How to Obtain the Maximum Properties Flexibility of 3D Printed Ketoprofen Tablets Using Only One Drug-Loaded Filament?
Abstract
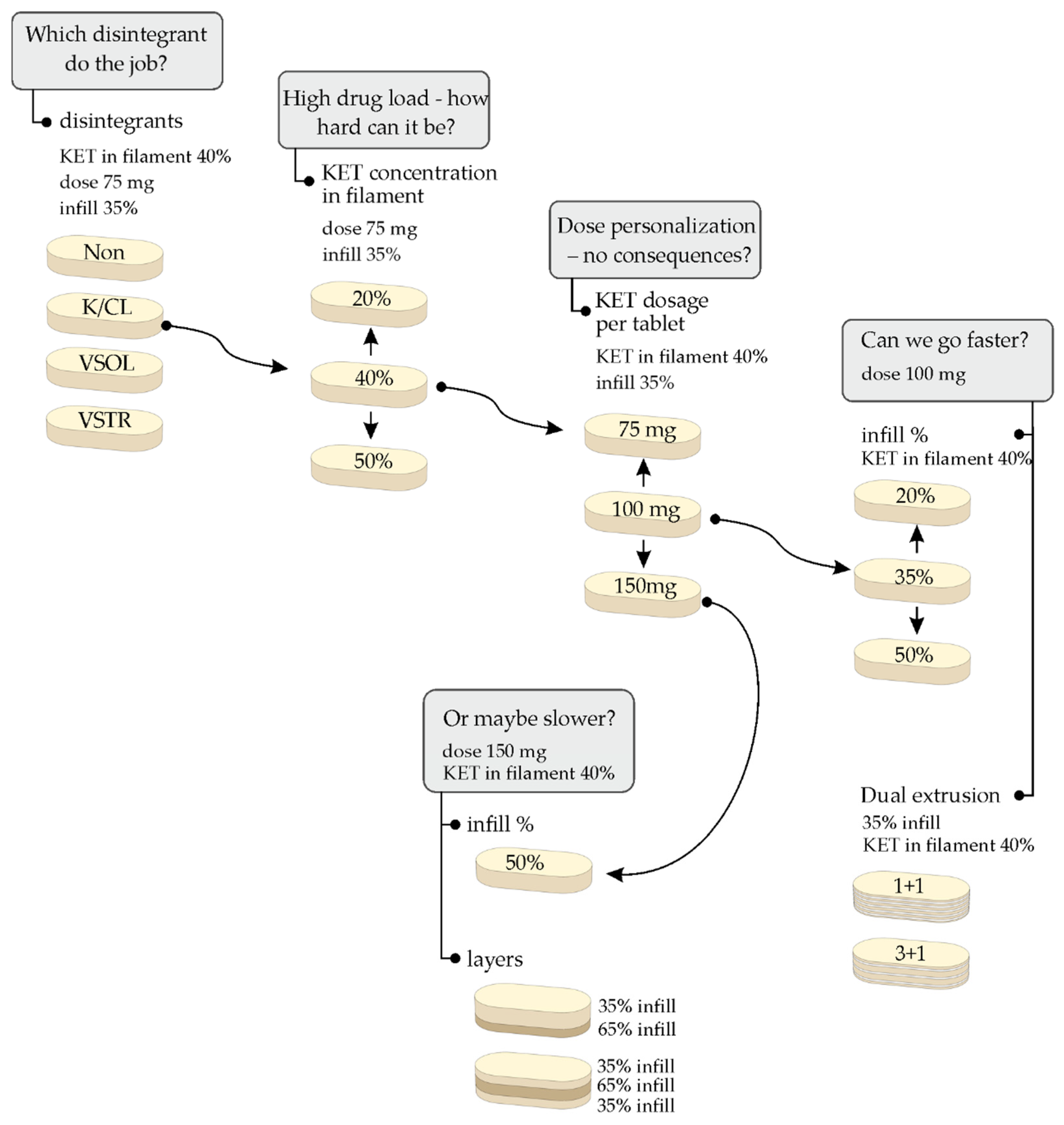
1. Introduction
2. Results and Discussion
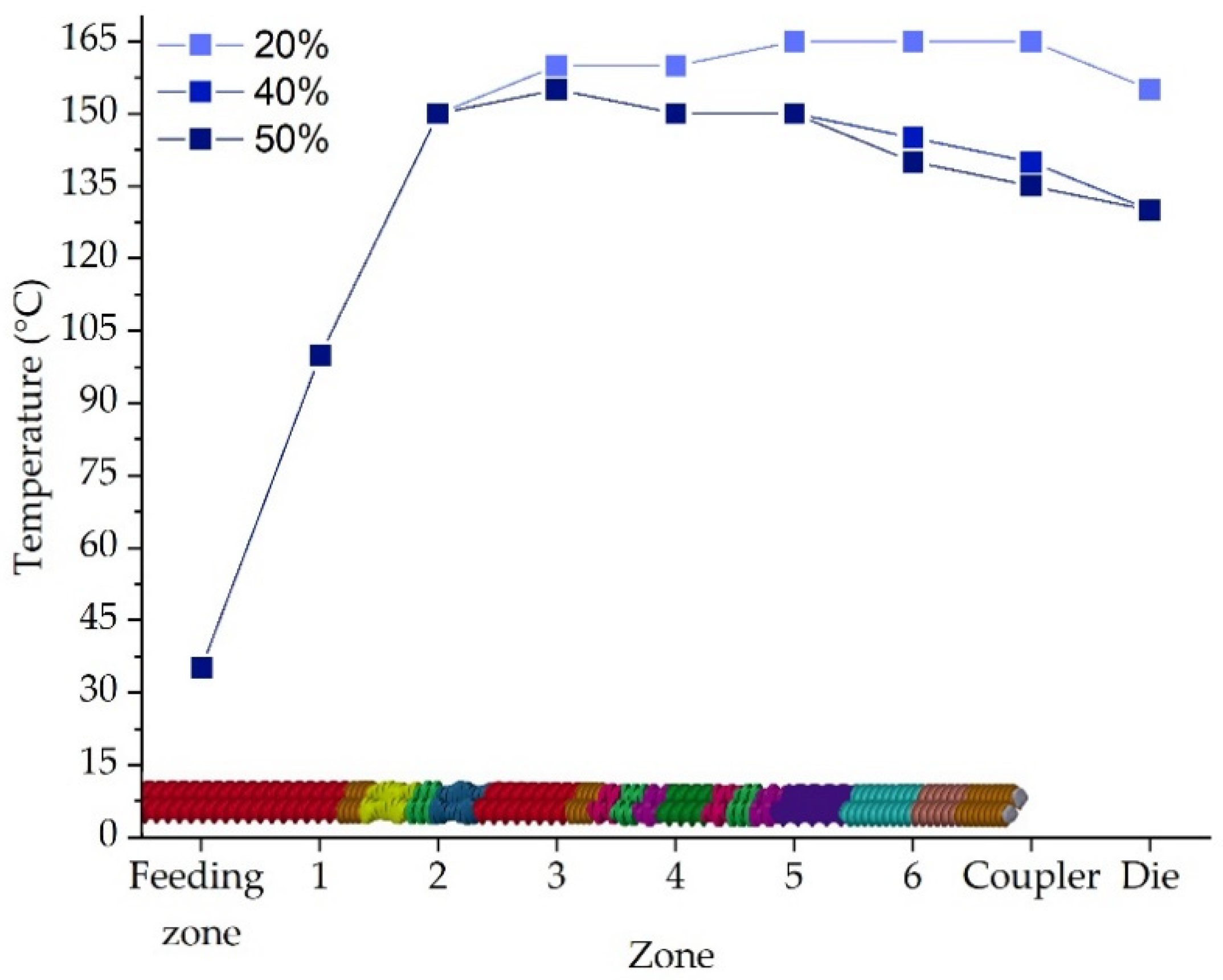
2.1. Extrusion Process and Filament Characteristics
2.2. 3D Printed Tablets
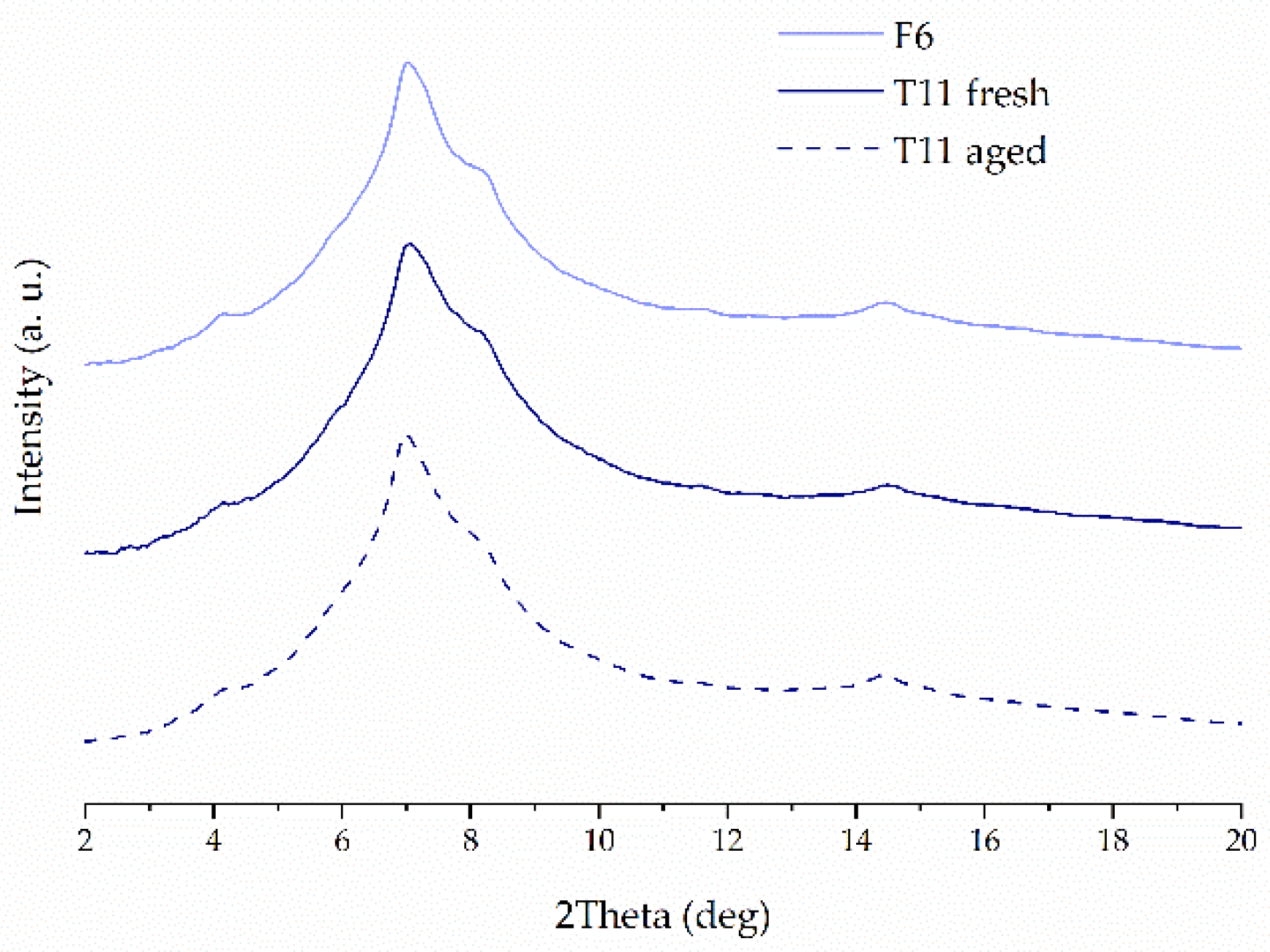
2.3. X-ray Powder Diffraction (XRPD)
2.4. Differential Scanning Calorimetry
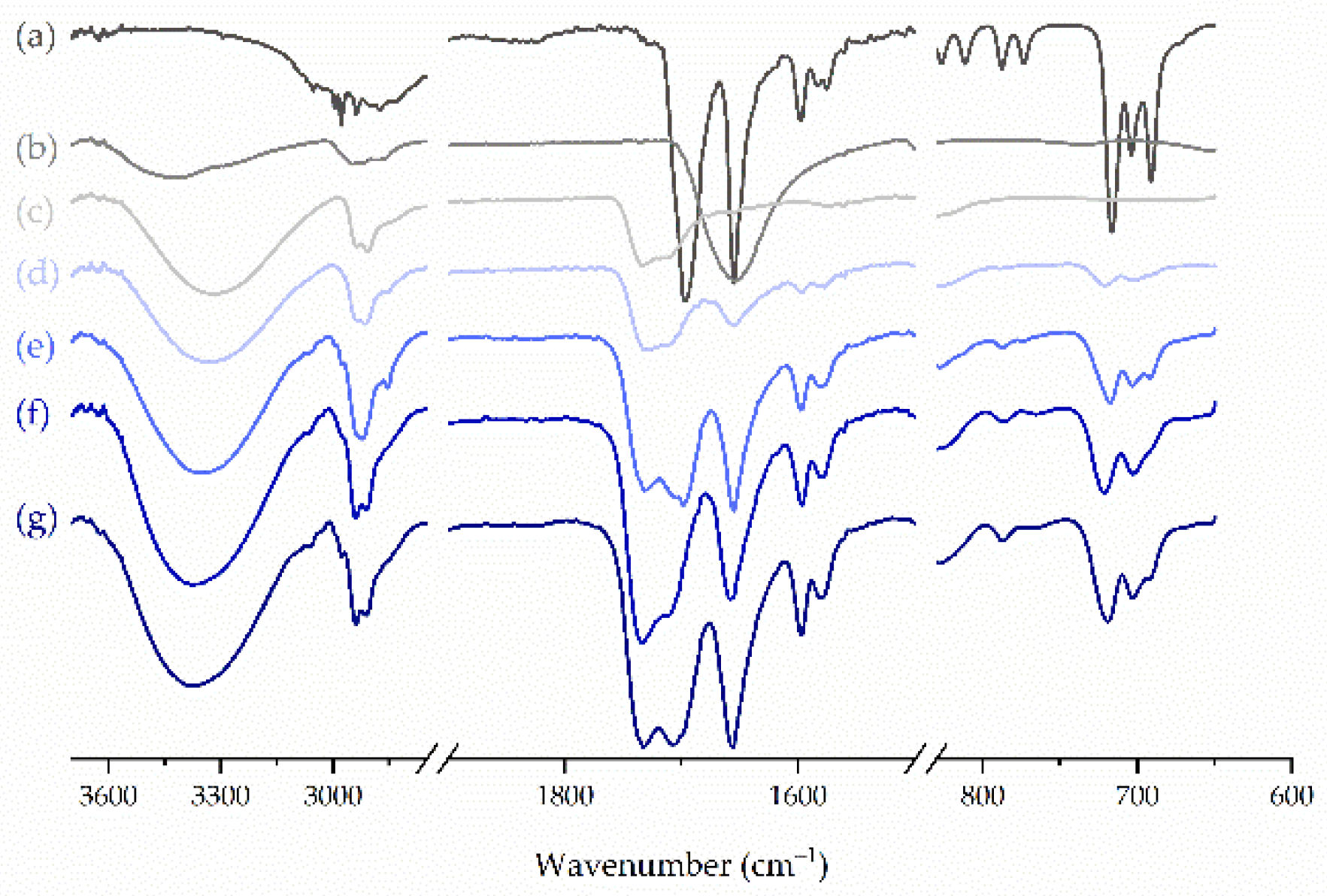
2.5. FTIR Spectroscopy
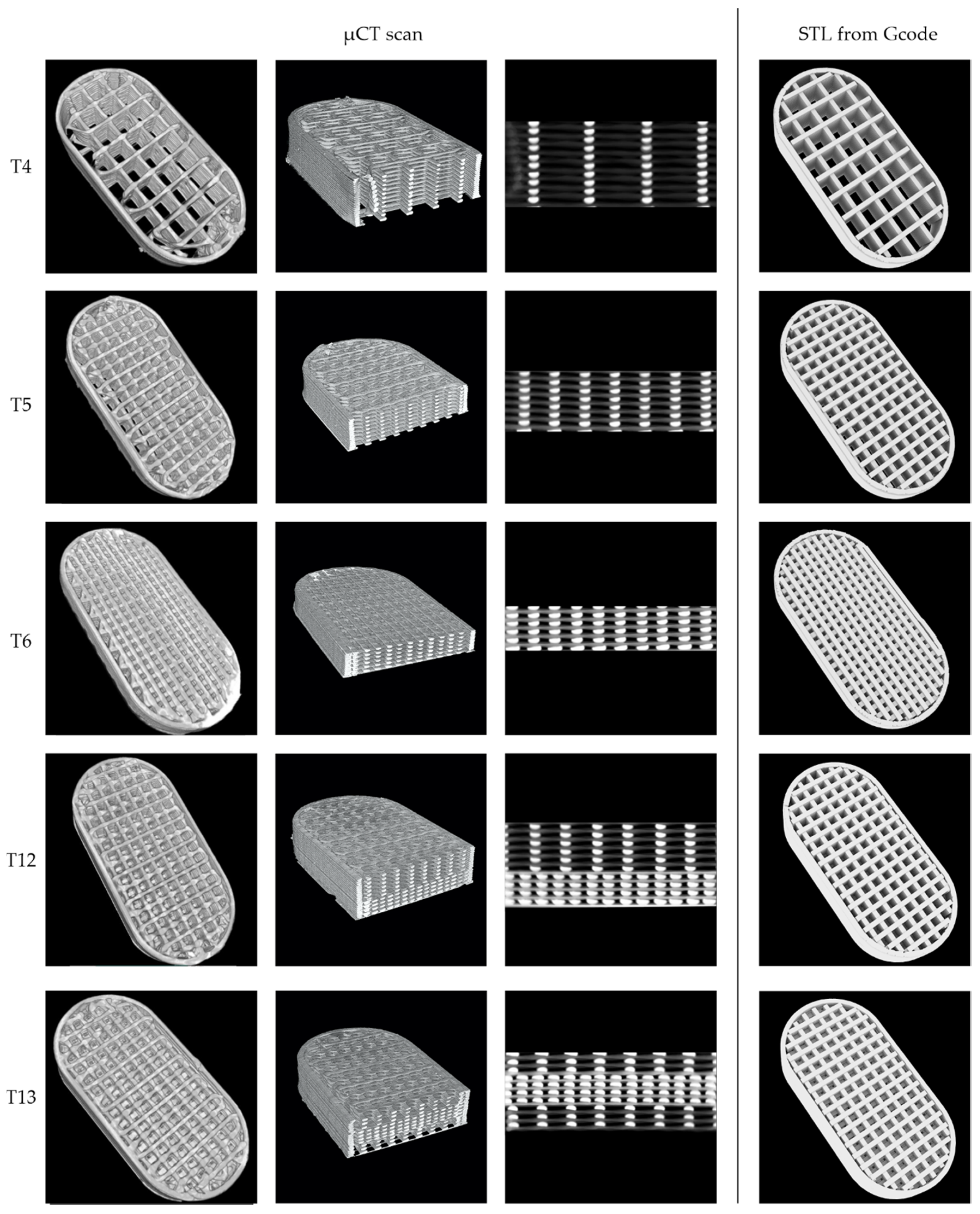
2.6. Micro-Computed Tomography
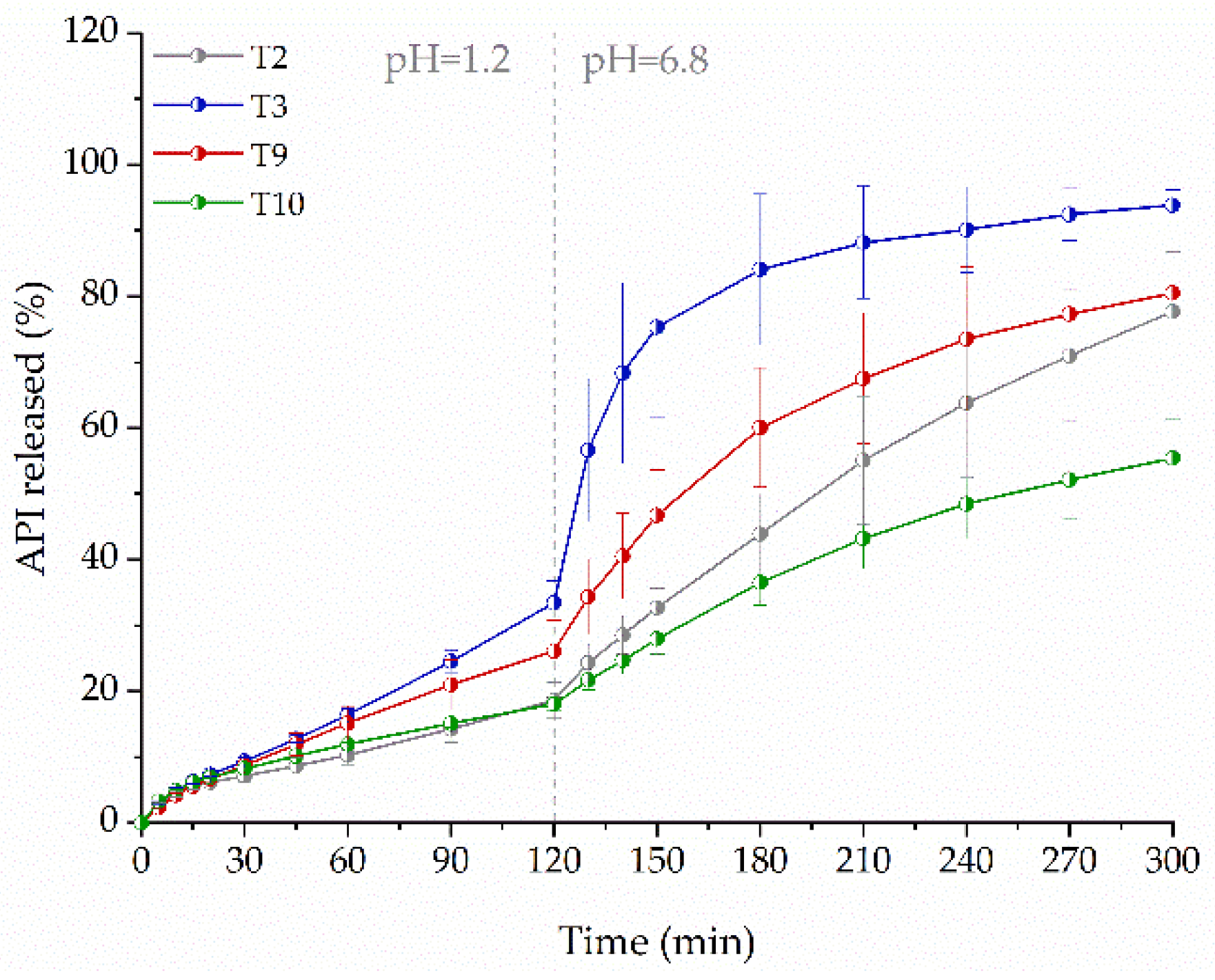
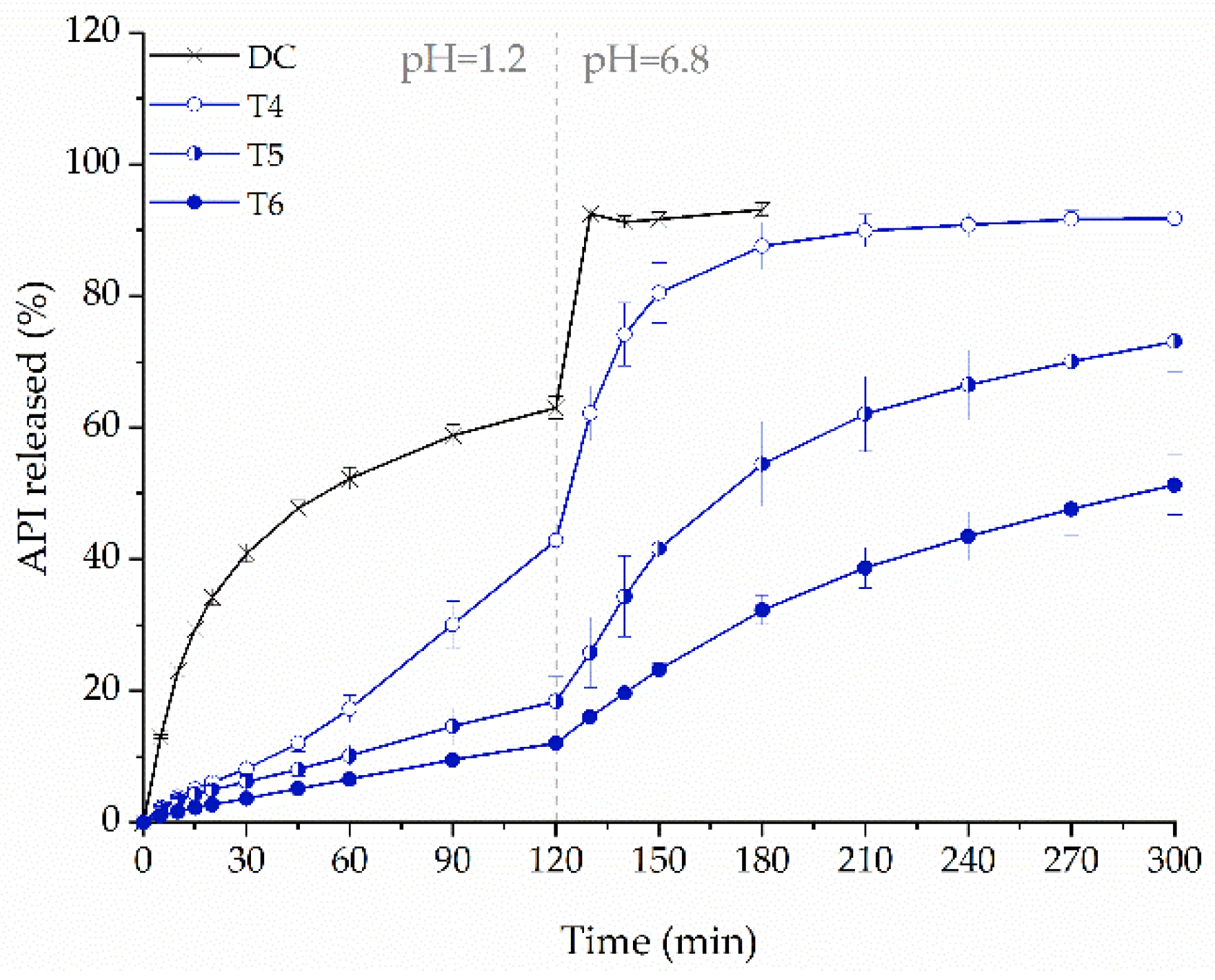
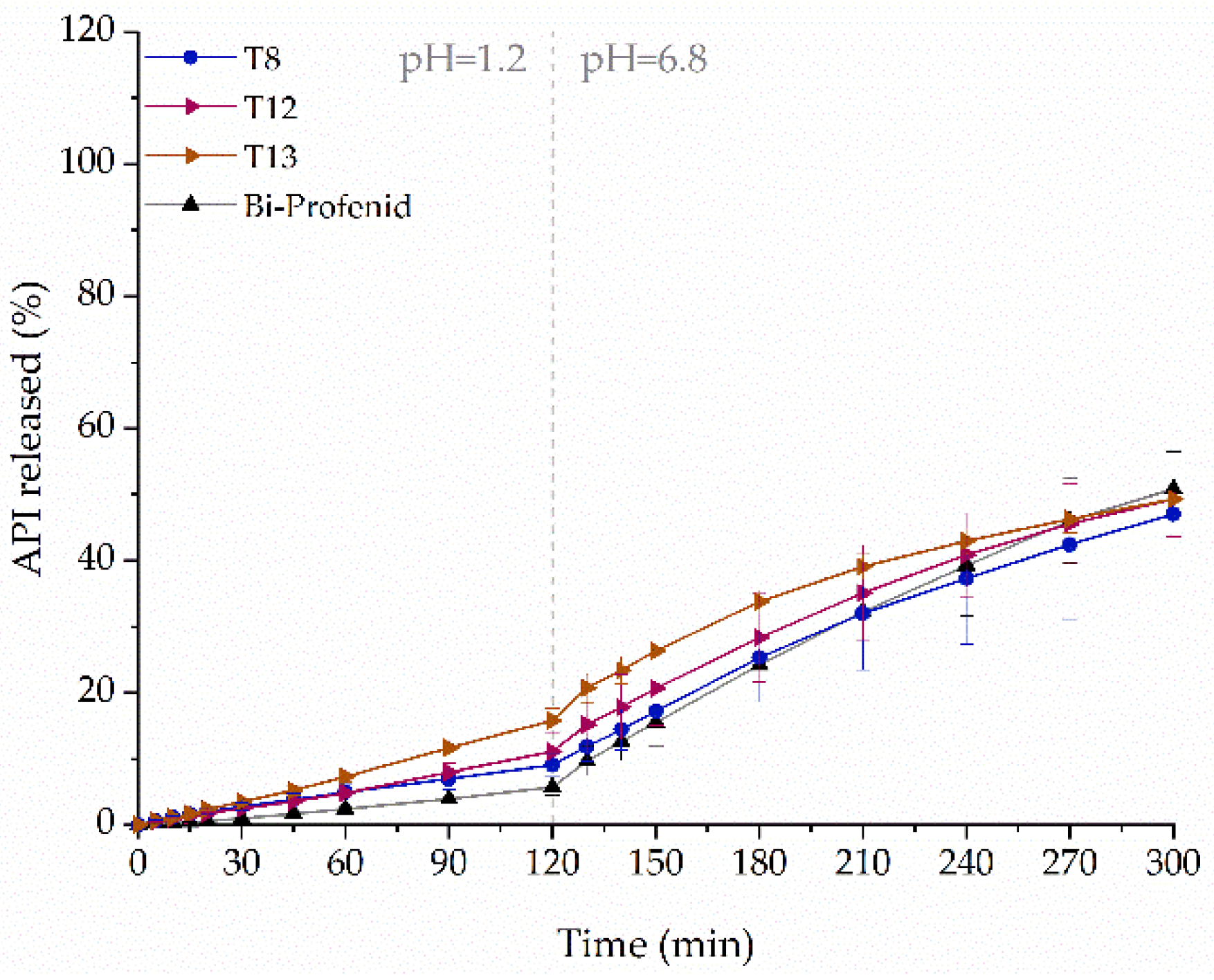
2.7. Influence of Tablets Formulation on the Dissolution Profile
3. Materials and Methods
3.1. Materials
3.2. Filaments Manufacturing by Hot-Melt Extrusion
3.3. Design and 3D Printing of Tablets
3.4. Preparation of Directly Compressed Tablets (DC Tablets)
3.5. Filament Properties’ Evaluation
3.6. Determination of Ketoprofen Content in the Filaments
3.7. FTIR Spectroscopy
3.8. Micro-Computed Tomography
3.9. Differential Scanning Calorimetry (DSC)
3.10. X-ray Powder Diffraction (XRPD)
3.11. Dissolution Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fernández-García, R.; Prada, M.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Oral Fixed-Dose Combination Pharmaceutical Products: Industrial Manufacturing Versus Personalized 3D Printing. Pharm. Res. 2020, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Öblom, H. 3D-Printed Drugs for Children—Are We Ready Yet? AAPS PharmSciTech 2017, 18, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Fatouros, D.G. Haptic Evaluation of 3D-Printed Braille-Encoded Intraoral Films. Eur. J. Pharm. Sci. 2021, 157, 105605. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Kumar, A.; Devi, G.; Sharada, N. A Review on Novel Approach to Pharmaceutical Drug Delivery: 3D Printing. IJPSR 2019, 10. [Google Scholar] [CrossRef]
- MEDTM 3D Printed Pharmaceutical Product Receives IND Clearance from the US FDA. Available online: Https://Www.Businesswire.Com/News/Home/20210209005529/En/MED%E2%84%A2-3D-Printed-Pharmaceutical-Product-Receives-IND-Clearance-From-the-US-FDA (accessed on 7 March 2021).
- Spritam. Available online: Https://Www.Spritam.Com/#/Patient (accessed on 7 March 2021).
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D Printed Orodispersible Films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional Drug Release from 3D Printed Mucoadhesive Buccal Films Using FDM Technology: In Vitro and Ex Vivo Evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Krause, J.; Müller, L.; Sarwinska, D.; Seidlitz, A.; Sznitowska, M.; Weitschies, W. 3D Printing of Mini Tablets for Pediatric Use. Pharmaceuticals 2021, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, H.E.; Tort, S.; Acartürk, F. An Effective Technology for the Development of Immediate Release Solid Dosage Forms Containing Low-Dose Drug: Fused Deposition Modeling 3D Printing. Pharm. Res. 2019, 36, 128. [Google Scholar] [CrossRef] [PubMed]
- Fanous, M.; Bitar, M.; Gold, S.; Sobczuk, A.; Hirsch, S.; Ogorka Funding, J.; Imanidis, G. Development of Immediate Release 3D-Printed Dosage Forms for Poorly Water-Soluble Drugs by Fused Deposition Modeling: Study of Morphology, Solid State and Dissolution. Int. J. Pharm. 2021, 120417. [Google Scholar] [CrossRef] [PubMed]
- Cotabarren, I.; Gallo, L. 3D Printing of PVA Capsular Devices for Modified Drug Delivery: Design and in Vitro Dissolution Studies. Drug Dev. Ind. Pharm. 2020, 1–11. [Google Scholar] [CrossRef]
- Öblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech 2019, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Katsiotis, C.S.; Bouropoulos, N.; Koutsopoulos, S.; Fatouros, D.G. FDM-Printed PH-Responsive Capsules for the Oral Delivery of a Model Macromolecular Dye. Pharm. Dev. Technol. 2020, 25, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Fabrication of Floating Capsule-in- 3D-Printed Devices as Gastro-Retentive Delivery Systems of Amoxicillin. J. Drug Deliv. Sci. Technol. 2020, 55, 101393. [Google Scholar] [CrossRef]
- Chen, D.; Xu, X.-Y.; Li, R.; Zang, G.-A.; Zhang, Y.; Wang, M.-R.; Xiong, M.-F.; Xu, J.-R.; Wang, T.; Fu, H.; et al. Preparation and In Vitro Evaluation of FDM 3D-Printed Ellipsoid-Shaped Gastric Floating Tablets with Low Infill Percentages. AAPS PharmSciTech 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Song, E.; Kwon, J.; Lee, J.-H.; Park, J.-B.; Kim, D. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Parietti, F.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A.; Maroni, A.; Zema, L. Lego-Inspired Capsular Devices for the Development of Personalized Dietary Supplements: Proof of Concept With Multimodal Release of Caffeine. J. Pharm. Sci. 2020, 109, 1990–1999. [Google Scholar] [CrossRef]
- Shaqour, B.; Samaro, A.; Verleije, B.; Beyers, K.; Vervaet, C.; Cos, P. Production of Drug Delivery Systems Using Fused Filament Fabrication: A Systematic Review. Pharmaceutics 2020, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D Printed Bilayer Oral Solid Dosage Form Combining Metformin for Prolonged and Glimepiride for Immediate Drug Delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef]
- Jamróz, W.; Pyteraf, J.; Kurek, M.; Knapik-Kowalczuk, J.; Szafraniec-Szczęsny, J.; Jurkiewicz, K.; Leszczyński, B.; Wróbel, A.; Paluch, M.; Jachowicz, R. Multivariate Design of 3D Printed Immediate-Release Tablets with Liquid Crystal-Forming Drug—Itraconazole. Materials 2020, 13, 4961. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef] [PubMed]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The Applicability of Pharmaceutical Polymeric Blends for the Fused Deposition Modelling (FDM) 3D Technique: Material Considerations–Printability–Process Modulation, with Consecutive Effects on in Vitro Release, Stability and Degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. 3D-Printed Network Structures as Controlled-Release Drug Delivery Systems: Dose Adjustment, API Release Analysis and Prediction. AAPS PharmSciTech 2018, 19, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D Printing Technology and Quality by Design Approach for Development of Age-Appropriate Pediatric Formulation of Baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, G.; Gretić, M.; Kezerić, K.; Petanjek, J.; Vukelić, E. Preparation of Filaments and the 3D Printing of Dronedarone HCl Tablets for Treating Cardiac Arrhythmias. AAPS PharmSciTech 2019, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Ponsar, H.; Wiedey, R.; Quodbach, J. Hot-Melt Extrusion Process Fluctuations and Their Impact on Critical Quality Attributes of Filaments and 3D-Printed Dosage Forms. Pharmaceutics 2020, 12, 511. [Google Scholar] [CrossRef]
- Than, Y.M.; Titapiwatanakun, V. Tailoring Immediate Release FDM 3D Printed Tablets Using a Quality by Design (QbD) Approach. Int. J. Pharm. 2021, 599, 120402. [Google Scholar] [CrossRef]
- Hussain, A.; Mahmood, F.; Arshad, M.S.; Abbas, N.; Qamar, N.; Mudassir, J.; Farhaj, S.; Nirwan, J.S.; Ghori, M.U. Personalised 3D Printed Fast-Dissolving Tablets for Managing Hypertensive Crisis: In-Vitro/In-Vivo Studies. Polymers 2020, 12, 3057. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. Fabrication of an Osmotic 3D Printed Solid Dosage Form for Controlled Release of Active Pharmaceutical Ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef]
- Kempin, W.; Domsta, V.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Development of a Dual Extrusion Printing Technique for an Acid- and Thermo-Labile Drug. Eur. J. Pharm. Sci. 2018, 123, 191–198. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Szafraniec-Szczęsny, J.; Czech, A.; Gawlak, K.; Knapik-Kowalczuk, J.; Leszczyński, B.; Wróbel, A.; Paluch, M.; Jachowicz, R. Speed It up, Slow It Down…An Issue of Bicalutamide Release from 3D Printed Tablets. Eur. J. Pharm. Sci. 2020, 143, 105169. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Kurek, M.; Czech, A.; Szafraniec, J.; Gawlak, K.; Jachowicz, R. 3D Printing of Tablets Containing Amorphous Aripiprazole by Filaments Co-Extrusion. Eur. J. Pharm. Biopharm. 2018, 131, 44–47. [Google Scholar] [CrossRef]
- Sadia, M.; Isreb, A.; Abbadi, I.; Isreb, M.; Aziz, D.; Selo, A.; Timmins, P.; Alhnan, M.A. From ‘Fixed Dose Combinations’ to ‘a Dynamic Dose Combiner’: 3D Printed Bi-Layer Antihypertensive Tablets. Eur. J. Pharm. Sci. 2018, 123, 484–494. [Google Scholar] [CrossRef]
- Đuranović, M.; Obeid, S.; Madžarević, M.; Cvijić, S.; Ibrić, S. Paracetamol Extended Release FDM 3D Printlets: Evaluation of Formulation Variables on Printability and Drug Release. Int. J. Pharm. 2021, 592, 120053. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D Printing of High Drug Loaded Dosage Forms Using Thermoplastic Polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef]
- Restrepo-Uribe, L.; Ioannidis, N.; del Pilar Noriega, M. Dissolution Improvement of an Active Pharmaceutical Ingredient in a Polymer Melt by Hot Melt Extrusion. J. Polym. Eng. 2019, 39, 186–196. [Google Scholar] [CrossRef]
- Tiţa, D.; Fuliaş, A.; Tiţa, B. Thermal Stability of Ketoprofen—Active Substance and Tablets: Part 1. Kinetic Study of the Active Substance under Non-Isothermal Conditions. J. Therm. Anal. Calorim. 2011, 105, 501–508. [Google Scholar] [CrossRef]
- Lobo-Guerrero, A. X-ray Analysis and Rietveld Refinement of Polyvinyl Alcohol. Mater. Lett. 2020, 265, 127434. [Google Scholar] [CrossRef]
- Mora, E.; Diogo, H.P.; Moura Ramos, J.J. The Slow Molecular Mobility in Amorphous Ketoprofen and Ibuprofen. J. Pharm. Sci. 2015, 104, 3833–3841. [Google Scholar] [CrossRef]
- Polyvinyl Alcohol in Hot Melt Extrusion to Improve the Solubility of Drugs. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/0/content/pdf/PS-PVA-HME-Improve-Solubility-03-2017_EN_MS.pdf (accessed on 21 May 2021).
- Chan, S.-Y.; Chung, Y.-Y.; Cheah, X.-Z.; Tan, E.Y.-L.; Quah, J. The Characterization and Dissolution Performances of Spray Dried Solid Dispersion of Ketoprofen in Hydrophilic Carriers. Asian J. Pharm. Sci. 2015, 10, 372–385. [Google Scholar] [CrossRef]
- Vueba, M.; Pina, M.; Veiga, F.; Sousa, J.; Decarvalho, L. Conformational Study of Ketoprofen by Combined DFT Calculations and Raman Spectroscopy. Int. J. Pharm. 2006, 307, 56–65. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; Shen, X.-X.; Zhang, X.-F.; Zhu, L.-M. Solid Dispersions of Ketoprofen in Drug-Loaded Electrospun Nanofibers. J. Dispers. Sci. Technol. 2010, 31, 902–908. [Google Scholar] [CrossRef]
- BASF—Kollidon® CL. Available online: Https://Pharmaceutical.Basf.Com/Global/En/Drug-Formulation/Products/Kollidon-Cl.Html (accessed on 25 March 2021).
- JRS Pharma—VIVASOL®. Available online: Https://Www.Jrspharma.Com/Pharma_en/Products-Services/Excipients/Superdisintegrants/Vivasol.Php (accessed on 25 March 2021).
- JRS Pharma—VIVASTAR®. Available online: Https://Www.Jrspharma.Com/Pharma_en/Products-Services/Excipients/Superdisintegrants/Vivastar.Php (accessed on 25 March 2021).
- Zarmpi, P.; Flanagan, T.; Meehan, E.; Mann, J.; Fotaki, N. Surface Dissolution UV Imaging for Characterization of Superdisintegrants and Their Impact on Drug Dissolution. Int. J. Pharm. 2020, 577, 119080. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical Cone-Beam Algorithm. J. Opt. Soc. Am. 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Doube, M.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Shefelbine, S.J. BoneJ: Free and Extensible Bone Image Analysis in ImageJ. Bone 2011, 10. [Google Scholar] [CrossRef] [PubMed]







| Sample Name | Formulation | Die Temperature (°C) | Torque ± SD (Nm) | API Content ± SD (%) | Diameter ± SD (mm) | Young Modulus ± SD (N/mm2) |
|---|---|---|---|---|---|---|
| Drug-Loaded Filaments | ||||||
| F1 | KET20_K/CL | 155 | 3.65 ± 0.12 | 20.99 ± 0.16 | 1.78 ± 0.02 | 2141.43 ± 75.39 |
| F2 | KET40 | 130 | 2.72 ± 0.29 | 37.88 ± 1.12 | 2.01 ± 0.02 | 618.08 ± 40.84 |
| F3 | KET40_K/CL | 130 | 2.70 ± 0.04 | 40.23 ± 0.89 | 1.72 ± 0.03 | 501.13 ± 46.22 |
| F4 | KET40_VSOL | 145 | 2.32 ± 0.12 | 40.52 ± 0.86 | 1.78 ± 0.07 | 466.85 ± 118.36 |
| F5 | KET40_VSTR | 145 | 2.16 ± 0.10 | 39.48 ± 0.89 | 1.83 ± 0.02 | 502.99 ± 66.09 |
| F6 | KET50_K/CL | 130 | 1.65 ± 0.05 | 48.39 ± 0.84 | 1.55 ± 0.06 | 187.93 ± 14.08 |
| Drug-Free Filament | ||||||
| PF | KIR_M | 175 | 2.52 ± 0.38 | - | 1.63 ± 0.05 | 1296.61 ± 61.35 |
| Sample Name | Filament Formulation | Dose (mg) | Infill Density (%) | Tablet Weight (mg) ± RSD (%) | Printing Temperature (°C) |
|---|---|---|---|---|---|
| T1 | KET20_K/CL | 75 | 35 | 383.46 ± 0.55 | 185 |
| T2 | KET40 | 75 | 35 | 182.04 ± 0.29 | 180 |
| T3 | KET40_K/CL | 75 | 35 | 181.73 ± 1.42 | 175 |
| T4 | 100 | 20 | 244.31 ± 2.33 | 175 | |
| T5 | 100 | 35 | 253.23 ± 0.99 | 175 | |
| T6 | 100 | 50 | 244.93 ± 2.12 | 175 | |
| T7 | 150 | 35 | 375.71 ± 1.01 | 175 | |
| T8 | 150 | 50 | 378.98 ± 1.00 | 175 | |
| T9 | KET40_VSOL | 75 | 35 | 191.33 ± 0.56 | 175 |
| T10 | KET40_VSTR | 75 | 35 | 179.50 ± 0.84 | 175 |
| T11 | KET50_K/CL | 75 | 35 | 157.22 ± 0.39 | 175 |
| T12 | KET40_K/CL 2L | 150 | 35/65 | 374.77 ± 2.27 | 175 |
| T13 | KET40_K/CL 3L | 150 | 35/65/35 | 384.77 ± 1.23 | 175 |
| T14 | KET40_K/CL 1 + 1 | 100 | 35 | 507.84 ± 2.61 | 190 |
| T15 | KET40_K/CL 3 + 1 | 100 | 35 | 338.55 ± 3.64 | 190 |
| Name | Dose (mg) | Length (mm) | Width (mm) | Height (mm) | Mass (mg) | Volume (mm3) | Surface (mm2) | Fraction | Printing Path Dimensions W × H (mm) |
|---|---|---|---|---|---|---|---|---|---|
| T4 | 100 | 19.88 | 10.04 | 3.39 | 243.8 | 186 | 1724 | 0.207 | 0.38 × 0.20 |
| T5 | 19.90 | 10.07 | 2.51 | 246.9 | 186 | 1854 | 0.343 | 0.40 × 0.19 | |
| T6 | 19.88 | 10.09 | 1.80 | 241.8 | 186 | 1626 | 0.541 | 0.42 × 0.16 | |
| T12 | 150 | 19.89 | 9.97 | 3.15 | 382.8 | 289 | 2661 | 0.437 | - |
| T13 | 19.89 | 10.06 | 3.13 | 383.3 | 298 | 2607 | 0.454 | - |
| Name | µCT | Blender | Photocentric Studio | |||
|---|---|---|---|---|---|---|
| Volume (mm3) | Surface (mm2) | Volume (mm3) | Surface (mm2) | Volume (mm3) | Surface (mm2) | |
| T4 | 186 | 1724 | 197 | 2034 | 197 | 2014 |
| T5 | 186 | 1854 | 217 | 2132 | 217 | 2090 |
| T6 | 186 | 1626 | 199 | 1759 | 199 | 1746 |
| T12 | 289 | 2661 | 339 | 2862 | 339 | 2716 |
| T13 | 298 | 2670 | 338 | 2823 | 338 | 2673 |
| Name | Formulation | Ketoprofen | Parteck® MXP | Kollidon® CL | Vivasol® | Vivastar® | Kollicoat® IR | Mannitol |
|---|---|---|---|---|---|---|---|---|
| Drug-Loaded Filaments | ||||||||
| F1 | KET20_K/CL | 20% | 76% | 4% | ||||
| F2 | KET40 | 40% | 60% | |||||
| F3 | KET40_K/CL | 40% | 56% | 4% | ||||
| F4 | KET40_VSOL | 40% | 56% | 4% | ||||
| F5 | KET40_VSTR | 40% | 56% | 4% | ||||
| F6 | KET50_K/CL | 50% | 46% | 4% | ||||
| Drug-Free Filament | ||||||||
| PF | KIR_M | 90% | 10% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyteraf, J.; Jamróz, W.; Kurek, M.; Szafraniec-Szczęsny, J.; Kramarczyk, D.; Jurkiewicz, K.; Knapik-Kowalczuk, J.; Tarasiuk, J.; Wroński, S.; Paluch, M.; et al. How to Obtain the Maximum Properties Flexibility of 3D Printed Ketoprofen Tablets Using Only One Drug-Loaded Filament? Molecules 2021, 26, 3106. https://doi.org/10.3390/molecules26113106
Pyteraf J, Jamróz W, Kurek M, Szafraniec-Szczęsny J, Kramarczyk D, Jurkiewicz K, Knapik-Kowalczuk J, Tarasiuk J, Wroński S, Paluch M, et al. How to Obtain the Maximum Properties Flexibility of 3D Printed Ketoprofen Tablets Using Only One Drug-Loaded Filament? Molecules. 2021; 26(11):3106. https://doi.org/10.3390/molecules26113106
Chicago/Turabian StylePyteraf, Jolanta, Witold Jamróz, Mateusz Kurek, Joanna Szafraniec-Szczęsny, Daniel Kramarczyk, Karolina Jurkiewicz, Justyna Knapik-Kowalczuk, Jacek Tarasiuk, Sebastian Wroński, Marian Paluch, and et al. 2021. "How to Obtain the Maximum Properties Flexibility of 3D Printed Ketoprofen Tablets Using Only One Drug-Loaded Filament?" Molecules 26, no. 11: 3106. https://doi.org/10.3390/molecules26113106
APA StylePyteraf, J., Jamróz, W., Kurek, M., Szafraniec-Szczęsny, J., Kramarczyk, D., Jurkiewicz, K., Knapik-Kowalczuk, J., Tarasiuk, J., Wroński, S., Paluch, M., & Jachowicz, R. (2021). How to Obtain the Maximum Properties Flexibility of 3D Printed Ketoprofen Tablets Using Only One Drug-Loaded Filament? Molecules, 26(11), 3106. https://doi.org/10.3390/molecules26113106












