Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process
Abstract
:1. Introduction
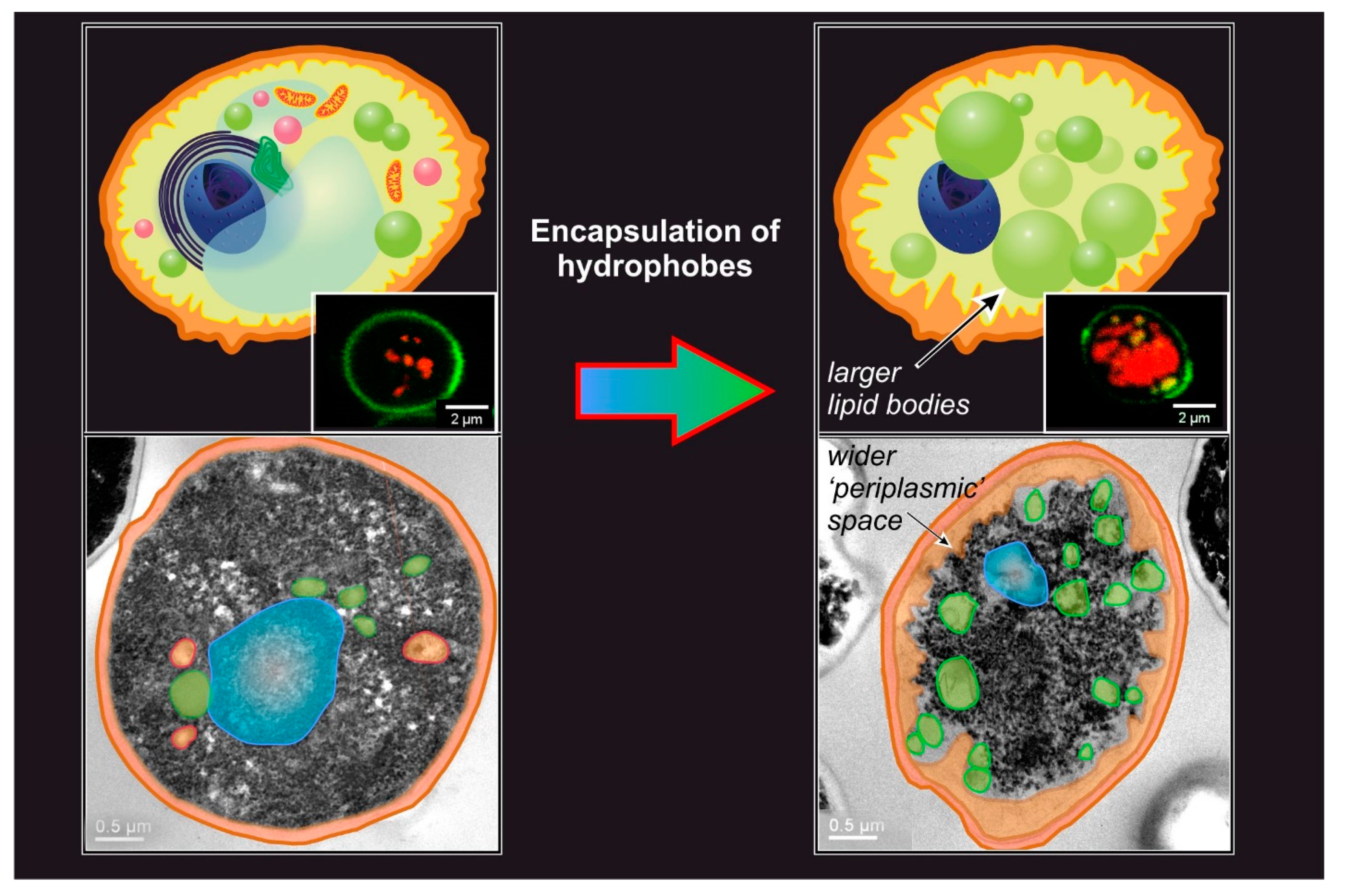
2. Yeast Cells and Their Barrier Structures
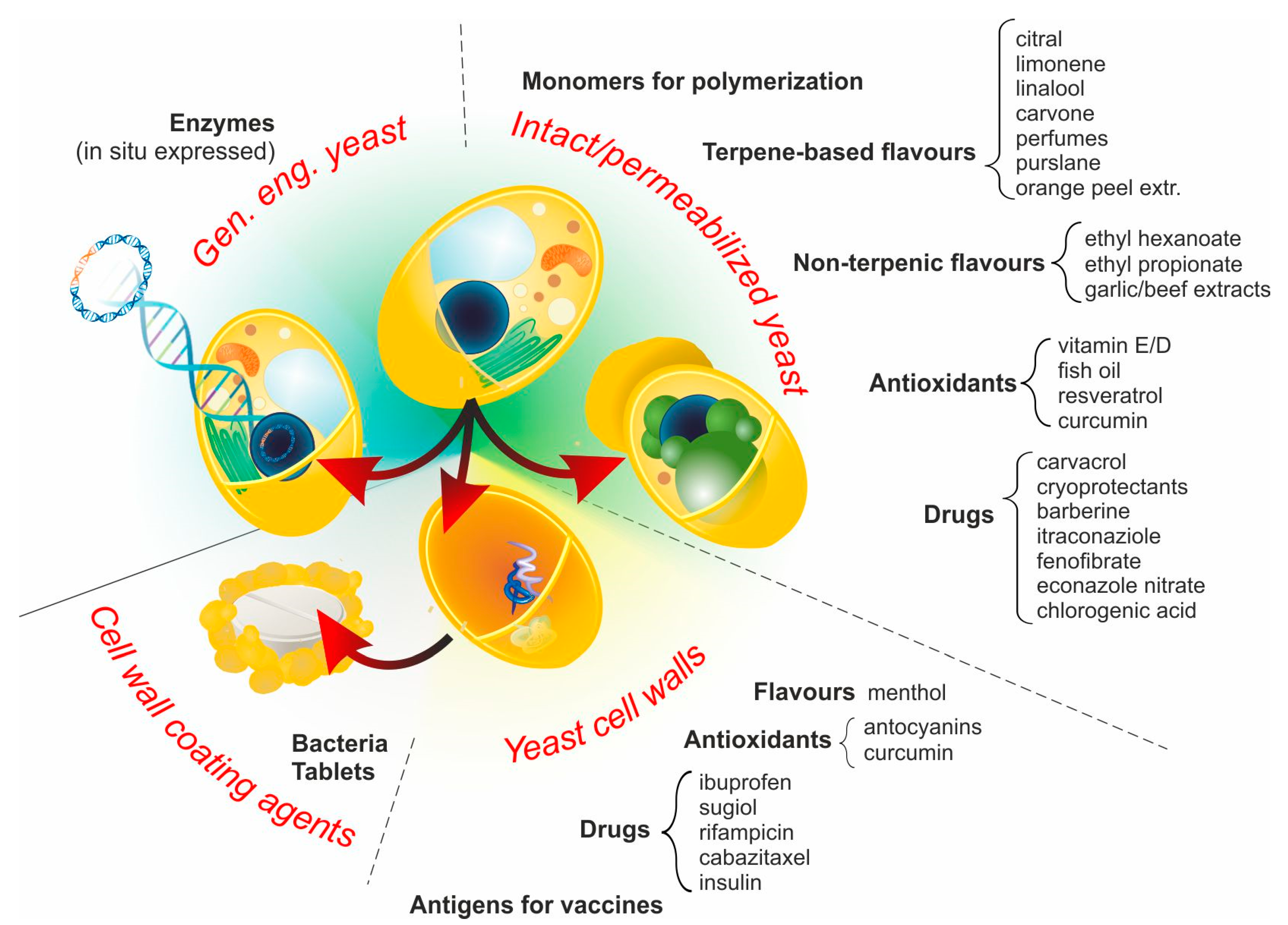
3. YBMCs–One Name, Different Materials
3.1. Intact Yeast Cells (No Pre-Treatment)
3.2. Permeabilized Yeast Cells
3.3. Yeast Cell Walls (YCWs)
3.4. Genetically Engineered Yeast
4. The Controlling Variables of Passive Encapsulation
- The yeast strain, the composition of the medium used to culture it (or the brewing process, in case of spent yeast), and the growth phase the cells are in are all rather variable parameters, but their influence on the encapsulation process is essentially untested.
- The analytical methods used to quantitate the processes also offer significant variability. For example, the amount of encapsulated hydrophobes is most commonly analyzed via HPLC or GC, but is sometimes obtained gravimetrically [41]; this analysis was performed after extraction of the yeast cells with organic solvents, but the variable nature of the solvent (e.g., Neobee [32], ethanol [91], methanol [31], methanol-chloroform [36], hexane, petroleum ether [89]) can be another source of variability. Last, before extraction, the cells are most often disrupted with bead mill disruption or via enzymatic digestion, with the former having been proven to be more efficient [31]. It is worth mentioning that a non-destructive and rapid quantification of hydrophobe encapsulation can be achieved via Nile Red lipid staining [31,130]. This method has some significant limitations: it requires an equilibration of at least 30 min [131] and a thorough calibration [31], which is critically necessary using different yeast strains (or other forms of microorganisms such as algae [132]) or operational conditions, since the results are quite dependent on cell morphology [133] and on whether an organic solvent was used as a carrier [134], leaving the fluorescence-free test as the most accurate approach [133].
5. Mechanistic Considerations
- In the previous section, we discussed how the permeation through the complex barrier comprising both wall and membrane should be interpreted as predominantly determined by their solubility in both environments. This would not apply if the hydrophobes permeate as oil droplets, since effectively, their molecules would not be solubilized;
- In a previous study [31], we demonstrated that when a low molecular weight compound (limonene) was mixed in variable amounts with a short polymer (a polysulfide), the encapsulation of the two in mixture was similar to when they were employed individually (i.e., high for limonene and low for the polysulfide); furthermore, this was irrespective of their ratio, and therefore also independent of the viscosity of the putative oil droplets; and
- The molecular weight threshold discussed in the last section can only be thought of in the context of the permeation of individual molecules.
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurtzman, C.P.; Fel, J.W.; Boekhout, T. (Eds.) Definition, classification and nomenclature of the yeasts. In The Yeasts, a Taxonomic Study; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Dashko, S.; Zhou, N.; Compagno, C.; Piskur, J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014, 14, 826–832. [Google Scholar] [CrossRef] [Green Version]
- Nadai, C.; Treu, L.; Campanaro, S.; Giacomini, A.; Corich, V. Different mechanisms of resistance modulate sulfite tolerance in wine yeasts. Appl. Microbiol. Biotechnol. 2016, 100, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Lata Panwar, S.; Smriti, K. Drug resistance in yeasts—An emerging scenario. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2002; Volume 46, pp. 155–201. [Google Scholar]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [Green Version]
- Breuer, U.; Harms, H. Debaryomyces hansenii—An extremophilic yeast with biotechnological potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose-containing polysaccharides wine composition. AMB Express 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, S.S.; Barrio, E.; Gafner, J.; Querol, A. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 2006, 6, 1221–1234. [Google Scholar] [CrossRef] [Green Version]
- Güneşer, O.; Demirkol, A.; Karagül Yüceer, Y.; Özmen Toğay, S.; İşleten Hoşoğlu, M.; Elibol, M. Bioflavour production from tomato and pepper pomaces by Kluyveromyces marxianus and Debaryomyces hansenii. Bioprocess Biosyst. Eng. 2015, 38, 1143–1155. [Google Scholar] [CrossRef]
- Löser, C.; Urit, T.; Gruner, E.; Bley, T. Efficient growth of Kluyveromyces marxianus biomass used as a biocatalyst in the sustainable production of ethyl acetate. Energy Sustain. Soc. 2015, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Kot, A.; Bzducha-Wróbel, A.; Błażejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- Bansal, R.A.; Tadros, S.; Bansal, A.S. Beer, Cider, and Wine Allergy. Case Rep. Immunol. 2017, 2017, 7958924. [Google Scholar] [CrossRef]
- Dermawan, J.K.T.; Ghosh, S.; Keating, M.K.; Gopalakrishna, K.V.; Mukhopadhyay, S. Candida pneumonia with severe clinical course, recovery with antifungal therapy and unusual pathologic findings: A case report. Medicine 2018, 97, e9650. [Google Scholar] [CrossRef] [PubMed]
- Shribman, S.; Noyce, A.; Gnanapavan, S.; Lambourne, J.; Harrison, T.; Schon, F. Cryptococcal meningitis in apparently immunocompetent patients: Association with idiopathic CD4+ lymphopenia. Pract. Neurol. 2018, 18, 166–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poley, M.; Koubek, R.; Walsh, L.; McGillen, B. Cryptococcal Meningitis in an Apparent Immunocompetent Patient. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619834578. [Google Scholar] [CrossRef] [Green Version]
- Schuytema, C.G.; Lata, G.F. Ergosterol synthesis and storage in the yeast Torulopsis lipofera. Arch. Biochem. Biophys. 1958, 75, 40–45. [Google Scholar] [CrossRef]
- Moon, N.J.; Hammond, E.G.; Glatz, B.A. Conversion of Cheese Whey and Whey Permeate to Oil and Single-Cell Protein. J. Dairy Sci. 1978, 61, 1537–1547. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.M.; Gaillardin, C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Liang, Y.N.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.-L.; Molina-Jouve, C.; Nicaud, J.-M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Zhao, H. Metabolic Engineering of Oleaginous Yeasts for Production of Fuels and Chemicals. Front. Microbiol. 2017, 8, 2185. [Google Scholar] [CrossRef] [Green Version]
- Walker, K.; Skelton, H.; Smith, K. Cutaneous lesions showing giant yeast forms of Blastomyces dermatitidis. J. Cutan. Pathol. 2002, 29, 616–618. [Google Scholar] [CrossRef] [Green Version]
- Shank, J.L. Encapsulation Process Utilizing Microorganisms and Products Produced Thereby. U.S. Patent US4001480A, 16 August 1976. [Google Scholar]
- Sultana, A.; Miyamoto, A.; Hy, Q.L.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Microencapsulation of flavors by spray drying using Saccharomyces cerevisiae. J. Food Eng. 2017, 199, 36–41. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Pen, G.; Long, S.; Yang, C. Yeast-cell-based microencapsulation of chlorogenic acid as a water-soluble antioxidant. J. Food Eng. 2007, 80, 1060–1067. [Google Scholar] [CrossRef]
- da Silva Lima, A.; Maciel, A.P.; Mendonça, C.d.J.S.; Costa Junior, L.M. Use of encapsulated carvacrol with yeast cell walls to control resistant strains of Rhipicephalus microplus (Acari: Ixodidae). Ind. Crops Prod. 2017, 108, 190–194. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef]
- Stenson, J.; Hartley, P.; Wang, C.; Thomas, C. Determining the Mechanical Properties of Yeast Cell Walls. Biotechnol. Prog. 2011, 27, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ciamponi, F.; Duckham, C.; Tirelli, N. Yeast cells as microcapsules. Analytical tools and process variables in the encapsulation of hydrophobes in S. cerevisiae. Appl. Microbiol. Biotechnol. 2012, 95, 1445–1456. [Google Scholar] [CrossRef]
- Dardelle, G.; Normand, V.; Steenhoudt, M.; Bouquerand, P.-E.; Chevalier, M.; Baumgartner, P. Flavour-Encapsulation and flavour-release performances of a commercial yeast-based delivery system. Food Hydrocoll. 2007, 21, 953–960. [Google Scholar] [CrossRef]
- Sultana, A.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Stability and release behavior of encapsulated flavor from spray-dried Saccharomyces cerevisiae and maltodextrin powder. Food Res. Int. 2018, 106, 809–816. [Google Scholar] [CrossRef]
- Kogan, G.; Pajtinka, M.; Babincova, M.; Miadokova, E.; Rauko, P.; Slamenova, D.; Korolenko, T.A. Yeast cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer? Neoplasma 2008, 55, 387–393. [Google Scholar] [PubMed]
- Kogan, G.; Staško, A.; Bauerová, K.; Polovka, M.; Šoltés, L.; Brezová, V.; Navarová, J.; Mihalová, D. Antioxidant properties of yeast (1→3)-β-d-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritis. Carbohydr. Polym. 2005, 61, 18–28. [Google Scholar] [CrossRef]
- Iassonova, D.R.; Hammond, E.G.; Beattie, S.E. Oxidative stability of polyunsaturated triacylglycerols encapsulated in oleaginous yeast. J. Am. Oil Chem. Soc. 2008, 85, 711–716. [Google Scholar] [CrossRef]
- Wu, J.; Guan, Y.; Zhong, Q. Yeast mannoproteins improve thermal stability of anthocyanins at pH 7.0. Food Chem. 2015, 172, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Beikzadeh, S.; Shojaee-Aliabadi, S.; Dadkhodazade, E.; Sheidaei, Z.; Abedi, A.-S.; Mirmoghtadaie, L.; Hosseini, S.M. Comparison of Properties of Breads Enriched with Omega-3 Oil Encapsulated in β-Glucan and Saccharomyces cerevisiae Yeast Cells. Appl. Food Biotechnol. 2019, 7, 11–20. [Google Scholar] [CrossRef]
- Paramera, E.I.; Konteles, S.J.; Karathanos, V.T. Stability and release properties of curcumin encapsulated in Saccharomyces cerevisiae, β-cyclodextrin and modified starch. Food Chem. 2011, 125, 913–922. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Czerniak, A.; Kubiak, P.; Białas, W.; Jankowski, T. Improvement of oxidative stability of menhaden fish oil by microencapsulation within biocapsules formed of yeast cells. J. Food Eng. 2015, 167, 2–11. [Google Scholar] [CrossRef]
- Bishop, J.R.P.; Nelson, G.; Lamb, J. Microencapsulation in yeast cells. J. Microencapsul. 1998, 15, 761–773. [Google Scholar] [CrossRef]
- Pannell, N.A. Microbial Encapsulation. International Patent Application No. EP0242135B1, 12 April 1986. [Google Scholar]
- Behan, J.M.; Perring, K.D. Fabric Softening Compositions Containing Microorganism-Encapsulated Perfume. U.S. Patent US5078904A, 7 January 1992. [Google Scholar]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef]
- McNeight, D.L. Nicotine Delivery Systems. International Patent Application No. EP1176961B1, 5 November 2003. [Google Scholar]
- Gordon, N.; Duckham, S.C.; Round, A.E. Targeted Delivery of Microbially Encapsulated Drugs. International Patent Application No. GB2394416A, 11 May 2000. [Google Scholar]
- Siegel, S.; Mavric, E.; Krammer, G. Encapsulated Vaccinium Extracts with Balanced Gastrointestinal Release. U.S. Patent US20090041872A1, 7 August 2008. [Google Scholar]
- Barra, J.; Dardelle, G.; Marty, M.; Castioni, N.V.; Wick, M.; Zampieri, D. Process for Encapsulating an Active Ingredient. International Patent Application No. WO2012084467A1, 28 June 2012. [Google Scholar]
- Paramera, E.I.; Karathanos, V.T.; Konteles, S.J. Yeast cells and yeast-based materials for microencapsulation. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: San Diego, CA, USA, 2014; Chapter 23; pp. 267–281. [Google Scholar] [CrossRef]
- Chorvatovičová, D.; Machová, E.; Šandula, J.; Kogan, G. Protective effect of the yeast glucomannan against cyclophosphamide-induced mutagenicity. Mutat. Res. Genet. Toxicol. Environ. 1999, 444, 117–122. [Google Scholar] [CrossRef]
- Fuller, E.; Duckham, C.; Wood, E. Disruption of Epithelial Tight Junctions by Yeast Enhances the Paracellular Delivery of a Model Protein. Pharm. Res. 2007, 24, 37–47. [Google Scholar] [CrossRef]
- Yang, W.; Yan, L.; Wu, C.; Zhao, X.; Tang, J. Fungal invasion of epithelial cells. Microbiol. Res. 2014, 169, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Wächtler, B.; Citiulo, F.; Jablonowski, N.; Förster, S.; Dalle, F.; Schaller, M.; Wilson, D.; Hube, B. Candida albicans-Epithelial Interactions: Dissecting the Roles of Active Penetration, Induced Endocytosis and Host Factors on the Infection Process. PLoS ONE 2012, 7, e36952. [Google Scholar] [CrossRef] [Green Version]
- Nicola, A.M.; Casadevall, A.; Goldman, D.L. Fungal killing by mammalian phagocytic cells. Curr. Opin. Microbiol. 2008, 11, 313–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Upadhyay, T.K.; Fatima, N.; Sharma, D.; Saravanakumar, V.; Sharma, R. Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. EXCLI J. 2017, 16, 210–228. [Google Scholar] [CrossRef]
- Stubbs, A.C.; Martin, K.S.; Coeshott, C.; Skaates, S.V.; Kuritzkes, D.R.; Bellgrau, D.; Franzusoff, A.; Duke, R.C.; Wilson, C.C. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat. Med. 2001, 7, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust Stimulation of Humoral and Cellular Immune Responses following Vaccination with Antigen-Loaded β-Glucan Particles. mBio 2010, 1, e00164-10. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Li, X.; Kang, T.; Meng, H.; Chen, Z.; Yang, L.; Wu, Y.; Wei, Y.; Gou, M. Efficient delivery of antigen to DCs using yeast-derived microparticles. Sci. Rep. 2015, 5, 10687. [Google Scholar] [CrossRef] [Green Version]
- De Smet, R.; Demoor, T.; Verschuere, S.; Dullaers, M.; Ostroff, G.; Leclercq, G.; Allais, L.; Pilette, C.; Dierendonck, M.; De Geest, B.; et al. Beta-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J. Control. Release 2013, 172, 671–678. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, E.; Caras, A.; Kut, L.; Castle, M.; Ostroff, G. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 143524. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Gao, C.; Liu, G.; Hu, J. MAP4K4: An emerging therapeutic target in cancer. Cell Biosci. 2016, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, X.; Chen, Y.; Dou, Y.; Zhou, X.; Li, L.; Li, C.; An, H.; Tao, H.; Hu, H.-Y.; et al. Bioinspired yeast microcapsules loaded with self-assembled nanotherapies for targeted treatment of cardiovascular disease. Mater. Today 2017, 20, 301–313. [Google Scholar] [CrossRef]
- Ghoneum, M.; Badr El-Din, N.K.; Noaman, E.; Tolentino, L. Saccharomyces cerevisiae, the Baker’s Yeast, suppresses the growth of Ehrlich carcinoma-bearing mice. Cancer Immunol. Immunother. 2008, 57, 581–592. [Google Scholar] [CrossRef]
- Nelson, G.; Duckham, S.C.; Crothers, M.E.D. Microencapsulation in Yeast Cells and Applications in Drug Delivery. In Polymeric Drug Delivery I; American Chemical Society: Washington, DC, USA, 2006; Volume 923, pp. 268–281. [Google Scholar]
- Klis, F.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- McLellan, W.L., Jr.; McDaniel, L.E.; Lampen, J.O. Purification of phosphomannanase and its action on the yeast cell wall. J. Bacteriol. 1970, 102, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [Green Version]
- Friis, J.; Ottolenghi, P. The genetically determined binding of alcian blue by a minor fraction of yeast cell walls. C. R. Trav. Lab. Carlsberg 1970, 37, 327–341. [Google Scholar]
- Singleton, D.R.; Masuoka, J.; Hazen, K.C. Surface hydrophobicity changes of two Candida albicans serotype B mnn4 delta mutants. Eukaryot. Cell 2005, 4, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Zlotnik, H.; Fernandez, M.P.; Bowers, B.; Cabib, E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 1984, 159, 1018–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, R.D.; Chaffin, W.L. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef]
- Gow, N.; Munro, C.; Latge, J.-P. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Kapteyn, J.C.; Van Den Ende, H.; Klis, F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1999, 1426, 373–383. [Google Scholar] [CrossRef]
- de Groot, P.W.; Ruiz, C.; Vázquez de Aldana, C.R.; Duenas, E.; Cid, V.J.; Del Rey, F.; Rodríquez-Peña, J.M.; Pérez, P.; Andel, A.; Caubín, J.; et al. A genomic approach for the identification and classification of genes involved in cell wall formation and its regulation in Saccharomyces cerevisiae. Comp. Funct. Genom. 2001, 2, 124–142. [Google Scholar] [CrossRef] [Green Version]
- Grillitsch, K.; Tarazona, P.; Klug, L.; Wriessnegger, T.; Zellnig, G.; Leitner, E.; Feussner, I.; Daum, G. Isolation and characterization of the plasma membrane from the yeast Pichia pastoris. BBA Biomembr. 2014, 1838, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Bagnat, M.; Keranen, S.; Shevchenko, A.; Shevchenko, A.; Simons, K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 2000, 97, 3254–3259. [Google Scholar] [CrossRef]
- Mollinedo, F. Lipid raft involvement in yeast cell growth and death. Front. Oncol. 2012, 2, 140. [Google Scholar] [CrossRef] [Green Version]
- Bagnat, M.; Simons, K. Cell surface polarization during yeast mating. Proc. Natl. Acad. Sci. USA 2002, 99, 14183–14188. [Google Scholar] [CrossRef] [Green Version]
- Thumm, M. Structure and function of the yeast vacuole and its role in autophagy. Microsc. Res. Tech. 2000, 51, 563–572. [Google Scholar] [CrossRef]
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. BBA Mol. Cell Res. 2009, 1793, 650–663. [Google Scholar] [CrossRef] [Green Version]
- Blanquet-Diot, S.; Garrait, G.; Beyssac, E.; Perrier, C.; Denis, S.; Hébrard, G.; Alric, M. Effects of cryoprotectants on the viability and activity of freeze dried recombinant yeasts as novel oral drug delivery systems assessed by an artificial digestive system. Eur. J. Pharm. Biopharm. 2005, 61, 32–39. [Google Scholar] [CrossRef]
- Sangwai, M.; Vavia, P. Effect of decisive formulation variables on bioencapsulation efficiency and integrity of yeast biocapsules for oral itraconazole delivery. J. Microencapsul. 2011, 28, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Gomes, B.; Weng, J.-L.; Ista, L.K.; Jesus, C.P.; David, M.R.; Ramalho-Ortigao, M.; Genta, F.A.; Matthews, S.K.; Durvasula, R.; et al. Yeast-encapsulated essential oils: A new perspective as an environmentally friendly larvicide. Parasite Vector 2020, 13, 19. [Google Scholar] [CrossRef]
- Czerniak, A.; Jankowski, T. Microencapsulation of α-tocopherol inside Saccharomyces cerevisiae yeast cells. ŻYWNOŚĆ Nauka Technol. Jakość 2013, 20, 151–164. [Google Scholar] [CrossRef]
- Kavosi, M.; Mohammadi, A.; Shojaee-Aliabadi, S.; Khaksar, R.; Seyede, M.H. Characterization and oxidative stability of purslane seed oil microencapsulated in yeast cells biocapsules. J. Sci. Food Agric. 2017, 98, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Dadkhodazade, E.; Mohammadi, A.; Shojaee-Aliabadi, S.; Mortazavian, A.M.; Mirmoghtadaie, L.; Hosseini, S.M. Yeast Cell Microcapsules as a Novel Carrier for Cholecalciferol Encapsulation: Development, Characterization and Release Properties. Food Biophys. 2018, 13, 404–411. [Google Scholar] [CrossRef]
- Salari, R.; Fazly Bazzaz, B.S.; Khashyarmanesh, Z. New aspects of Saccharomyces cerevisiae as a novel carrier for berberine. DARU J. Pharm. Sci. 2013, 21, 73. [Google Scholar] [CrossRef] [Green Version]
- Paramera, E.; Konteles, S.; Karathanos, V. Microencapsulation of curcumin in cells of Saccharomyces cerevisiae. Food Chem. 2011, 125, 892–902. [Google Scholar] [CrossRef]
- Ruphuy, G.; Saloň, I.; Tomas, J.; Šalamúnová, P.; Hanuš, J.; Štěpánek, F. Encapsulation of poorly soluble drugs in yeast glucan particles by spray drying improves dispersion and dissolution properties. Int. J. Pharm. 2020, 576, 118990. [Google Scholar] [CrossRef] [PubMed]
- Errenst, C.; Petermann, M.; Kilzer, A. Encapsulation of limonene in yeast cells using the concentrated powder form technology. J. Supercrit. Fluid 2021, 168, 105076. [Google Scholar] [CrossRef]
- Morris, G.J.; Winters, L.; Coulson, G.E.; Clarke, K.J. Effect of osmotic stress on the ultrastructure and viability of the yeast Saccharomyces cerevisiae. J. Gen. Microbiol. 1986, 132, 2023–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Song, L.; Guan, J.; Sun, C.; Zhou, D.; Zhu, B. Encapsulation of Antarctic krill oil in yeast cell microcarriers: Evaluation of oxidative stability and in vitro release. Food Chem. 2021, 338, 128089. [Google Scholar] [CrossRef]
- Young, S.; Nitin, N. Thermal and oxidative stability of curcumin encapsulated in yeast microcarriers. Food Chem. 2019, 275, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar] [CrossRef]
- Champagne, C.P.; Barrette, J.; Goulet, J. Interaction between pH, autolysis promoters and bacterial contamination on the production of yeast extracts. Food Res. Int. 1999, 32, 575–583. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Utilisation of spent brewer’s yeast for yeast extract production by autolysis: The effect of temperature. Food Bioprod. Process. 2008, 86, 317–321. [Google Scholar] [CrossRef]
- Shi, G.; Liu, Y.; He, Z.; Zhou, J. Chemical treatment and chitosan coating of yeast cells to improve the encapsulation and controlled release of bovine serum albumin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1207–1215. [Google Scholar] [CrossRef]
- Chow, C.-K.; Palecek, S. Enzyme Encapsulation in Permeabilized Saccharomyces cerevisiae Cells. Biotechnol. Prog. 2004, 20, 449–456. [Google Scholar] [CrossRef]
- Pham-Hoang, B.N.; Voilley, A.; Waché, Y. Molecule structural factors influencing the loading of flavoring compounds in a natural-preformed capsule: Yeast cells. Colloids Surface B 2016, 148, 220–228. [Google Scholar] [CrossRef]
- Ma, G.; Zhao, Z.; Liu, H. Yeast Cells Encapsulating Polymer Nanoparticles as Trojan Particles via in Situ Polymerization inside Cells. Macromolecules 2016, 49, 1545–1551. [Google Scholar] [CrossRef]
- Kilcher, G.; Delneri, D.; Duckham, C.; Tirelli, N. Probing (macro)molecular transport through cell walls. Faraday Discuss. 2008, 139, 199–212. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Xie, Q.; Li, J.; Li, B.; Xiong, X. Characterization of yeast cells as a microencapsulation wall material by Fourier-transform infrared spectroscopy. Vib. Spectrosc. 2010, 53, 289–295. [Google Scholar] [CrossRef]
- Laroche, C.; Gervais, P. Achievement of rapid osmotic dehydration at specific temperatures could maintain high Saccharomyces cerevisiae viability. Appl. Microbiol. Biotechnol. 2003, 60, 743–747. [Google Scholar] [CrossRef]
- Normand, V.; Dardelle, G.; Bouquerand, P.E.; Nicolas, L.; Johnston, D.J. Flavor encapsulation in yeasts: Limonene used as a model system for characterization of the release mechanism. J. Agric. Food Chem. 2005, 53, 7532–7543. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, G. Drug Delivery Product and Methods. U.S. Patent US20050281781A1, 22 December 2005. [Google Scholar]
- Ren, T.; Gou, J.; Sun, W.; Tao, X.; Tan, X.; Wang, P.; Zhang, Y.; He, H.; Yin, T.; Tang, X. Entrapping of Nanoparticles in Yeast Cell Wall Microparticles for Macrophage-Targeted Oral Delivery of Cabazitaxel. Mol. Pharm. 2018, 15. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Han, S.; Dou, Y.; Liu, M.; Zhang, L.; Guo, J.; Shi, Q.; Gong, G.; Wang, R.; et al. Yeast Microcapsule-Mediated Targeted Delivery of Diverse Nanoparticles for Imaging and Therapy via the Oral Route. Nano Lett. 2017, 17, 1056–1064. [Google Scholar] [CrossRef]
- Soto, E.R.; Ostroff, G.R. Characterization of Multilayered Nanoparticles Encapsulated in Yeast Cell Wall Particles for DNA Delivery. Bioconjug. Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef]
- Soto, E.; Kim, Y.S.; Lee, J.; Kornfeld, H.; Ostroff, G. Glucan Particle Encapsulated Rifampicin for Targeted Delivery to Macrophages. Polymers 2010, 2, 681–689. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Polysaccharide dual coating of yeast capsules for stabilization of anthocyanins. Food Chem. 2021, 357, 129652. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiang, S.; Xia, F.; Xiongwei, H.; He, H.; Yin, Z.; Qi, J.; Lu, Y.; Wu, W. Glucan microparticles thickened with thermosensitive gels as potential carriers for oral delivery of insulin. J. Mater. Chem. B 2016, 4, 4040. [Google Scholar] [CrossRef]
- Scariot, D.; Volpato, H.; Fernandes, N.; Soares, E.; Ueda-Nakamura, T.; Dias-Filho, B.; Din, Z.; Rodrigues-Filho, E.; Rubira, A.; Borges, O.; et al. Activity and Cell-Death Pathway in Leishmania infantum Induced by Sugiol: Vectorization Using Yeast Cell Wall Particles Obtained From Saccharomyces cerevisiae. Front. Cell Infect. Microbiol. 2019, 9, 208. [Google Scholar] [CrossRef]
- Marson, G.V.; Saturno, R.P.; Comunian, T.A.; Consoli, L.; da Costa Machado, M.T.; Hubinger, M.D. Maillard conjugates from spent brewer’s yeast by-product as an innovative encapsulating material. Food Res. Int. 2020, 136, 109365. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Van, L.; Duc, T.; Le, H. Encapsulation of Lactobacillus Acidophilus in Yeast Cell Walls (Saccharomyces cerevisiae) for Improving Survival in Gastrointestinal Conditions. Vietnam J. Sci. Technol. 2016, 54, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M.; Maghsoudlou, Y.; Ghorbani, M. The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Res. Int. 2017, 96, 19–26. [Google Scholar] [CrossRef]
- Yuasa, H.; Kaneshige, J.; Ozeki, T.; Kasai, T.; Eguchi, T.; Ishiwaki, N. Application of acid-treated yeast cell wall (AYC) as a pharmaceutical additive. II: Effects of curing on the medicine release from AYC-coated tablets. Int. J. Pharm. 2000, 209, 69–77. [Google Scholar] [CrossRef]
- Blanquet-Diot, S.; Marol-Bonnin, S.; Beyssac, E.; Pompon, D.; Renaud, M.; Alric, M. The ‘biodrug’ concept: An innovative approach to therapy. Trends Biotechnol. 2001, 19, 393–400. [Google Scholar] [CrossRef]
- Hitzeman, R.A.; Hagie, F.E.; Levine, H.L.; Goeddel, D.V.; Ammerer, G.; Hall, B.D. Expression of a human gene for interferon in yeast. Nature 1981, 293, 717–722. [Google Scholar] [CrossRef]
- Stephenne, J. Production in yeast versus mammalian cells of the first recombinant DNA human vaccine and its proved safety, efficacy, and economy: Hepatitis B vaccine. Adv. Biotechnol. Process. 1990, 14, 279–299. [Google Scholar]
- Kim, H.; Yoo, S.J.; Kang, H.A. Yeast synthetic biology for the production of recombinant therapeutic proteins. FEMS Yeast Res. 2015, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branduardi, P.; Fossati, T.; Sauer, M.; Pagani, R.; Mattanovich, D.; Porro, D. Biosynthesis of vitamin C by yeast leads to increased stress resistance. PLoS ONE 2007, 2, e1092. [Google Scholar] [CrossRef]
- van der Hoek, S.A.; Darbani, B.; Zugaj, K.E.; Prabhala, B.K.; Biron, M.B.; Randelovic, M.; Medina, J.B.; Kell, D.B.; Borodina, I. Engineering the Yeast Saccharomyces cerevisiae for the Production of L-(+)-Ergothioneine. Front. Bioeng. Biotechnol. 2019, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.M.; Prakash, S. Therapeutic uses of microencapsulated genetically engineered cells. Mol. Med. Today 1998, 4, 221–227. [Google Scholar] [CrossRef]
- Urban, P.; Werck-Reichhart, D.; Teutsch, H.G.; Durst, F.; Regnier, S.; Kazmaier, M.; Pompon, D. Characterization of recombinant plant cinnamate 4-hydroxylase produced in yeast: Kinetic and spectral properties of the major plant P450 of the phenylpropanoid pathway. Eur. J. Biochem. 1994, 222, 843–850. [Google Scholar] [CrossRef]
- Renaud, M.; Bonnin, S.; Alric, M.; Blanquet, S.; Pompon, D. Micro-organisms active in the digestive environment. International Patent Application No. WO200198461-A1; FR2810337-A1, 20 June 2000. [Google Scholar]
- Miranda, C.; Bettencourt, S.; Pozdniakova, T.; Pereira, J.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Modified high-throughput Nile red fluorescence assay for the rapid screening of oleaginous yeasts using acetic acid as carbon source. BMC Microbiol. 2020, 20, 60. [Google Scholar] [CrossRef]
- Ramírez-Castrillón, M.; Jaramillo-Garcia, V.P.; Lopes Barros, H.; Pegas Henriques, J.A.; Stefani, V.; Valente, P. Nile Red Incubation Time Before Reading Fluorescence Greatly Influences the Yeast Neutral Lipids Quantification. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Alemán-Nava, G.S.; Cuellar-Bermudez, S.P.; Cuaresma, M.; Bosma, R.; Muylaert, K.; Ritmann, B.E.; Parra, R. How to use Nile Red, a selective fluorescent stain for microalgal neutral lipids. J. Microbiol. Methods 2016, 128, 74–79. [Google Scholar] [CrossRef]
- Hicks, R.H.; Chuck, C.J.; Scott, R.J.; Leak, D.J.; Henk, D.A. Comparison of Nile Red and Cell Size Analysis for High-Throughput Lipid Estimation Within Oleaginous Yeast. Eur. J. Lipid Sci. Technol. 2019, 121, 1800355. [Google Scholar] [CrossRef] [Green Version]
- Poli, J.S.; Lützhøft, H.-C.H.; Karakashev, D.B.; Valente, P.; Angelidaki, I. An environmentally-friendly fluorescent method for quantification of lipid contents in yeast. Bioresour. Technol. 2014, 151, 388–391. [Google Scholar] [CrossRef]
- Simonin, H.; Beney, L.; Gervais, P. Controlling the membrane fluidity of yeasts during coupled thermal and osmotic treatments. Biotechnol. Bioeng. 2008, 100, 325–333. [Google Scholar] [CrossRef]
- Stirke, A.; Celiesiute-Germaniene, R.; Zimkus, A.; Zurauskiene, N.; Simonis, P.; Dervinis, A.; Ramanavicius, A.; Balevicius, S. The link between yeast cell wall porosity and plasma membrane permeability after PEF treatment. Sci. Rep. 2019, 9, 14731. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, G.; Katsimichas, A.; Tsimogiannis, D.; Oreopoulou, V.; Taoukis, P. Cell permeabilization processes for improved encapsulation of oregano essential oil in yeast cells. J. Food Eng. 2021, 294, 110408. [Google Scholar] [CrossRef]
- Stirke, A.; Zimkus, A.; Ramanaviciene, A.; Balevičius, S.; Zurauskiene, N.; Saulis, G.; Chaustova, L.; Stankevic, V.; Ramanavicius, A. Electric Field-Induced Effects on Yeast Cell Wall Permeabilization. Bioelectromagnetics 2014, 35, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Galutzov, B.; Teissié, J. Electric field mediated loading of macromolecules in intact yeast cells is critically controlled at the wall level. BBA Biomembr. 1995, 1240, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Young, S.; Dea, S.; Nitin, N. Vacuum facilitated infusion of bioactives into yeast microcarriers: Evaluation of a novel encapsulation approach. Food Res. Int. 2017, 100, 100–112. [Google Scholar] [CrossRef]
- Pedrini, M.; Dupont, S.; Câmara, A.; Beney, L.; Gervais, P. Osmoporation: A simple way to internalize hydrophilic molecules into yeast. Appl. Microbiol. Biotechnol. 2013, 98, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, R.; Louden, L.; Gerhardt, P. Porosity of the yeast cell wall and membrane. J. Bacteriol. 1974, 118, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Oh, Y.S.; Kim, S.-J. The possible involvement of cell surface in aliphatic hydrocarbon utilization by an oil-degrading Yeast, Yarrowia lipolytica 180. J. Microbiol. Biotechnol. 2000, 10, 333–337. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: Effect of temperature. Process Biochem. 2000, 36, 431–439. [Google Scholar] [CrossRef]
- Guler, U.; Sarioglu, M. Mono and binary component biosorption of Cu(II), Ni(II), and Methylene Blue onto raw and pretreated S. cerevisiae: Equilibrium and kinetics. Desalin. Water Treat. 2014, 52, 4871–4888. [Google Scholar] [CrossRef]
- Göksungur, M.Y.; Uren, S.; Güvenç, U. Biosorption of Cadmium and Lead Ions by Ethanol Treated Waste Baker’s Yeast Biomass. Bioresour. Technol. 2005, 96, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Dönmez, G. A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere 2003, 50, 1075–1083. [Google Scholar] [CrossRef]
- Bell, J.; Tsezos, M. Removal of Hazardous Organic Pollutants by Adsorption on Microbial Biomass. Water Sci. Technol. 1987, 19, 409–416. [Google Scholar] [CrossRef]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- De Nobel, J.G.; Barnett, J.A. Passage of molecules through yeast cell walls: A brief essay-review. Yeast 1991, 7, 313–323. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123. https://doi.org/10.3390/molecules26113123
Coradello G, Tirelli N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules. 2021; 26(11):3123. https://doi.org/10.3390/molecules26113123
Chicago/Turabian StyleCoradello, Giulia, and Nicola Tirelli. 2021. "Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process" Molecules 26, no. 11: 3123. https://doi.org/10.3390/molecules26113123
APA StyleCoradello, G., & Tirelli, N. (2021). Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules, 26(11), 3123. https://doi.org/10.3390/molecules26113123





