | HMC-1 | Inhibit TNF-α, IL-8, IL-6, GM-CSF, and COX-2 expression and NF-kB activation | [106] | ||
| monocytes | monocytes to HUVEC | Reduce TNF-α, production | [105] | ||
| Vitexin | macrophages | RAW 264.7 | Inhibit TNF-α, IL-1β, NO, PGE2 and increase in IL-10 release | [113] | |
| neutrophils | Human peripheral blood neutrophils | Reduce NO, TNF-α, and MPO production | [112] | ||
| mast cells | RBL-2H3 | Prevent degranulation | [111] | ||
| Diosmin | macrophages | RAW264.7 | Reduce NO, PGE2, IL-6, IL-12, TNF-α production | [120] | |
| Flavanones | Naringenin | macrophages | U937 | Regulate activation | [129] |
| neutrophils | Human peripheral blood neutrophils | Regulate microbicidal activity | [130] | ||
| dendritic cell | BMDC | Reduce maturation | [131] | ||
| Hesperidin | macrophages | RAW264.7 | Modulate M1 polarization | [70] | |
| neutrophils | Human peripheral blood neutrophils | Reduce generate superoxide radical | [140] | ||
| mast cells | HMC-1 | Reduce TNF-α and IL-1β production | [141] | ||
| Chalcone | Trans-chalcone | macrophages | BMDM | Reduce IL-1β production | [42] |
| Hesperidin methyl chalcone | macrophages | RAW264.7 | Reduce IL-33, TNF-α, and IL-6 levels | [141] | |
| Flavan-3-ols | Epigallocatechin-3-gallate | macrophages | RAW 264.7 | Reduce NO, prostaglandin PGE2 and COX-2 production | [147] |
| neutrophils | Murine peritoneal neutrophils | Reduce chemotaxis | [150] | ||
| dendritic cell | Human dendritic cell | Differentiation and maturation | [148] | ||
| mast cells | RBL-2H3 | Inhibit degranulation | [149] | ||
| Flavonoid/Flavonoid-Based Compound | n | Treatment | Duration | Outcomes | Refs |
|---|---|---|---|---|---|
| Flavanones, anthocyanins, flavan-3-ols, flavonols, flavones, and polymers | 49,281 men in the HPFS and 80,336 women from the NSH | Food frequency questionnaire | 20–22 years of follow-up | Intake of some flavonoids may reduce Parkinson disease risk, particularly in men | [193] |
| Hesperidin | 24 | 500 mg, daily | 3 weeks | Increased flow-mediated dilation and reduced concentrations of circulating inflammatory biomarkers | [195] |
| 100 | Daflon 500 mg (3 tablets bid. the first 4 days and 2 tablets bid. the following 3 days) | 7 days | Clinical severity, inflammation, congestion, edema, prolapse, duration, and severity of hemorrhoidal episode diminished | [196] | |
| 120 | Daflon 500 mg, 2 tablets, daily | 2 months | The overall symptom score decreased when compared to placebo | [197] | |
| 105 | Daflon 500 mg, 2 tablets, daily | 4 weeks + follow-up for 6 months | Improvement in pain, heaviness, bleeding, pruritus, and mucosal discharge from baseline | [198] | |
| 56 | 379 mg of green tea extract | 3 months | Improvements in blood pressure, insulin resistance, inflammation and oxidative stress, and lipid profile in patients with obesity-related hypertension | [199] | |
| Quercetin | 50 | 500 mg, daily | 8 weeks | Improvements in clinical symptoms, disease activity, hs-TNFα, and health assessment questionnaire in women with RA | [200] |
| Apigenin | 100 | 2 mL of an oleogel preparation of reformulated traditional chamomile oil | Topical application, once | Pain, nausea, vomiting, photophobia, and phonophobia significantly decreased in patients with migraine without aura | [201] |
| Silymarin (Livergol®, Goldaruo pharmaceutical, Iran) | 44 | 420 mg, daily | 90 days | Joint swelling, tenderness, and pain were reduced | [202] |
| Pycnogenol® (Horphag Research Ltd., UK, Geneve, Switzerland) | 67 | 220 mg, daily | 3 weeks | Patients with OA decreased C reactive protein levels and reduced use of painkillers and non-steroidal anti-inflammatory drugs | [203] |
| 100 | 150 mg/day | 3 months | Patients with OA presented relief from daily pain, stiffness, and physical function | [204] | |
| 37 | 150 mg/day | 3 months | Alleviating OA symptoms and reducing the need for NSAIDs or COX-2 inhibitors administration | [205] | |
| Alvocidib or Flavopiridol (Tolero Pharmaceuticals, Inc.) | 10 | 30-min loading dose of 30 mg/m (2) followed by a 4-h infusion of 30 mg/m (2) once weekly | 3 weeks every 5 weeks, twice | Reduction in tumor burden on chronic lymphocytic leukemia patients | [206] |
| Formulations Containing Flavonoids | Flavonoids | Biological Effects |
|---|---|---|
| Microparticles | Quercetin [69] Rutin [266] | ↑ bioavailability ↑ absorption ↑ solubility ↑ stability ↑ efficacy |
| Nanoparticles | Quercetin [267] Naringenin [268] Fisetin [269] | |
| SNEDDS | Naringenin [270] Quercetin [271] Rutin [272,273] | |
| Liposomal | Quercetin [274,275] Rutin [274] Kaempferol [275] Luteolin [275] | |
| Inclusion Complex | Quercetin [276] Rutin [277] Naringenin [278] | |
| Micelles | Quercetin [279] Rutin [280] | |
| Solid Dispersion | Naringenin [281] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. https://doi.org/10.3390/molecules25030762
Ferraz CR, Carvalho TT, Manchope MF, Artero NA, Rasquel-Oliveira FS, Fattori V, Casagrande R, Verri WA Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules. 2020; 25(3):762. https://doi.org/10.3390/molecules25030762
Chicago/Turabian StyleFerraz, Camila R., Thacyana T. Carvalho, Marília F. Manchope, Nayara A. Artero, Fernanda S. Rasquel-Oliveira, Victor Fattori, Rubia Casagrande, and Waldiceu A. Verri, Jr. 2020. "Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development" Molecules 25, no. 3: 762. https://doi.org/10.3390/molecules25030762
APA StyleFerraz, C. R., Carvalho, T. T., Manchope, M. F., Artero, N. A., Rasquel-Oliveira, F. S., Fattori, V., Casagrande, R., & Verri, W. A., Jr. (2020). Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules, 25(3), 762. https://doi.org/10.3390/molecules25030762







