Ferrous and Ferric Ion-Facilitated Dilute Acid Pretreatment of Lignocellulosic Biomass under Anaerobic or Aerobic Conditions: Observations of Fe Valence Interchange and the Role of Fenton Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. TEM Imaging of Dilute Acid/Fe3+ Ions-Pretreated Corn Stover under Anaerobic Setup Condition
2.2. Efficiency of DA/Fe Pretreatment under Anaerobic Condition: Fe3+ Is Nearly as Effective as Fe2+ in Enhancing DA Pretreatment of Biomass
2.3. Fe Ion Interconversion and Specification in Hydrolysates After DA/Fe Pretreatment Under Anaerobic Condition
2.4. Impacts of Oxygen on the Pretreatment Efficiency and the Fe Valence Changes
2.4.1. Pretreatment Efficiency under Aerobic Conditions
2.4.2. Fe3+ to Fe2+ Conversion under Aerobic Conditions
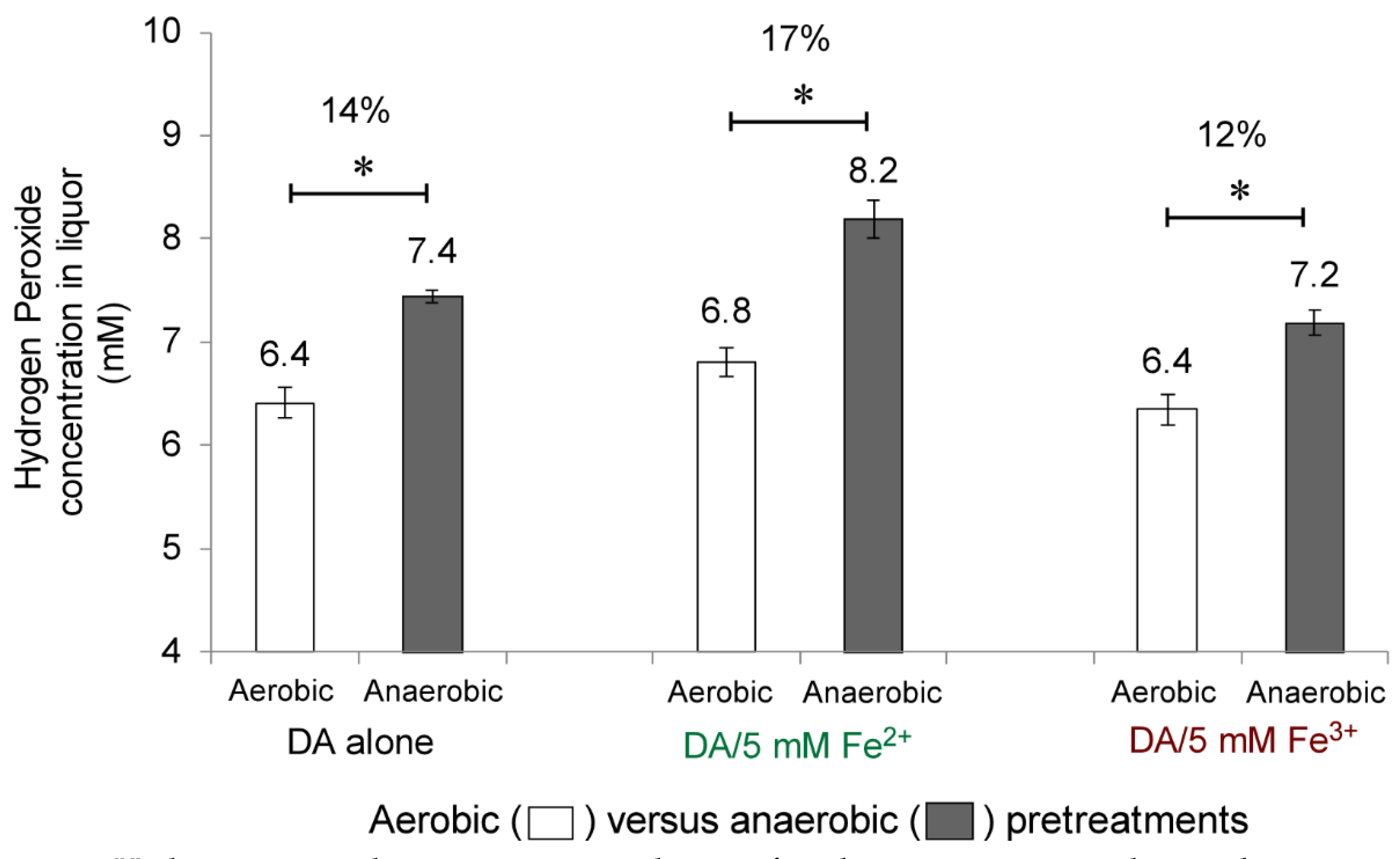
2.5. Detection of Hydrogen Peroxide
2.6. A Possible Role of Thermal Fenton Reaction in DA/Fe Pretreatments
2.7. Future Studies on the Effect of IRON Facilitated DA Pretreatment on Components of Biomass
3. Materials and Methods
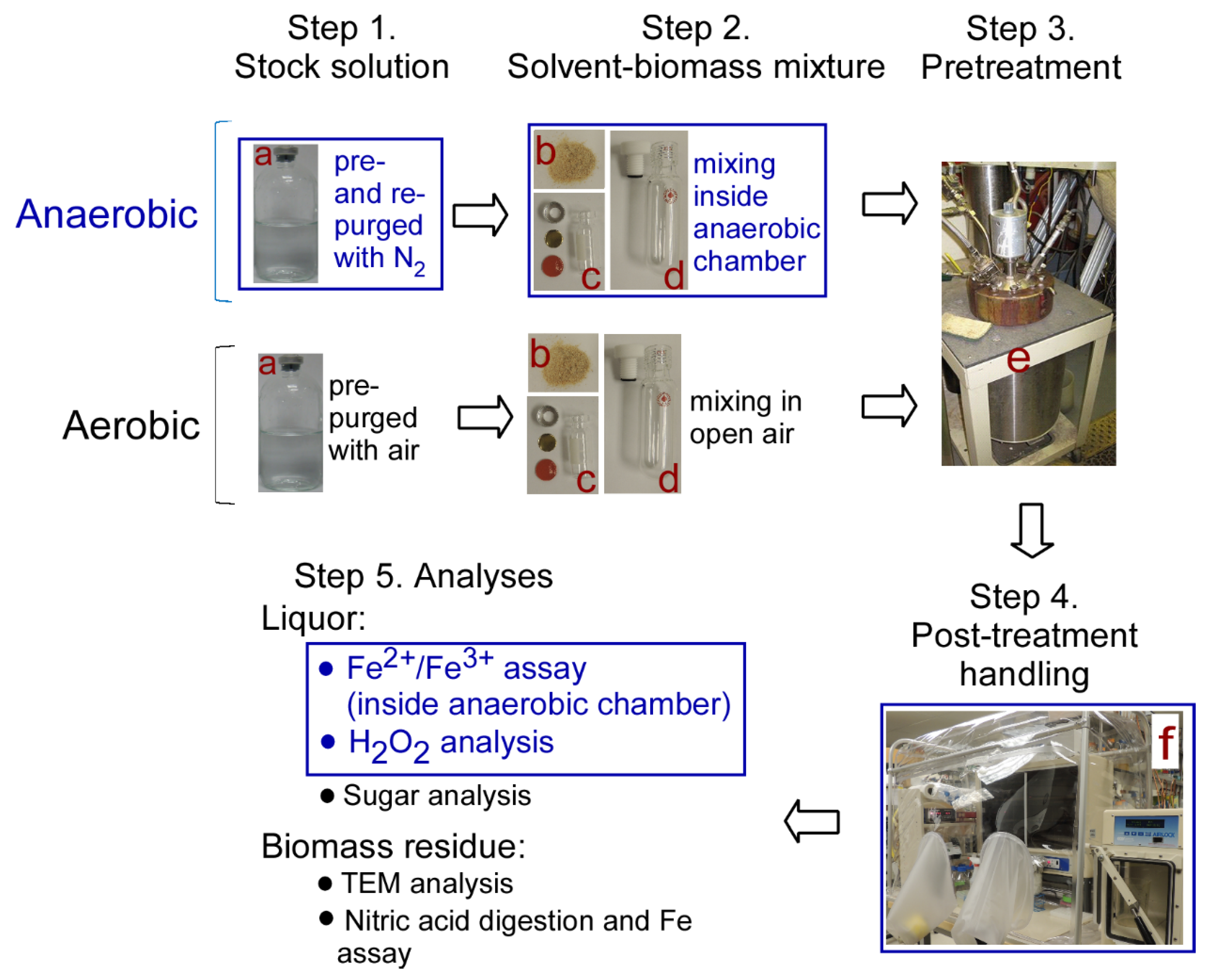
3.1. Main Experimental Steps andAapparatus
3.1.1. Step 1. Solvent Stock Solution
3.1.2. Step 2. Biomass–Solvent Mixtures
3.1.3. Step 3. Pretreatment Conditions
3.1.4. Step 4. Post-Handling of Pretreated Samples
3.2. Transmission Electron Microscopy
3.3. HPLC Analysis for the Sugars in Liquors After Pretreatment
3.4. Measurement of Fe2+ and Fe3+ Ions in Liquors After Pretreatment
3.5. Nitric Acid Digestion of Biomass Residue and the Measurement of Total Fe Ions
3.6. Measurement of Hydrogen Peroxide
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | corn stover |
| DA | dilute acid |
References
- Fengel, D.; Wegener, G. Wood: Chemistry, ultrastructure, reactions; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B.; Montague, L.; Slayton, A.; Lukas, J. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; No. NREL/TP-510-32438; National Renewable Energy Laboratory: Golden, CO, USA, 2002.
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; No. NREL/TP-510-47764; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Wyman, C.E. What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol. 2007, 25. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.; Lee, K.; Tribe, D. Kinetics of Alcohol Production by Zymomonas mobilis at High Sugar Concentrations. Biotechnol. Lett. 1979, 1, 165. [Google Scholar] [CrossRef]
- Schell, D.J.F.J.; Newman, M.; McMillan, J.D. Dilute-Sulfuric Acid Pretreatment of Corn Stover in Pilot-Scale Reactor: Investigation of Yields, Kinetics, and Enzymatic Digestibilities of Solids. Appl. Biochem. Biotechnol. 2003, 105, 69–85. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, A.J. Catalytic Conversion of Renewable Biomass Resources to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Schell, D.; Farmer, J.; Newman, M.; McMillan, J. Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor. Biotechnol. Fuels Chem. 2003, 105, 69–85. [Google Scholar]
- Chen, X.; Shekiro, J.; Franden, M.A.; Wang, W.; Zhang, M.; Kuhn, E.; Johnson, D.K.; Tucker, M.P. The impacts of deacetylation prior to dilute acid pretreatment on the bioethanol process. Biotechnol. Biofuels 2012, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, Q.; Tucker, M. Dilute acid/metal salt hydrolysis of lignocellulosics. U.S. Patent No. 6,423,145, 23 July 2002. [Google Scholar]
- Soudham, V.P.; Brandberg, T.; Mikkola, J.-P.; Larsson, C. Detoxification of acid pretreated spruce hydrolysates with ferrous sulfate and hydrogen peroxide improves enzymatic hydrolysis and fermentation. Bioresour. Technol. 2014, 166, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Donohoe, B.S.; Vinzant, T.B.; Ciesielski, P.N.; Wang, W.; Gedvilas, L.M.; Zeng, Y.; Johnson, D.K.; Ding, S.Y.; Himmel, M.E.; et al. Elucidating the role of ferrous ion cocatalyst in enhancing dilute acid pretreatment of lignocellulosic biomass. Biotechnol. Biofuels 2011, 4, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielski, P.N.; Wang, W.; Chen, X.; Vinzant, T.B.; Tucker, M.P.; Decker, S.R.; Himmel, M.E.; Johnson, D.K.; Donohoe, B.S. Effect of mechanical disruption on the effectiveness of three reactors used for dilute acid pretreatment of corn stover Part 2: Morphological and structural substrate analysis. Biotechnol. Biofuels 2014, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenstein, J.; Reddy Kamireddy, S.; Tucker, M.P.; Ji, Y. Oligomer saccharide reduction during dilute acid pretreatment co-catalyzed with Lewis acids on corn stover biomass. Int. J. Agric. Biol. Eng. 2013, 6, 54–62. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, P.N.; Matthews, J.F.; Tucker, M.P.; Beckham, G.T.; Crowley, M.F.; Himmel, M.E.; Donohoe, B.S. 3D Electron Tomography of Pretreated Biomass Informs Atomic Modeling of Cellulose Microfibrils. ACS Nano 2013, 7, 8011–8019. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, S1674–S1679. [Google Scholar] [CrossRef]
- Gan, S.; Ng, H.K. Inorganic chelated modified-Fenton treatment of polycyclic aromatic hydrocarbon (PAH)-contaminated soils. Chem. Eng. J. 2012, 180, 1–8. [Google Scholar]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Banos, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B: Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Donohoe, B.S.; Ciesielski, P.N.; Vinzant, T.B. Preservation and Preparation of Lignocellulosic Biomass Samples for Multi-scale Microscopy Analysis. In Biomass Conversion: Methods and Protocols; Himmel, M.E., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 31–47. [Google Scholar]
- Wei, H.; Layzell, D. Adenylate-coupled ion movement. A mechanism for the control of nodule permeability to O2 diffusion. Plant Physiol. 2006, 141, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opwis, K.; Knittel, D.; Bahners, T.; Schollmeyer, E. Thin film coating of textile materials. Part II: Enzyme. In Contact Angle, Wettability and Adhesion; Mittal, K.L., Ed.; CRC Press: Boston, MA, USA, 2006; Volume 4. [Google Scholar]
- Steinberg, S.M. High-performance liquid chromatography method for determination of hydrogen peroxide in aqueous solution and application to simulated Martian soil and related materials. Environ. Monit. Assess. 2013, 185, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Pretreatments (Anaerobic) | Glu Release | Increase | Xyl Release | Increase |
|---|---|---|---|---|
| g per 100 g CS | % over Ctrl | g per 100 g CS | % over Ctrl | |
| DA alone (Ctrl) | 3.17 ± 0.06 | NA | 16.81 ± 0.47 | - |
| DA/5 mM Fe2+ | 3.58 ± 0.01 | 17.1% * | 18.94 ± 0.27 | 16.5% * |
| DA/10 mM Fe2+ | 3.75 ± 0.04 | 23.8% ** | 19.96 ± 0.26 | 24.4% ** |
| DA/5 mM Fe3+ | 3.56 ± 0.09 | 15.9% * | 18.88 ± 0.45 | 16.1% * |
| DA/10 mM Fe3+ | 3.68 ± 0.04 | 21.0% ** | 19.25 ± 0.37 | 19.0% ** |
| Pretreatments (Aerobic) | Glu Release | Increase | Xyl Release | Increase |
|---|---|---|---|---|
| g per 100 g CS | % over Ctrl | g per 100 g CS | % over Ctrl | |
| DA alone (Ctrl) | 3.03 ± 0.08 | NA | 14.87 ± 0.73 | NA |
| DA/5 mM Fe2+ | 3.24 ± 0.05 | 7.1% | 16.24 ± 0.11 | 9.2% * |
| DA/10 mM Fe2+ | 3.56 ± 0.03 | 17.4% * | 17.67 ± 0.24 | 18.8% ** |
| DA/5 mM Fe3+ | 3.21 ± 0.05 | 6.0% | 16.00 ± 0.10 | 7.6% |
| DA/10 mM Fe3+ | 3.50 ± 0.05 | 15.6% * | 17.01 ± 0.49 | 14.4% * |
| Pretreatments | Description of Components |
|---|---|
| Small scale DA/Fe pretreatment in 2-mL glass vials | |
| DA alone (Ctrl) | 100 mg CS + 1 mL 0.5% H2SO4 |
| DA/5 mM Fe2+ | 100 mg CS + 1 mL 5 mM FeCl2 in 0.5% H2SO4 |
| DA/10 mM Fe2+ | 100 mg CS + 1 mL 10 mM FeCl2 in 0.5% H2SO4 |
| DA/5 mM Fe3+ | 100 mg CS + 1 mL 5 mM FeCl3 in 0.5% H2SO4 |
| DA/10 mM Fe3+ | 100 mg CS + 1 mL 10 mM FeCl3 in 0.5% H2SO4 |
| Intermediate scale DA/Fe pretreatment in 65-mL pressure tubes | |
| DA alone (Ctrl) | 3 g CS + 30 mL 0.5% H2SO4 |
| DA/10 mM Fe2+ | 3 g CS + 30 mL 10 mM FeCl2 in 0.5% H2SO4 |
| DA/10 mM Fe3+ | 3 g CS + 30 mL 10 mM FeCl3 in 0.5% H2SO4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Wang, W.; Ciesielski, P.N.; Donohoe, B.S.; Zhang, M.; Himmel, M.E.; Chen, X.; Tucker, M.P. Ferrous and Ferric Ion-Facilitated Dilute Acid Pretreatment of Lignocellulosic Biomass under Anaerobic or Aerobic Conditions: Observations of Fe Valence Interchange and the Role of Fenton Reaction. Molecules 2020, 25, 1427. https://doi.org/10.3390/molecules25061427
Wei H, Wang W, Ciesielski PN, Donohoe BS, Zhang M, Himmel ME, Chen X, Tucker MP. Ferrous and Ferric Ion-Facilitated Dilute Acid Pretreatment of Lignocellulosic Biomass under Anaerobic or Aerobic Conditions: Observations of Fe Valence Interchange and the Role of Fenton Reaction. Molecules. 2020; 25(6):1427. https://doi.org/10.3390/molecules25061427
Chicago/Turabian StyleWei, Hui, Wei Wang, Peter N. Ciesielski, Bryon S. Donohoe, Min Zhang, Michael E. Himmel, Xiaowen Chen, and Melvin P. Tucker. 2020. "Ferrous and Ferric Ion-Facilitated Dilute Acid Pretreatment of Lignocellulosic Biomass under Anaerobic or Aerobic Conditions: Observations of Fe Valence Interchange and the Role of Fenton Reaction" Molecules 25, no. 6: 1427. https://doi.org/10.3390/molecules25061427
APA StyleWei, H., Wang, W., Ciesielski, P. N., Donohoe, B. S., Zhang, M., Himmel, M. E., Chen, X., & Tucker, M. P. (2020). Ferrous and Ferric Ion-Facilitated Dilute Acid Pretreatment of Lignocellulosic Biomass under Anaerobic or Aerobic Conditions: Observations of Fe Valence Interchange and the Role of Fenton Reaction. Molecules, 25(6), 1427. https://doi.org/10.3390/molecules25061427







