Photophysical Study and Biological Applications of Synthetic Chalcone-Based Fluorescent Dyes
Abstract
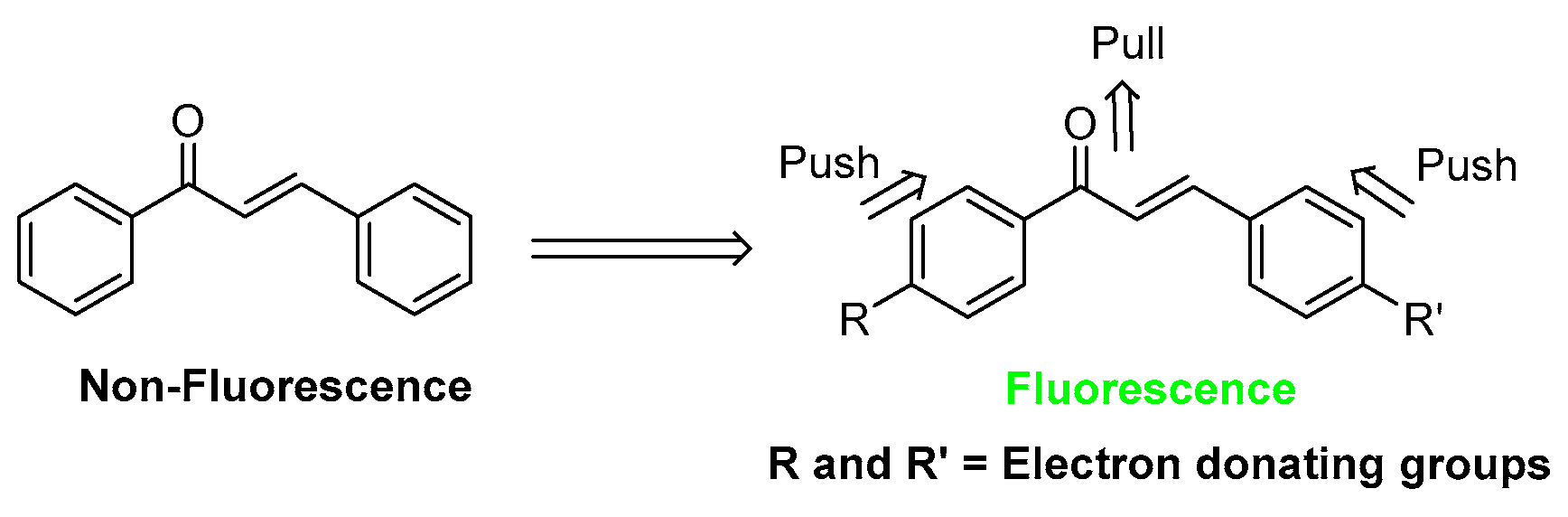
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Chalcones 3a–f
2.2. Photophysical Properties of Chalcones 3a–f
2.3. pH Effects of Chalcones 3a–f by Fluorescence Spectroscopic Analysis
2.4. Photostability of Chalcones 3a–f
2.5. Cell Viability
2.6. Confocal Imaging
2.7. Antibacterial Activity of the Synthesized Chalcones (3a–f)
3. Materials and Methods
3.1. Material and Instrumentation
3.2. Synthesis
3.3. Photophysical Properties
3.4. Study of the Effect of pH
3.5. Photo-Stability Test
3.6. Confocal Imaging
3.7. Cell Viability Assay
3.8. Antibacterial Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shin, S.P.; Choi, Y.M.; Kim, W.H.; Hong, S.P.; Park, J.M.; Kim, J.; Kwon, O.; Lee, E.H.; Hahm, K.B. A double blind, place-bo-controlled, randomized clinical trial that breast milk derived-Lactobacillus gasseri BNR17 mitigated diarrhea-dominant ir-ritable bowel syndrome. J. Clin. Biochem. Nutr. 2018, 62, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring Pharmacological Significance of Chalcone Scaffold: A Review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-F.; Liu, S.-S.; Song, Z.-Q.; Xu, T.-C.; Liu, C.-S.; Hou, Y.-G.; Huang, R.; Wu, S.-H. Naturally Occurring Flavonoids and Isoflavonoids and Their Microbial Transformation: A Review. Molecules 2020, 25, 5112. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Bok, V.V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Luo, Y.; Song, R.; Li, Y.; Zhang, S.; Liu, Z.-J.; Fu, J.; Zhu, H.-L. Design, synthesis, and biological evaluation of chalcone oxime derivatives as potential immunosuppressive agents. Bioorganic Med. Chem. Lett. 2012, 22, 3039–3043. [Google Scholar] [CrossRef]
- Pilatova, M.; Varinska, L.; Perjesi, P.; Sarissky, M.; Mirossay, L.; Solar, P.; Ostro, A.; Mojzis, J. In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicol. Vitr. 2010, 24, 1347–1355. [Google Scholar] [CrossRef]
- Lavis, L.D.; Raines, R.T. Bright Building Blocks for Chemical Biology. ACS Chem. Biol. 2014, 9, 855–866. [Google Scholar] [CrossRef]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Karuppusamy, A.; Vandana, T.; Kannan, P. Pyrene based chalcone materials as solid state luminogens with aggrega-tion-induced enhanced emission properties. J. Photochem. Photobiol. A Chem. 2017, 345, 11–20. [Google Scholar] [CrossRef]
- Shanker, N.; Dilek, O.; Mukherjee, K.; McGee, D.W.; Bane, S.L. Aurones: small molecule visible range fluorescent probes suitable for biomacromolecules. J. Fluoresc. 2011, 21, 2173–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Fayed, T.; Awad, M.K. Dual emission of chalcone-analogue dyes emitting in the red region. Chem. Phys. 2004, 303, 317–326. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kang, N.-Y.; Park, S.-J.; Yun, S.-W.; Chandran, Y.; Chang, Y.-T. Development of a fluorescent chalcone library and its application in the discovery of a mouse embryonic stem cell probe. Chem. Commun. 2012, 48, 6681. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, X.; Guo, Z.; Tang, J.; Shen, Y.; James, T.D.; Tian, H.; Zhu, W. In vivo and in situ tracking cancer chemotherapy by highly photostable NIR fluorescent theranostic prodrug. J. Am. Chem. Soc. 2014, 136, 3579–3588. [Google Scholar] [CrossRef]
- Bricks, J.L.; Kachkovskii, A.D.; Slominskii, Y.L.; Gerasov, A.O.; Popov, S.V. Molecular design of near infrared polymethine dyes: A review. Dyes Pigments 2015, 121, 238–255. [Google Scholar] [CrossRef]
- Henary, M.; Levitz, A. Synthesis and applications of unsymmetrical carbocyanine dyes. Dyes Pigments 2013, 99, 1107–1116. [Google Scholar] [CrossRef]
- Panigrahi, M.; Dash, S.; Patel, S.; Mishra, B.K. Syntheses of cyanines: A review. Tetrahedron 2012, 68, 781–805. [Google Scholar] [CrossRef]
- Mora-Huertas, C.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Reisch, A.; Klymchenko, A.S. Fluorescent Polymer Nanoparticles Based on Dyes: Seeking Brighter Tools for Bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Zhang, Y.; Xu, B.; Tian, W. Fluorescent nanoparticles based on AIE fluorogens for bioimaging. Nanoscale 2016, 8, 2471–2487. [Google Scholar] [CrossRef] [PubMed]
- Mdee, L.K.; Yeboah, S.O.; Abegaz, B.M. Rhuschalcones II-VI, five new bichalcones from the root bark of Rhus pyroides. J. Nat. Prod. 2003, 66, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Long, C.; Forster, R.J.; Keyes, T.E. Near IR emitting BODIPY fluorophores with mega-stokes shifts. Chem. Commun. 2012, 48, 5617–5619. [Google Scholar] [CrossRef]
- Shahverdi, A.; Fazeli, M.; Rafii, F.; Kakavand, M.; Jamalifar, H.; Hamedi, J. Inhibition of Nitrofurantoin Reduction by Menthol Leads to Enhanced Antimicrobial Activity. J. Chemother. 2003, 15, 449–453. [Google Scholar] [CrossRef]
- Krawczyk, P.; Pietrzak, M.; Janek, T.; Jędrzejewska, B.; Cysewski, P. Spectroscopic and nonlinear optical properties of new chalcone fluorescent probes for bioimaging applications: a theoretical and experimental study. J. Mol. Model. 2016, 22, 125. [Google Scholar] [CrossRef]
- Gaber, M.; Fayed, T.A.; El-Daly, S.A.; El-Sayed, Y.S. Spectral properties and inclusion of a hetero-chalcone analogue in orga-nized media of micellar solutions and beta-cyclodextrin. Photochem. Photobiol. Sci. 2008, 7, 257–262. [Google Scholar] [CrossRef]
- Morão, L.G.; Lorenzoni, A.S.G.; Chakraborty, P.; Ayusso, G.M.; Cavalca, L.B.; Santos, M.B.; Marques, B.C.; Dilarri, G.; Zamuner, C.; Regasini, L.O.; et al. Investigating the Modes of Action of the Antimicrobial Chalcones BC1 and T9A. Molecules 2020, 25, 4596. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Sączewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Tomašič, T.; Skok, Ž.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef]
- Tomasch, M.; Schwed, J.S.; Weizel, L.; Stark, H. Novel Chalcone-Based Fluorescent Human Histamine H3 Receptor Ligands as Pharmacological Tools. Front. Syst. Neurosci. 2012, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Guo, C.; Guo, S.; Wang, L.; Shi, D. Design and Synthesis of a Fluorescent Probe with a Large Stokes Shift for Detecting Thiophenols and Its Application in Water Samples and Living Cells. Molecules 2019, 24, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rurack, K.; Bricks, J.L.; Reck, G.; Radeglia, A.R.; Resch-Genger, U. Chalcone-Analogue Dyes Emitting in the Near-Infrared (NIR): Influence of Donor−Acceptor Substitution and Cation Complexation on Their Spectroscopic Properties and X-ray Structure. J. Phys. Chem. A 2000, 104, 3087–3109. [Google Scholar] [CrossRef]
- Wei, Y.; Qin, G.; Wang, W.; Bian, W.; Shuang, S.; Dong, C. Development of fluorescent FeIII sensor based on chalcone. J. Lumin- 2011, 131, 1672–1676. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Elzupir, A.; AlSalhi, M.; Alaamer, A.S. Influence of functional groups on the photophysical properties of dimethylamino chalcones as laser dyes. Opt. Mater. 2018, 76, 216–221. [Google Scholar] [CrossRef]
- Elzupir, A.; Ali, M.; Hussein, R.; Ibrahem, M.; Al-Muhanna, M.K.; Ibnaouf, K. Molecular structure, frontier molecular orbital and spectral analysis of dimethylamino chalcones efficient lasing dyes. J. Mol. Struct. 2019, 1178, 285–289. [Google Scholar] [CrossRef]
- A Dumont, J.; Euwart, D.; Mei, B.; Estes, S.; Kshirsagar, R.R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit. Rev. Biotechnol. 2016, 36, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Baquerizo, A.; Bañares, R.; Saliba, F. Current Clinical Status of the Extracorporeal Liver Support Devices. In Transplantation of the Liver; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 1463–1487. [Google Scholar]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef]
- Kar, S.; Mishra, R.K.; Pathak, A.; Dikshit, A.; Golakoti, N.R.; Rao, G.N. In silico modeling and synthesis of phenyl and thienyl analogs of chalcones for potential leads as anti-bacterial agents. J. Mol. Struct. 2018, 1156, 433–440. [Google Scholar] [CrossRef]
- Bhatia, N.M. Solution Phase Combinatorial Synthesis and Screening of Mini Libraries of Arylchalcones for Antibacterial Activity. Sci. Pharm. 2008, 76, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Prasad, Y.R.; Rani, V.J.; Rao, A.S. In vitro Antioxidant Activity and Scavenging Effects of Some Synthesized 4¢-Aminochalcones. Asian J. Chem. 2013, 25, 52–58. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, P.; Lu, J.; Xing, C. Characterization of the Fluorescence Properties of 4-Dialkylaminochalcones and Investi-gation of the Cytotoxic Mechanism of Chalcones. Arch. Pharm. 2016, 349, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.; Taslimi, P.; Ozaslan, M.S.; Oztaskin, N.; Çetinkaya, Y.; Gulçin, I.; Beydemir, Ş.; Goksu, S. Antidiabetic potential: In vitro inhibition effects of bromophenol and diarylmethanones derivatives on metabolic enzymes. Arch. Pharm. 2018, 351, e1800263. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Ma, Y.H.; Li, Y.H.; Zhang, Y.P.; Tian, H.C.; Huang, Y.C.; Li, Y.; Chen, W.; Yang, L.J. Design, Synthesis, and An-ticancer Activity of Novel Trimethoxyphenyl-Derived Chalcone-Benzimidazolium Salts. ACS Omega 2019, 4, 20381–20393. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, D.; Wei, G.; Zhou, Q.; Gan, X. Synthesis and anticancer activity of chalcone–quinoxalin conjugates. Synth. Commun. 2021, 51, 1363–1372. [Google Scholar] [CrossRef]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K. Chalcone derivatives and their antibacterial activities: Current development. Bioorganic Chem. 2019, 91, 103133. [Google Scholar] [CrossRef]
- Amole, K.L.; Bello, I.A.; Oyewale, A.O. Synthesis, Characterization and Antibacterial Activities of New Fluorinated Chalcones. Chem. Afr. 2019, 2, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Prasad, Y.R.; Rao, A.L.; Rambabu, R. Synthesis and Antimicrobial Activity of Some Chalcone Derivatives. E-J. Chem. 2008, 5, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Adithya, K.S.; Shankar, P.; Jagadeesh Babu, N.; Srivastava, S.; Nageswara Rao, G. Nonlinear optical studies and structure-activity relationship of chalcone derivatives with in silico insights. J. Mol. Struct. 2017, 1139, 294–302. [Google Scholar] [CrossRef]







| Solvents | Dye | Photophysical Properties | ||||
|---|---|---|---|---|---|---|
| λabs a (nm) | λem b (nm) | ∆λ c (nm) | Φf d (n = 3) | ε e (M−1 cm−1) | ||
| DMSO | 3a | 417 | 532 | 115 | 0.69 ± 0.04 | 4.6 × 104 |
| 3b | 431 | 560 | 129 | 0.34 ± 0.04 | 3.1 × 104 | |
| 3c | 413 | 520 | 107 | 0.61 ± 0.04 | 1.0 × 104 | |
| 3d | 422 | 516 | 94 | 0.10 ± 0.02 | 4.6 × 104 | |
| 3e | 420 | 539 | 119 | 0.50 ± 0.02 | 3.2 × 104 | |
| 3f | 412 | 512 | 100 | 0.21 ± 0.03 | 3.5 × 104 | |
| MeOH | 3a | 417 | 549 | 132 | 0.013 ± 0.006 | 3.0 × 104 |
| 3b | 428 | 567 | 139 | 0.012 ± 0.007 | 2.9 × 104 | |
| 3c | 414 | 546 | 132 | 0.017 ± 0.011 | 1.0 × 104 | |
| 3d | 423 | 555 | 132 | 0.024 ± 0.015 | 3.7 × 104 | |
| 3e | 417 | 547 | 130 | 0.012 ± 0.007 | 3.2 × 104 | |
| 3f | 414 | 549 | 135 | 0.027 ± 0.011 | 3.1 × 104 | |
| PBS (3%w/w tween 80) | 3a | 416 | 529 | 113 | 0.15 ± 0.02 | 3.3 × 104 |
| 3b | 427 | 520 | 93 | 0.11 ± 0.02 | 3.7 × 104 | |
| 3c | 411 | 526 | 115 | 0.11 ± 0.02 | 1.3 × 104 | |
| 3d | 422 | 542 | 120 | 0.17 ± 0.03 | 4.4 × 104 | |
| 3e | 420 | 521 | 101 | 0.18 ± 0.03 | 2.8 × 104 | |
| 3f | 412 | 524 | 112 | 0.18 ± 0.02 | 3.4 × 104 | |
| Cell Lines | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 3d | 3e | 3f | |
| HEK-293 | >100 | >100 | >100 | >100 | 96 | >100 |
| HepG2 | 56 | 45 | 100 | 56 | 54 | 54 |
| Compounds | E. coli 780 | S. aureus 1466 | ||
|---|---|---|---|---|
| MIC (μM) | MBC (μM) | MIC (μM) | MBC (μM) | |
| 3a | 375 ± 0.01 | 750 ± 0.02 | 375 ± 0.03 | 1000 ± 0.09 |
| 3b | 250 ± 0.02 | 750 ± 0.02 | 375 ± 0.01 | 1000 ± 0.01 |
| 3c | 250 ± 0.01 | 750 ± 0.01 | 375 ± 0.01 | 1000 ± 0.01 |
| 3d | 250 ± 0.01 | 375 ± 0.01 | 250 ± 0.01 | 1000 ± 0.01 |
| 3e | 375 ± 0.01 | 1000 ± 0.02 | 375 ± 0.03 | 1000 ± 0.02 |
| 3f | 375 ± 0.01 | 750 ± 0.01 | 375 ± 0.01 | 1000 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangngae, S.; Chansaenpak, K.; Nootem, J.; Ngivprom, U.; Aryamueang, S.; Lai, R.-Y.; Kamkaew, A. Photophysical Study and Biological Applications of Synthetic Chalcone-Based Fluorescent Dyes. Molecules 2021, 26, 2979. https://doi.org/10.3390/molecules26102979
Wangngae S, Chansaenpak K, Nootem J, Ngivprom U, Aryamueang S, Lai R-Y, Kamkaew A. Photophysical Study and Biological Applications of Synthetic Chalcone-Based Fluorescent Dyes. Molecules. 2021; 26(10):2979. https://doi.org/10.3390/molecules26102979
Chicago/Turabian StyleWangngae, Sirilak, Kantapat Chansaenpak, Jukkrit Nootem, Utumporn Ngivprom, Sirimongkon Aryamueang, Rung-Yi Lai, and Anyanee Kamkaew. 2021. "Photophysical Study and Biological Applications of Synthetic Chalcone-Based Fluorescent Dyes" Molecules 26, no. 10: 2979. https://doi.org/10.3390/molecules26102979
APA StyleWangngae, S., Chansaenpak, K., Nootem, J., Ngivprom, U., Aryamueang, S., Lai, R.-Y., & Kamkaew, A. (2021). Photophysical Study and Biological Applications of Synthetic Chalcone-Based Fluorescent Dyes. Molecules, 26(10), 2979. https://doi.org/10.3390/molecules26102979







