Assessment of Astaxanthin Accumulation in Hepatocytes of Atlantic Salmon Fed Different Diets Using NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
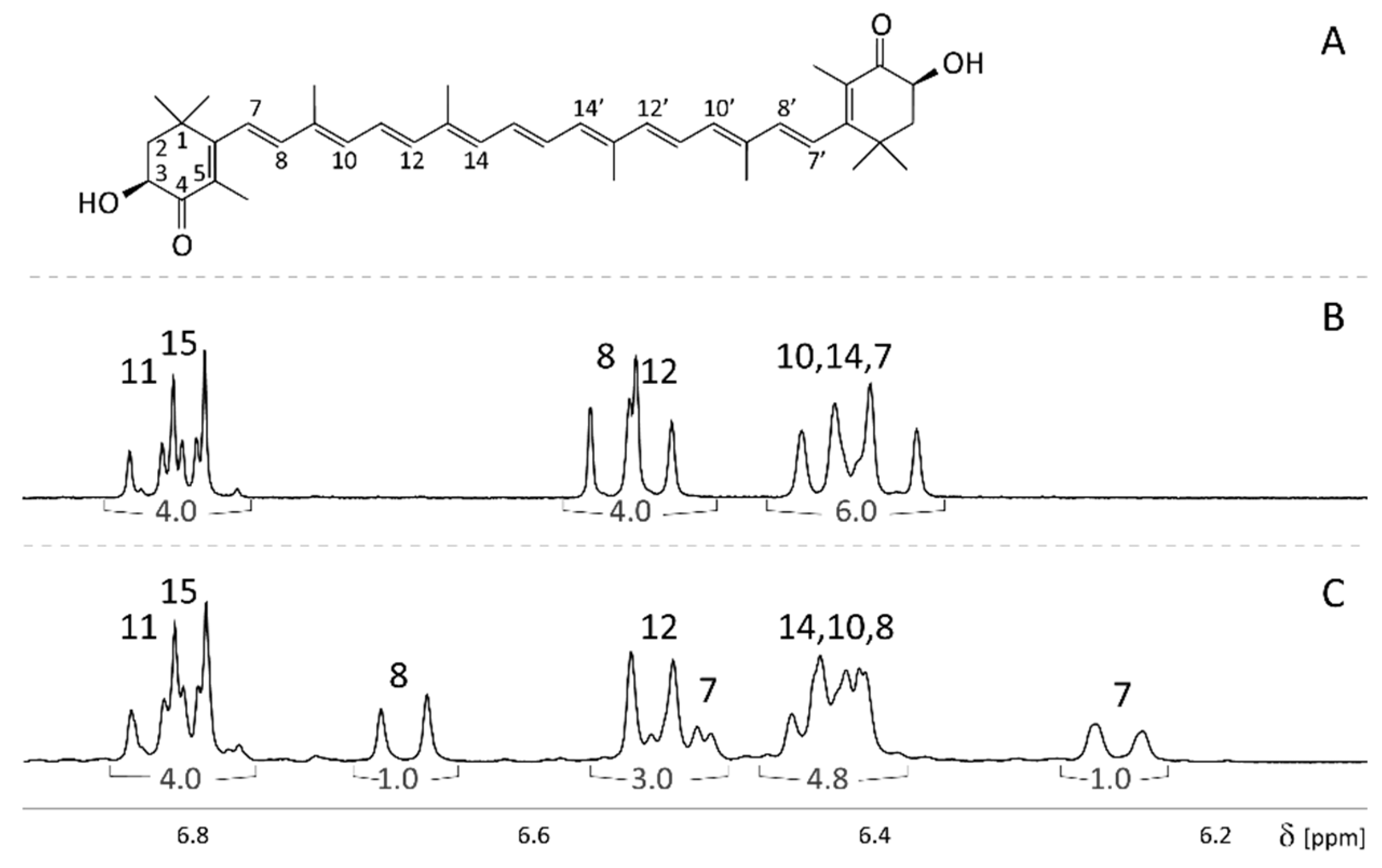
2.1. Assignment of Natural and 7,7′,8,8′-13C-Enriched Astaxanthin
2.2. Astaxanthin Detection and Quantification in Hepatocytes
3. Materials and Methods
3.1. Experimental Design
3.2. Salmon Feeding
3.3. Hepatocytes Isolation
3.4. Astaxanthin Extraction from Hepatocytes
3.5. NMR Sample Preparation
3.6. NMR Data Acquisition and Processing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torrissen, O.; Christiansen, R. Requirements for carotenoids in fish diets. J. Appl. Ichthyol. 1995, 11, 225–230. [Google Scholar] [CrossRef]
- Shahidi, F.; Brown, J.A. Carotenoid pigments in seafoods and aquaculture. Crit. Reveiws Food Sci. 1998, 38, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Pfeiffer, A.-M.; Schöner, F.-J.; Smith-Lemmon, L. Pigmenting efficacy of astaxanthin and canthaxanthin in fresh-water reared Atlantic salmon, Salmo salar. Anim. Feed Sci. Technol. 2002, 99, 97–106. [Google Scholar] [CrossRef]
- Storebakken, T.; No, H.K. Pigmentation of rainbow trout. Aquaculture 1992, 100, 209–229. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Torrissen, O.J. Pigmentation of salmonids: Interactions of astaxanthin and canthaxanthin on pigment deposition in rainbow trout. Aquaculture 1989, 79, 363–374. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Berge, G. Apparent digestibility coefficients and accumulation of astaxanthin E/Z isomers in Atlantic salmon (Salmo salar L.) and Atlantic halibut (Hippoglossus hippoglossus L.). Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2000, 127, 423–432. [Google Scholar] [CrossRef]
- Steine, G.; Alfnes, F.; RØRÅ, M.B. The effect of color on consumer WTP for farmed salmon. Mar. Resour. Econ. 2005, 20, 211–219. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the safety and efficacy of CAROPHYLL® Stay-Pink (astaxanthin dimethylsuccinate) as feed additive for salmon and trout. EFSA J. 2007, 574, 1–25. [Google Scholar]
- Rajasingh, H.; Øyehaug, L.; Våge, D.I.; Omholt, S.W. Carotenoid dynamics in Atlantic salmon. BMC Biol. 2006, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrissen, O.J.; Ingebrigtsen, K. Tissue distribution of 14C-astaxanthin in the Atlantic salmon (Salmo salar). Aquaculture 1992, 108, 381–385. [Google Scholar] [CrossRef]
- Englert, G. NMR Spectroscopy. In Carotenoids. Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1994; Volume 1B, p. 360. [Google Scholar]
- Englert, G.; Noack, K.; Broger, E.A.; Glinz, E.; Vecchi, M.; Zell, R. Synthesis, Isolation, and Full Spectroscopic Characterization of Eleven (Z)-Isomers of (3R, 3′ R)-Zeaxanthin. Helv. Chim. Acta 1991, 74, 969–982. [Google Scholar] [CrossRef]
- Page, G.; Davies, S. Tissue astaxanthin and canthaxanthin distribution in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2006, 143, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Muller, N.; Pritchard, D.E. C13 splittings in proton magnetic resonance spectra. I. Hydrocarbons. J. Chem. Physics 1959, 31, 768–771. [Google Scholar] [CrossRef]
- Goldstein, J.H.; Watts, V.S.; Rattet, L.S. 13CH Satellite NMR Spectra. Prog. Nucl. Magn. Reson. Spectrosc. 1971, 8, 104–162. [Google Scholar] [CrossRef]
- Kjær, M.A.; Ruyter, B.; Berge, G.M.; Sun, Y.; Østbye, T.-K.K. Regulation of the omega-3 fatty acid biosynthetic pathway in Atlantic salmon hepatocytes. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| Ingredient (%) | Product Name, Supplier | D1 | D2 | D3 | D4 |
|---|---|---|---|---|---|
| Fishmeal | Norse-LT, Vedde AS, Langevåg, Norway | 58.70 | 7.50 | 7.50 | 7.50 |
| Fish oil | NorSalmOil, Pelagia, Egersund, Norway | 22.00 | 6.50 | 3.30 | 0.50 |
| Wheat | Norgesmøllene AS, Bergen, Norway | 13.50 | 10.00 | 10.00 | 10.00 |
| Wheat gluten | Amytex 100, Tereos Syral, Aalst, Belgium | - | 22.45 | 22.45 | 22.45 |
| Soy protein concentrate | Alpha-Soy Pro 650, Agrokorn, Videbaek, Denmark | - | 26.00 | 26.00 | 26.00 |
| Rapeseed oil | Crude rapeseed oil, Emmelev, Otterup, Denmark | - | 19.40 | 19.40 | 19.40 |
| Mineralmix | Nofima Mineralpremix, Vilomix, Hønefoss, Norway | 0.59 | 0.59 | 0.59 | 0.59 |
| Vitaminmix | Nofima Vitmainpremix, Vilomix, Hønefoss, Norway | 2.00 | 2.00 | 2.00 | 2.00 |
| MSP (26% P) | 2.50 | 2.50 | 2.50 | 2.50 | |
| Carophyll pink (10% Astaxanthin) | Carophyll pink, Vilomix, Hønefoss, Norway | 0.05 | 0.05 | 0.05 | 0.05 |
| Yttrium oxide | Diyttrium trioxide 99.9%, VWR, Oslo, Norway | 0.01 | 0.01 | 0.01 | 0.01 |
| Betafine | Betafine, delivered by Vilomix, Hønefoss, Norway | 0.50 | 0.50 | 0.50 | 0.50 |
| DL-Methionine | DL-Methionine, delivered by Vilomix, Hønefoss, Norway | 0.20 | 0.80 | 0.80 | 0.80 |
| L-Lysine | L-lysine, delivered by Vilomix, Hønefoss, Norway | - | 1.70 | 1.70 | 1.70 |
| Threonine | L-Threonine, delivered by Vilomix, Hønefoss, Norway | - | 0.10 | 0.10 | 0.10 |
| Marine phospholipids | Marine phospholipids, TripleNine, Esbjerg, Denmark | - | - | 3.20 | - |
| DHA | Incromega DHA 500TG, Croda Nordica AB, Limhamn, Sweeden | - | - | - | 6.00 |
| Mineralmix | Nofima Mineralpremix, Vilomix, Hønefoss, Norway | 0.59 | 0.59 | 0.59 | 0.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumilina, E.; Ciampa, A.; Ytrestøyl, T.; Dikiy, A. Assessment of Astaxanthin Accumulation in Hepatocytes of Atlantic Salmon Fed Different Diets Using NMR Spectroscopy. Molecules 2020, 25, 1769. https://doi.org/10.3390/molecules25081769
Shumilina E, Ciampa A, Ytrestøyl T, Dikiy A. Assessment of Astaxanthin Accumulation in Hepatocytes of Atlantic Salmon Fed Different Diets Using NMR Spectroscopy. Molecules. 2020; 25(8):1769. https://doi.org/10.3390/molecules25081769
Chicago/Turabian StyleShumilina, Elena, Alessandra Ciampa, Trine Ytrestøyl, and Alexander Dikiy. 2020. "Assessment of Astaxanthin Accumulation in Hepatocytes of Atlantic Salmon Fed Different Diets Using NMR Spectroscopy" Molecules 25, no. 8: 1769. https://doi.org/10.3390/molecules25081769
APA StyleShumilina, E., Ciampa, A., Ytrestøyl, T., & Dikiy, A. (2020). Assessment of Astaxanthin Accumulation in Hepatocytes of Atlantic Salmon Fed Different Diets Using NMR Spectroscopy. Molecules, 25(8), 1769. https://doi.org/10.3390/molecules25081769




