Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases
Abstract
:1. Introduction
2. Types of Dendrimers
2.1. PAMAM Dendrimers
2.2. PPI Dendrimers
2.3. PAMAM Core–Shell Tecto Dendrimers
2.4. Chiral Dendrimers
2.5. Frechet-Type Dendrimers
2.6. Liquid Crystalline Dendrimers
2.7. Peptide Dendrimers
2.8. Hybrid Dendrimers
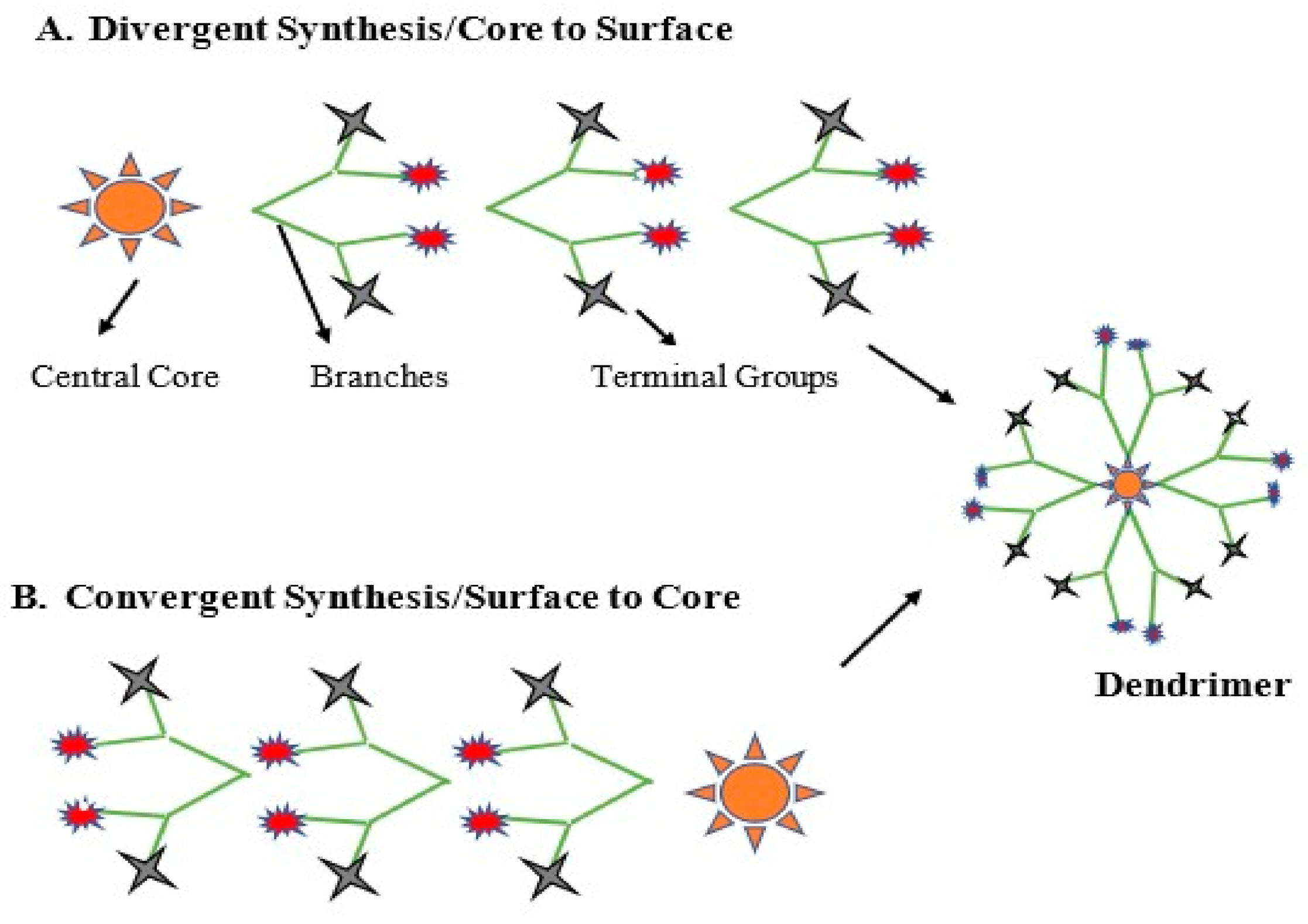
3. Synthesis Routes
3.1. Divergent Method
3.2. Convergent Method
3.3. Click Chemistry
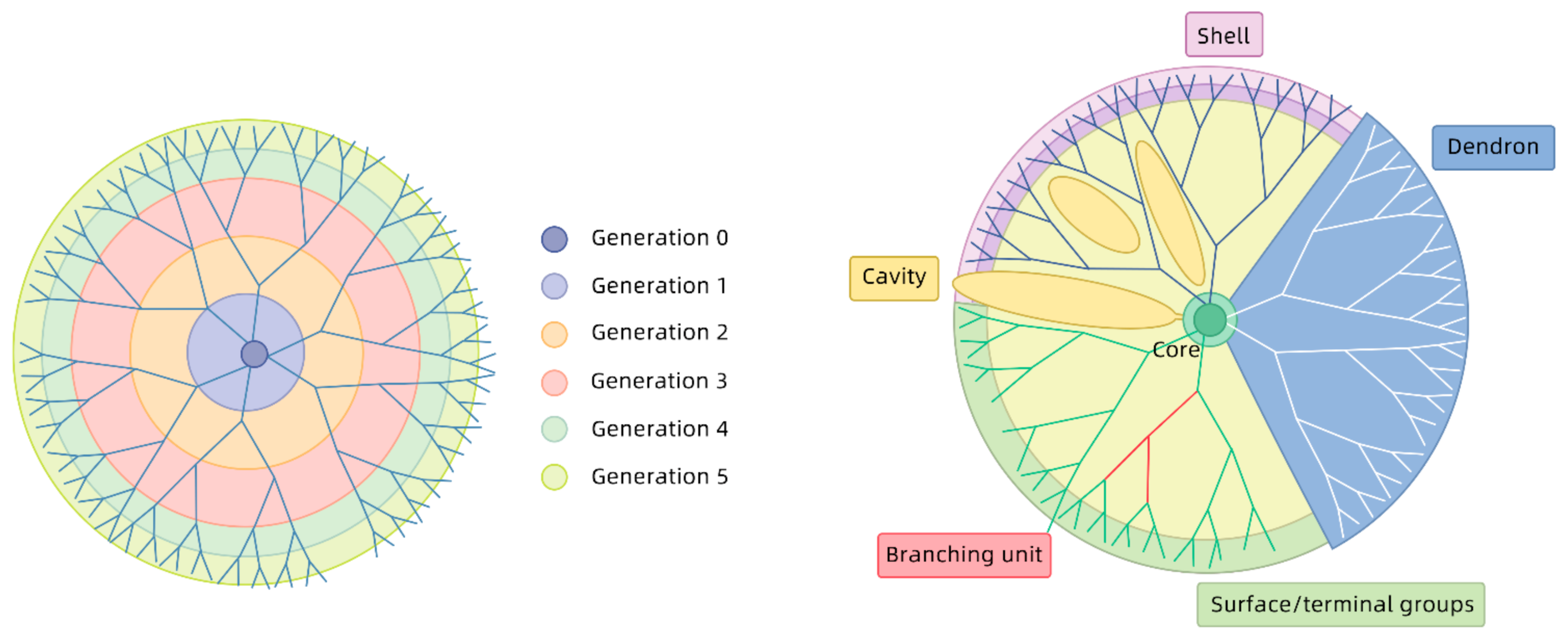
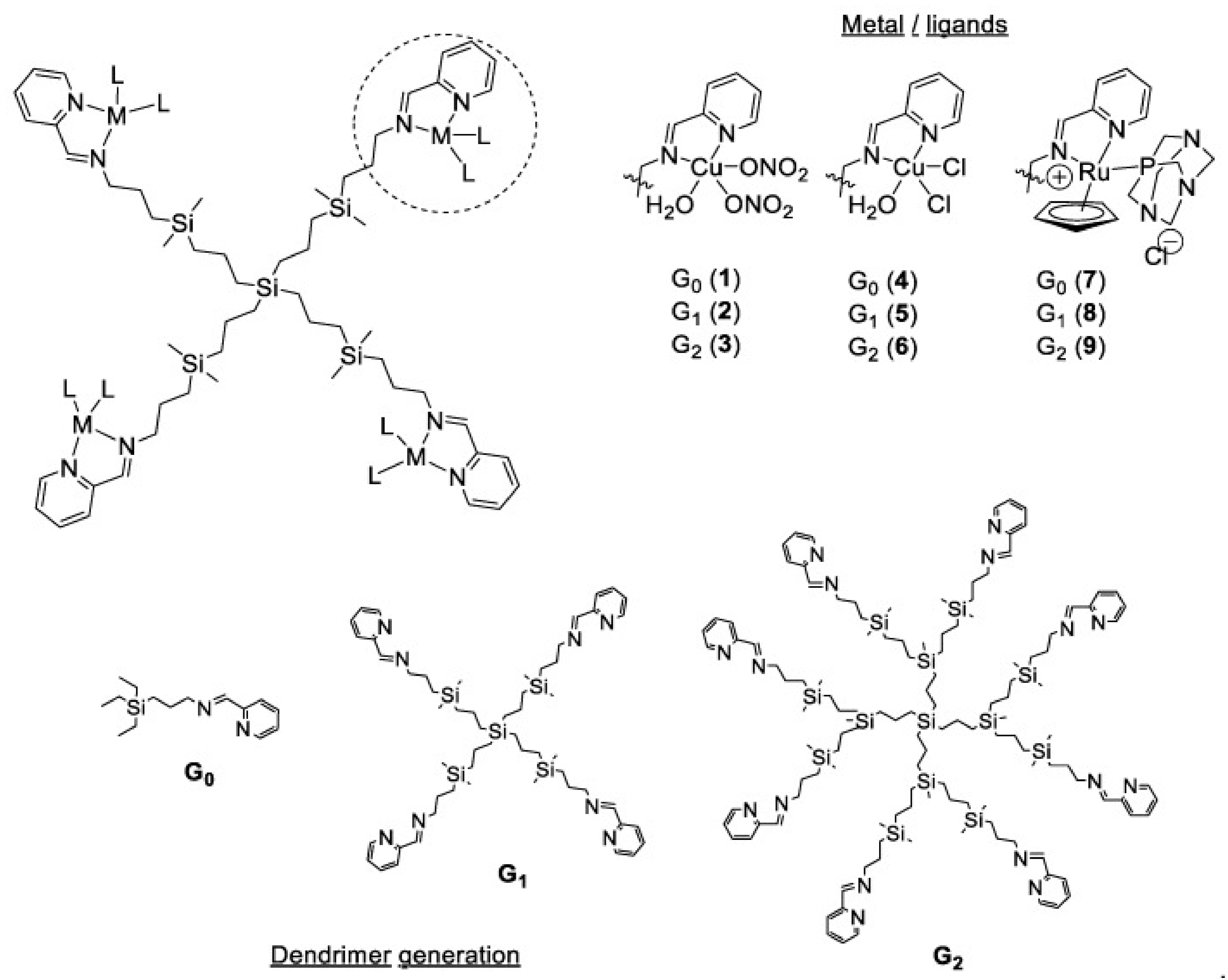
3.4. Generation of Dendrimers
4. Application of Dendrimers in Treatment of Infectious Diseases
4.1. Dendrimers as Antiviral Agents
4.1.1. Human Immunodeficiency Virus (HIV)
4.1.2. Coronaviruses
4.1.3. Ebola Virus
4.1.4. Influenza Virus
4.1.5. Herpes Simplex Virus
4.1.6. Other Viruses
4.2. Dendrimers as Antibacterial Agents
4.2.1. Gram Negative Bacteria
4.2.2. Gram Positive Bacteria
4.3. Dendrimers as Antiparasite Agents
4.3.1. Malaria
4.3.2. Leishmaniasis
4.3.3. Toxoplasmosis
5. Problems in Using Dendrimers
| Problems/Undesirable Effects | Methods to Resolve Undesirable Effects | References |
|---|---|---|
| Interaction with cell membranes due to their cationic charge causing cell lysis |
| [182,183,188,203,204] |
| Hematological toxicity due to its cationic charge | PEGylation, acetylation or carboxylation of the terminal groups | [180,181] |
| Rapid Renal Clearance | PEGylation to increase circulation time | [195,196,197,198] |
| Slow, complex and costly synthetic process | Orthogonal coupling and click chemistry are ways to resolve this slow, complex and costly process | [201,202] |
| Inability to penetrate the blood brain barrier (BBB) | Use of mixed-surface dendrimers have proven to be effective in delivering the cargo across BBB | [194] |
6. Use of Dendrimers as Diagnostics
6.1. Research Progress and Applications of Dendrimers in SPECT Imaging
6.2. Application of Dendrimers in CT Imaging
6.3. Application of Dendrimers in MR Imaging
6.4. Application of PAMAM Dendrimers in CT/MR Bimodal Imaging
6.5. Application of Dendrimers in ELISA-Like Assays
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K.; et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Dendrimers in Nanomedicine: History, Concept and Properties of Dendrimers. In Dendrimers in Nanomedicine; CRC Press: Boca Raton, FL, USA, 2021; pp. 41–51. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shen, M.; Rodrigues, J.; Mignani, S.; Majoral, J.-P.; Shi, X. Superstructured poly (amidoamine) dendrimer-based nanoconstructs as platforms for cancer nanomedicine: A concise review. Coord. Chem. Rev. 2020, 421, 213463. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef] [Green Version]
- Kretzmann, J.A.; Ho, D.; Evans, C.W.; Plani-Lam, J.H.C.; Garcia-Bloj, B.; Mohamed, A.E.; O’Mara, M.L.; Ford, E.; Tan, D.E.K.; Lister, R.; et al. Synthetically controlling dendrimer flexibility improves delivery of large plasmid DNA. Chem. Sci. 2017, 8, 2923–2930. [Google Scholar] [CrossRef] [Green Version]
- Samad, A.; Alam, M.I.; Saxena, K. Dendrimers: A class of polymers in the nanotechnology for the delivery of active pharmaceuticals. Curr. Pharm. Des. 2009, 15, 2958–2969. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.V.; Santos, S.D.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekowski, S.; Buczkowski, A.; Palecz, B.; Gabryelak, T. Interaction of polyamidoamine (PAMAM) succinamic acid dendrimers generation 4 with human serum albumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 706–710. [Google Scholar] [CrossRef]
- Idris, A.O.; Mamba, B.; Feleni, U. Poly (propylene imine) dendrimer: A potential nanomaterial for electrochemical application. Mater. Chem. Phys. 2020, 244, 122641. [Google Scholar] [CrossRef]
- Dwivedi, N.; Shah, J.; Mishra, V.; Mohd Amin, M.C.; Iyer, A.K.; Tekade, R.K.; Kesharwani, P. Dendrimer-mediated approaches for the treatment of brain tumor. J. Biomater. Sci. Polym. Ed. 2016, 27, 557–580. [Google Scholar] [CrossRef]
- Kaur, D.; Jain, K.; Mehra, N.K.; Kesharwani, P.; Jain, N.K. A review on comparative study of PPI and PAMAM dendrimers. J. Nanoparticle Res. 2016, 18, 1–14. [Google Scholar] [CrossRef]
- Gupta, R.; Mehra, N.K.; Jain, N.K. Development and characterization of sulfasalazine loaded fucosylated PPI dendrimer for the treatment of cytokine-induced liver damage. Eur. J. Pharm. Biopharm. 2014, 86, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Kong, L.; Wang, L.; Fan, Y.; Shen, M.; Shi, X. Construction of core-shell tecto dendrimers based on supramolecular host-guest assembly for enhanced gene delivery. J. Mater. Chem. B 2017, 5, 8459–8466. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Fan, Y.; Shi, M.; Yang, Y.; Wang, L.; Peng, Y.; Shen, M.; Shi, X. Core-shell tecto dendrimers formed via host-guest supramolecular assembly as pH-responsive intelligent carriers for enhanced anticancer drug delivery. Nanoscale 2019, 11, 22343–22350. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Gao, Y.; Chen, J.; Wang, L.; Bányai, I.; Shen, M.; Shi, X. Physicochemical aspects of zwitterionic core-shell tecto dendrimers characterized by a thorough NMR investigation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126466. [Google Scholar] [CrossRef]
- Quintana, S.; García, M.Á.; Marina, M.L.; Gómez, R.; de la Mata, F.J.; Ortega, P. Synthesis of chiral carbosilane dendrimers with l-cysteine and N-acetyl-l-cysteine on their surface and their application as chiral selectors for enantiomer separation by capillary electrophoresis. Tetrahedron Asymmetry 2017, 28, 1797–1802. [Google Scholar] [CrossRef]
- Seebach, D.; Rheiner, P.B.; Greiveldinger, G.; Butz, T.; Sellner, H. Chiral dendrimers. Dendrimers 1998, 125–164. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Jiang, W.; Liu, H.; Sun, B. Chiral Recognition for Chromatography and Membrane-Based Separations: Recent Developments and Future Prospects. Molecules 2021, 26, 1145. [Google Scholar] [CrossRef]
- Thirunarayanan, A.; Raja, S.; Mohanraj, G.; Rajakumar, P. Synthesis of chiral core based triazole dendrimers with m-terphenyl surface unit and their antibacterial studies. RSC Adv. 2014, 4, 41778–41783. [Google Scholar] [CrossRef]
- Fréchet, J.M. Dendrimers and supramolecular chemistry. Proc. Natl. Acad. Sci. USA 2002, 99, 4782–4787. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, K.; Hölter, D.; Stühn, B.; Mülhaupt, R.; Frey, H. A mesogen-functionized carbosilane dendrimer: A dendritic liquid crystalline polymer. Adv. Mater. 1996, 8, 414–416. [Google Scholar] [CrossRef]
- Frey, H.; Lorenz, K.; Mülhaupt, R.; Rapp, U.; Mayer-Posner, F.J. Dendritic polyols based on carbosilanes-lipophilic dendrimers with hydrophilic skin. Macromol. Symp. 1996, 102, 19–26. [Google Scholar] [CrossRef]
- Aya, S.; Haba, O.; Yonetake, K.; Araoka, F. Anchoring and molecular conformation of liquid crystalline dendrimer. J. Mol. Liq. 2021, 321, 114379. [Google Scholar] [CrossRef]
- Tabatabaei Mirakabad, F.S.; Khoramgah, M.S.; Keshavarz, F.K.; Tabarzad, M.; Ranjbari, J. Peptide dendrimers as valuable biomaterials in medical sciences. Life Sci. 2019, 233, 116754. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, T.N.; Stach, M.; He, R.; Gan, B.H.; Javor, S.; Heitz, M.; Ma, L.; Cai, X.; Chen, P.; Wei, D.; et al. Lipidated Peptide Dendrimers Killing Multidrug-Resistant Bacteria. J. Am. Chem. Soc. 2018, 140, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Manikkath, J.; Hegde, A.R.; Kalthur, G.; Parekh, H.S.; Mutalik, S. Influence of peptide dendrimers and sonophoresis on the transdermal delivery of ketoprofen. Int. J. Pharm. 2017, 521, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekade, R.K.; Tekade, M.; Kumar, M.; Chauhan, A.S. Dendrimer-stabilized smart-nanoparticle (DSSN) platform for targeted delivery of hydrophobic antitumor therapeutics. Pharm. Res. 2015, 32, 910–928. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Tintaru, A.; Peng, L. Self-assembling supramolecular dendrimers for biomedical applications: Lessons learned from poly (amidoamine) dendrimers. Acc. Chem. Res. 2020, 53, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Reis, R.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Huang, A.-T.; Kao, C.-L.; Peng, L. Poly (amidoamine) dendrimers: Covalent and supramolecular synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Hawker, C.; Fréchet, J.M. A new convergent approach to monodisperse dendritic macromolecules. J. Chem. Soc. Chem. Commun. 1990, 1010–1013. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [Green Version]
- Bondareva, J.; Kolotylo, M.; Rozhkov, V.; Burilov, V.; Lukin, O. A convergent approach to sulfonimide-based dendrimers and dendrons. Tetrahedron Lett. 2020, 61, 152011. [Google Scholar] [CrossRef]
- Wooley, K.L.; Hawker, C.J.; Fréchet, J. Hyperbranched macromolecules via a novel double-stage convergent growth approach. J. Am. Chem. Soc. 1991, 113, 4252–4261. [Google Scholar] [CrossRef]
- Wu, P.; Feldman, A.K.; Nugent, A.K.; Hawker, C.J.; Scheel, A.; Voit, B.; Pyun, J.; Fréchet, J.M.; Sharpless, K.B.; Fokin, V.V. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper (I)-catalyzed ligation of azides and alkynes. Angew. Chem. 2004, 116, 4018–4022. [Google Scholar] [CrossRef]
- Gupta, V.; Nayak, S. Dendrimers: A Review on Synthetic Approaches. J. Appl. Pharm. Sci. 2015, 5, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Hayes, W.; Rannard, S. Dendrimers: A new class of nanoscopic containers and delivery devices. Eur. Polym. J. 2003, 39, 1741–1771. [Google Scholar] [CrossRef]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majoros, I.J.; Thomas, T.P.; Mehta, C.B.; Baker, J.R., Jr. Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J. Med. Chem. 2005, 48, 5892–5899. [Google Scholar] [CrossRef]
- Thomas, T.P.; Majoros, I.J.; Kotlyar, A.; Kukowska-Latallo, J.F.; Bielinska, A.; Myc, A.; Baker, J.R., Jr. Targeting and inhibition of cell growth by an engineered dendritic nanodevice. J. Med. Chem. 2005, 48, 3729–3735. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, R.; Cao, X.; Shen, M.; Shi, X. Encapsulation of 2-methoxyestradiol within multifunctional poly(amidoamine) dendrimers for targeted cancer therapy. Biomaterials 2011, 32, 3322–3329. [Google Scholar] [CrossRef]
- Peng, C.; Zheng, L.; Chen, Q.; Shen, M.; Guo, R.; Wang, H.; Cao, X.; Zhang, G.; Shi, X. PEGylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomography. Biomaterials 2012, 33, 1107–1119. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, Z.-R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Milhem, O.M.; Myles, C.; McKeown, N.B.; Attwood, D.; D’Emanuele, A. Polyamidoamine Starburst dendrimers as solubility enhancers. Int. J. Pharm. 2000, 197, 239–241. [Google Scholar] [CrossRef]
- Devarakonda, B.; Hill, R.A.; Liebenberg, W.; Brits, M.; de Villiers, M.M. Comparison of the aqueous solubilization of practically insoluble niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int. J. Pharm. 2005, 304, 193–209. [Google Scholar] [CrossRef]
- Rengaraj, A.; Subbiah, B.; Haldorai, Y.; Yesudhas, D.; Yun, H.J.; Kwon, S.; Choi, S.; Han, Y.-K.; Kim, E.-S.; Shenpagam, H.; et al. PAMAM/5-fluorouracil drug conjugate for targeting E6 and E7 oncoproteins in cervical cancer: A combined experimental/in silico approach. RSC Adv. 2017, 7, 5046–5054. [Google Scholar] [CrossRef] [Green Version]
- Na, M.; Yiyun, C.; Tongwen, X.; Yang, D.; Xiaomin, W.; Zhenwei, L.; Zhichao, C.; Guanyi, H.; Yunyu, S.; Longping, W. Dendrimers as potential drug carriers. Part II. Prolonged delivery of ketoprofen by in vitro and in vivo studies. Eur. J. Med. Chem. 2006, 41, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Kahaie-Khosrowshahi, A. A comparative study on solubility improvement of tetracycline and dexamethasone by poly (propylene imine) and polyamidoamine dendrimers: An insight into cytotoxicity and cell proliferation. J. Biomed. Mater. Res. Part A 2020, 108, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Kuncha, M.; Naidu, V.G.M.; Bansal, V.; Sistla, R.; Adams, D.J. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep. 2016, 6, 23179. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.A.; Fricke, C.H.; Bonorino, C.B.; Bogo, M.R.; Nardi, N.B. An efficient gene transfer system for hematopoietic cell line using transient and stable vectors. J. Biotechnol. 2001, 88, 159–165. [Google Scholar] [CrossRef]
- Pan, J.; Mendes, L.P.; Yao, M.; Filipczak, N.; Garai, S.; Thakur, G.A.; Sarisozen, C.; Torchilin, V.P. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur. J. Pharm. Biopharm. 2019, 136, 18–28. [Google Scholar] [CrossRef]
- Mahajan, B.; Berzofsky, J.A.; Boykins, R.A.; Majam, V.; Zheng, H.; Chattopadhyay, R.; de la Vega, P.; Moch, J.K.; Haynes, J.D.; Belyakov, I.M.; et al. Multiple antigen peptide vaccines against Plasmodium falciparum malaria. Infect. Immun. 2010, 78, 4613–4624. [Google Scholar] [CrossRef] [Green Version]
- Karpenko, L.I.; Apartsin, E.K.; Dudko, S.G.; Starostina, E.V.; Kaplina, O.N.; Antonets, D.V.; Volosnikova, E.A.; Zaitsev, B.N.; Bakulina, A.Y.; Venyaminova, A.G.; et al. Cationic Polymers for the Delivery of the Ebola DNA Vaccine Encoding Artificial T-Cell Immunogen. Vaccines 2020, 8, 718. [Google Scholar] [CrossRef]
- Sakamoto, J.; Koyama, T.; Miyamoto, D.; Yingsakmongkon, S.; Hidari, K.I.; Jampangern, W.; Suzuki, T.; Suzuki, Y.; Esumi, Y.; Nakamura, T.; et al. Systematic syntheses of influenza neuraminidase inhibitors: A series of carbosilane dendrimers uniformly functionalized with thioglycoside-type sialic acid moieties. Bioorg. Med. Chem. 2009, 17, 5451–5464. [Google Scholar] [CrossRef]
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R.; et al. SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS ONE 2011, 6, e24095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliev, G.; Ashraf, G.M.; Tarasov, V.V.; Chubarev, V.N.; Leszek, J.; Gasiorowski, K.; Makhmutovа, A.; Baeesa, S.S.; Avila-Rodriguez, M.; Ustyugov, A.A.; et al. Alzheimer’s Disease—Future Therapy Based on Dendrimers. Curr. Neuropharmacol. 2019, 17, 288–294. [Google Scholar] [CrossRef]
- Geiger, B.C.; Wang, S.; Padera, R.F., Jr.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Fruchon, S.; Poupot, R. The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells. Molecules 2018, 23, 1272. [Google Scholar] [CrossRef] [Green Version]
- Projan, S.J. Whither antibacterial drug discovery? Drug Discov. Today 2008, 13, 279–280. [Google Scholar] [CrossRef]
- Castonguay, A.; Ladd, E.; van de Ven, T.G.; Kakkar, A. Dendrimers as bactericides. New J. Chem. 2012, 36, 199–204. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Swanson, D.R.; Tomalia, D.A.; Hagnauer, G.L.; McManus, A.T. Dendrimer− silver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001, 1, 18–21. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazniewska, J.; Milowska, K.; Gabryelak, T. Dendrimers—Revolutionary drugs for infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T. Longer-term immunologic effects and side effects of successful antiretroviral therapy. Clin. Infect. Dis. 1999, 29, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.; Matthews, B.R.; Holan, G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–318. [Google Scholar] [CrossRef]
- Baert, L.; van’t Klooster, G.; Dries, W.; François, M.; Wouters, A.; Basstanie, E.; Iterbeke, K.; Stappers, F.; Stevens, P.; Schueller, L.; et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 2009, 72, 502–508. [Google Scholar] [CrossRef]
- Fiandra, L.; Colombo, M.; Mazzucchelli, S.; Truffi, M.; Santini, B.; Allevi, R.; Nebuloni, M.; Capetti, A.; Rizzardini, G.; Prosperi, D.; et al. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine 2015, 11, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Sattentau, Q.J. The role of the CD4 antigen in HIV infection and immune pathogenesis. Aids 1988, 2 (Suppl. 1), S11–S16. [Google Scholar] [CrossRef]
- Sattentau, Q.J.; Weiss, R.A. The CD4 antigen: Physiological ligand and HIV receptor. Cell 1988, 52, 631–633. [Google Scholar] [CrossRef]
- Guerrero-Beltran, C.; Rodriguez-Izquierdo, I.; Serramia, M.J.; Araya-Durán, I.; Márquez-Miranda, V.; Gomez, R.; de la Mata, F.J.; Leal, M.; González-Nilo, F.; Muñoz-Fernández, M.A. Anionic Carbosilane Dendrimers Destabilize the GP120-CD4 Complex Blocking HIV-1 Entry and Cell to Cell Fusion. Bioconjug. Chem. 2018, 29, 1584–1594. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Serramía, M.J.; Tager, A.M.; Vrbanac, V.; Gómez, R.; De La Mata, F.J.; Jiménez, J.L.; Muñoz-Fernández, M. Prevention vaginally of HIV-1 transmission in humanized BLT mice and mode of antiviral action of polyanionic carbosilane dendrimer G2-S16. Nanomedicine 2015, 11, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Crespo, D.; Sánchez-Rodríguez, J.; Serramía, M.J.; Gómez, R.; De La Mata, F.J.; Jiménez, J.L.; Muñoz-Fernández, M. Triple combination of carbosilane dendrimers, tenofovir and maraviroc as potential microbicide to prevent HIV-1 sexual transmission. Nanomedicine 2015, 10, 899–914. [Google Scholar] [CrossRef]
- García-Broncano, P.; Ceña-Diez, R.; de la Mata, F.J.; Gómez, R.; Resino, S.; Muñoz-Fernández, M. Efficacy of carbosilane dendrimers with an antiretroviral combination against HIV-1 in the presence of semen-derived enhancer of viral infection. Eur. J. Pharm. 2017, 811, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Perisé-Barrios, A.J.; Jiménez, J.L.; Domínguez-Soto, A.; de la Mata, F.J.; Corbí, A.L.; Gomez, R.; Muñoz-Fernandez, M. Carbosilane dendrimers as gene delivery agents for the treatment of HIV infection. J. Control. Release 2014, 184, 51–57. [Google Scholar] [CrossRef]
- Pavan, G.M.; Albertazzi, L.; Danani, A. Ability to adapt: Different generations of PAMAM dendrimers show different behaviors in binding siRNA. J. Phys. Chem. B 2010, 114, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Neff, C.P.; Liu, X.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Aboellail, T.; Huang, Y.; Du, Q.; et al. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011, 19, 2228–2238. [Google Scholar] [CrossRef] [Green Version]
- Christ, F.; Thys, W.; De Rijck, J.; Gijsbers, R.; Albanese, A.; Arosio, D.; Emiliani, S.; Rain, J.C.; Benarous, R.; Cereseto, A.; et al. Transportin-SR2 imports HIV into the nucleus. Curr. Biol. 2008, 18, 1192–1202. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.; Li, M.-J.; Palmer, B.; Remling, L.; Li, S.; Yam, P.; Yee, J.-K.; Rossi, J.; Zaia, J.; Akkina, R. Safety and efficacy of a lentiviral vector containing three anti-HIV genes—CCR5 ribozyme, tat-rev siRNA, and TAR decoy—In SCID-hu mouse–derived T cells. Mol. Ther. 2007, 15, 1182–1188. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral Potential of Nanoparticles-Can Nanoparticles Fight against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Mweetwa, L.L.; Ntemi, P.V.; Chikukwa, M.T.R.; Matafwali, S.K.; Mwila, C.; Mudenda, S.; Katandula, J.; Walker, R.B. Nano-Biomimetic Drug Delivery Vehicles: Potential Approaches for COVID-19 Treatment. Molecules 2020, 25, 5952. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef]
- Islam, M.A.; Xu, Y.; Tao, W.; Ubellacker, J.M.; Lim, M.; Aum, D.; Lee, G.Y.; Zhou, K.; Zope, H.; Yu, M.; et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2018, 2, 850–864. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Jia, Y.; Farbiak, L.; Zhou, K.; Zhang, S.; Wei, Y.; Zhu, H.; Siegwart, D.J. Dendrimer-Based Lipid Nanoparticles Deliver Therapeutic FAH mRNA to Normalize Liver Function and Extend Survival in a Mouse Model of Hepatorenal Tyrosinemia Type I. Adv. Mater. 2018, 30, e1805308. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, S.; Wei, T.; Cheng, Q.; Siegwart, D.J. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J. Control. Release 2020, 325, 198–205. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.K.; Salunkhe, S.; Agrawal, M.; Kali, M.; Singhvi, G.; Tiwari, S.; Saraf, S.; Saraf, S.; Alexander, A. Understanding the Pharmaceutical Aspects of Dendrimers for the Delivery of Anticancer Drugs. Curr. Drug Targets 2020, 21, 528–540. [Google Scholar] [CrossRef]
- Kandeel, M.; Al-Taher, A.; Park, B.K.; Kwon, H.J.; Al-Nazawi, M. A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J. Med. Virol. 2020, 92, 1665–1670. [Google Scholar] [CrossRef]
- Khaitov, M.; Nikonova, A.; Shilovskiy, I.; Kozhikhova, K.; Kofiadi, I.; Vishnyakova, L.; Nikolskii, A.; Gattinger, P.; Kovchina, V.; Barvinskaia, E.; et al. Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 2021. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J. Ebola Virus Disease: A Review of Its Past and Present. Anesth. Analg. 2015, 121, 798–809. [Google Scholar] [CrossRef]
- Alvarez, C.P.; Lasala, F.; Carrillo, J.; Muñiz, O.; Corbí, A.L.; Delgado, R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 2002, 76, 6841–6844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, G.; Reeves, J.D.; Grogan, C.C.; Vandenberghe, L.H.; Baribaud, F.; Whitbeck, J.C.; Burke, E.; Buchmeier, M.J.; Soilleux, E.J.; Riley, J.L.; et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 2003, 305, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, J.; Delgado, R. Glycodendritic structures: Promising new antiviral drugs. J. Antimicrob. Chemother. 2004, 54, 579–581. [Google Scholar] [CrossRef] [Green Version]
- Lasala, F.; Arce, E.; Otero, J.R.; Rojo, J.; Delgado, R. Mannosyl glycodendritic structure inhibits DC-SIGN-mediated Ebola virus infection in cis and in trans. Antimicrob. Agents Chemother. 2003, 47, 3970–3972. [Google Scholar] [CrossRef] [Green Version]
- Luczkowiak, J.; Sattin, S.; Sutkevičiūtė, I.; Reina, J.J.; Sánchez-Navarro, M.; Thépaut, M.; Martínez-Prats, L.; Daghetti, A.; Fieschi, F.; Delgado, R.; et al. Pseudosaccharide functionalized dendrimers as potent inhibitors of DC-SIGN dependent Ebola pseudotyped viral infection. Bioconjug. Chem. 2011, 22, 1354–1365. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigal, G.B.; Mammen, M.; Dahmann, G.; Whitesides, G.M. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus: The strong inhibition reflects enhanced binding through cooperative polyvalent interactions. J. Am. Chem. Soc. 1996, 118, 3789–3800. [Google Scholar] [CrossRef]
- Zanini, D.; Roy, R. Practical Synthesis of Starburst PAMAM α-Thiosialodendrimers for Probing Multivalent Carbohydrate—Lectin Binding Properties. J. Org. Chem. 1998, 63, 3486–3491. [Google Scholar] [CrossRef]
- Günther, S.C.; Maier, J.D.; Vetter, J.; Podvalnyy, N.; Khanzhin, N.; Hennet, T.; Stertz, S. Antiviral potential of 3′-sialyllactose- and 6′-sialyllactose-conjugated dendritic polymers against human and avian influenza viruses. Sci. Rep. 2020, 10, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, K.; Matsubara, T.; Muramatsu, Y.; Ezure, M.; Koyama, T.; Matsuoka, K.; Kuriyama, R.; Kori, H.; Sato, T. Synthesis and influenza virus inhibitory activities of carbosilane dendrimers peripherally functionalized with hemagglutinin-binding Peptide. J. Med. Chem. 2014, 57, 8332–8339. [Google Scholar] [CrossRef] [PubMed]
- Landers, J.J.; Cao, Z.; Lee, I.; Piehler, L.T.; Myc, P.P.; Myc, A.; Hamouda, T.; Galecki, A.T.; Baker, J.R., Jr. Prevention of influenza pneumonitis by sialic Acid-conjugated dendritic polymers. J. Infect. Dis. 2002, 186, 1222–1230. [Google Scholar] [CrossRef]
- Langeland, N.; Moore, L.J.; Holmsen, H.; Haarr, L. Interaction of polylysine with the cellular receptor for herpes simplex virus type 1. J. Gen. Virol. 1988, 69 Pt 6, 1137–1145. [Google Scholar] [CrossRef]
- Aguilar, J.S.; Rice, M.; Wagner, E.K. The polysulfonated compound suramin blocks adsorption and lateral difusion of herpes simplex virus type-1 in vero cells. Virology 1999, 258, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Luganini, A.; Nicoletto, S.F.; Pizzuto, L.; Pirri, G.; Giuliani, A.; Landolfo, S.; Gribaudo, G. Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers. Antimicrob. Agents Chemother. 2011, 55, 3231–3239. [Google Scholar] [CrossRef] [Green Version]
- Tarallo, R.; Carberry, T.P.; Falanga, A.; Vitiello, M.; Galdiero, S.; Galdiero, M.; Weck, M. Dendrimers functionalized with membrane-interacting peptides for viral inhibition. Int. J. Nanomed. 2013, 8, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Carberry, T.P.; Tarallo, R.; Falanga, A.; Finamore, E.; Galdiero, M.; Weck, M.; Galdiero, S. Dendrimer functionalization with a membrane-interacting domain of herpes simplex virus type 1: Towards intracellular delivery. Chemistry 2012, 18, 13678–13685. [Google Scholar] [CrossRef]
- Ceña-Diez, R.; Vacas-Córdoba, E.; García-Broncano, P.; de la Mata, F.J.; Gómez, R.; Maly, M.; Muñoz-Fernández, M. Prevention of vaginal and rectal herpes simplex virus type 2 transmission in mice: Mechanism of antiviral action. Int. J. Nanomed. 2016, 11, 2147–2162. [Google Scholar] [CrossRef] [Green Version]
- Ceña-Díez, R.; Sepúlveda-Crespo, D.; Maly, M.; Muñoz-Fernández, M.A. Dendrimeric based microbicides against sexual transmitted infections associated to heparan sulfate. RSC Adv. 2016, 6, 46755–46764. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Jiménez, J.L.; Gómez, R.; De La Mata, F.J.; Majano, P.L.; Muñoz-Fernández, M.Á.; Gastaminza, P. Polyanionic carbosilane dendrimers prevent hepatitis C virus infection in cell culture. Nanomedicine 2017, 13, 49–58. [Google Scholar] [CrossRef]
- Lakshminarayanan, A.; Reddy, B.U.; Raghav, N.; Ravi, V.K.; Kumar, A.; Maiti, P.K.; Sood, A.K.; Jayaraman, N.; Das, S. A galactose-functionalized dendritic siRNA-nanovector to potentiate hepatitis C inhibition in liver cells. Nanoscale 2015, 7, 16921–16931. [Google Scholar] [CrossRef] [Green Version]
- Dutta, T.; Burgess, M.; McMillan, N.A.; Parekh, H.S. Dendrosome-based delivery of siRNA against E6 and E7 oncogenes in cervical cancer. Nanomedicine 2010, 6, 463–470. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Debele, T.A.; Chou, H.Y.; Tsai, H.C. IL-6 Antibody and RGD Peptide Conjugated Poly(amidoamine) Dendrimer for Targeted Drug Delivery of HeLa Cells. J. Phys. Chem. B 2016, 120, 123–130. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.; Villén, J.; Giralt, E.; Andreu, D. Synthetic approaches to multivalent lipopeptide dendrimers containing cyclic disulfide epitopes of foot-and-mouth disease virus. Bioconjug. Chem. 2003, 14, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Razinkov, V.; Gazumyan, A.; Nikitenko, A.; Ellestad, G.; Krishnamurthy, G. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 2001, 8, 645–659. [Google Scholar] [CrossRef] [Green Version]
- Shaunak, S.; Thomas, S.; Gianasi, E.; Godwin, A.; Jones, E.; Teo, I.; Mireskandari, K.; Luthert, P.; Duncan, R.; Patterson, S.; et al. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat. Biotechnol. 2004, 22, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Teo, I.; Toms, S.M.; Marteyn, B.; Barata, T.S.; Simpson, P.; Johnston, K.A.; Schnupf, P.; Puhar, A.; Bell, T.; Tang, C.; et al. Preventing acute gut wall damage in infectious diarrhoeas with glycosylated dendrimers. EMBO Mol. Med. 2012, 4, 866–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahori, N.; Lee, R.T.; Nishimura, S.; Pagé, D.; Roy, R.; Lee, Y.C. Inhibition of adhesion of type 1 fimbriated Escherichia coli to highly mannosylated ligands. Chembiochem 2002, 3, 836–844. [Google Scholar] [CrossRef]
- Wang, B.; Navath, R.S.; Menjoge, A.R.; Balakrishnan, B.; Bellair, R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int. J. Pharm. 2010, 395, 298–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Baldauf, K.J.; Royal, J.M.; Hamorsky, K.T.; Matoba, N. Cholera toxin B: One subunit with many pharmaceutical applications. Toxins 2015, 7, 974–996. [Google Scholar] [CrossRef] [Green Version]
- Arosio, D.; Vrasidas, I.; Valentini, P.; Liskamp, R.M.; Pieters, R.J.; Bernardi, A. Synthesis and cholera toxin binding properties of multivalent GM1 mimics. Org. Biomol. Chem. 2004, 2, 2113–2124. [Google Scholar] [CrossRef]
- Vrasidas, I.; de Mol, N.J.; Liskamp, R.M.J.; Pieters, R.J. Synthesis of Lactose Dendrimers and Multivalency Effects in Binding to the Cholera Toxin B Subunit. Eur. J. Org. Chem. 2001, 2001, 4685–4692. [Google Scholar] [CrossRef]
- Thompson, J.P.; Schengrund, C.L. Inhibition of the adherence of cholera toxin and the heat-labile enterotoxin of Escherichia coli to cell-surface GM1 by oligosaccharide-derivatized dendrimers. Biochem. Pharm. 1998, 56, 591–597. [Google Scholar] [CrossRef]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. PAMAM-dendrimer Enhanced Antibacterial Effect of Vancomycin Hydrochloride Against Gram-Negative Bacteria. J. Pharm. Pharm. Sci. 2018, 22, 10–21. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, P.O.; Gagnaire, J.; Botelho-Nevers, E.; Grattard, F.; Carricajo, A.; Lucht, F.; Pozzetto, B.; Berthelot, P. Detection and clinical relevance of Staphylococcus aureus nasal carriage: An update. Expert. Rev. Anti-Infect. Ther. 2014, 12, 75–89. [Google Scholar] [CrossRef]
- Felczak, A.; Wrońska, N.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Appelhans, D.; Voit, B.; Różalska, S.; Lisowska, K. Antimicrobial activity of poly (propylene imine) dendrimers. New J. Chem. 2012, 36, 2215–2222. [Google Scholar] [CrossRef]
- Tsuchido, Y.; Horiuchi, R.; Hashimoto, T.; Ishihara, K.; Kanzawa, N.; Hayashita, T. Rapid and Selective Discrimination of Gram-Positive and Gram-Negative Bacteria by Boronic Acid-Modified Poly(amidoamine) Dendrimer. Anal. Chem. 2019, 91, 3929–3935. [Google Scholar] [CrossRef] [PubMed]
- Sanz Del Olmo, N.; Peña González, C.E.; Rojas, J.D.; Gómez, R.; Ortega, P.; Escarpa, A.; de la Mata, F.J. Antioxidant and Antibacterial Properties of Carbosilane Dendrimers Functionalized with Polyphenolic Moieties. Pharmaceutics 2020, 12, 698. [Google Scholar] [CrossRef]
- Llamazares, C.; Sanz Del Olmo, N.; Ortega, P.; Gómez, R.; Soliveri, J.; de la Mata, F.J.; García-Gallego, S.; Copa-Patiño, J.L. Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm. Biomolecules 2019, 9, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svenningsen, S.W.; Frederiksen, R.F.; Counil, C.; Ficker, M.; Leisner, J.J.; Christensen, J.B. Synthesis and Antimicrobial Properties of a Ciprofloxacin and PAMAM-dendrimer Conjugate. Molecules 2020, 25, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beare, N.A.; Lewallen, S.; Taylor, T.E.; Molyneux, M.E. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol. 2011, 6, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.F.; Fauci, A.S. Malaria control, elimination, and eradication: The role of the evolving biomedical research agenda. J. Infect. Dis. 2009, 200, 1639–1643. [Google Scholar] [CrossRef]
- Douglas, N.M.; Anstey, N.M.; Buffet, P.A.; Poespoprodjo, J.R.; Yeo, T.W.; White, N.J.; Price, R.N. The anaemia of Plasmodium vivax malaria. Malar. J. 2012, 11, 135. [Google Scholar] [CrossRef] [Green Version]
- Hartman, T.K.; Rogerson, S.J.; Fischer, P.R. The impact of maternal malaria on newborns. Ann. Trop. Paediatr. 2010, 30, 271–282. [Google Scholar] [CrossRef]
- Aditya, N.P.; Vathsala, P.G.; Vieira, V.; Murthy, R.S.; Souto, E.B. Advances in nanomedicines for malaria treatment. Adv. Colloid Interface Sci. 2013, 201–202, 1–17. [Google Scholar] [CrossRef]
- Movellan, J.; Urbán, P.; Moles, E.; de la Fuente, J.M.; Sierra, T.; Serrano, J.L.; Fernàndez-Busquets, X. Amphiphilic dendritic derivatives as nanocarriers for the targeted delivery of antimalarial drugs. Biomaterials 2014, 35, 7940–7950. [Google Scholar] [CrossRef]
- Fröhlich, T.; Hahn, F.; Belmudes, L.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Couté, Y.; Marschall, M.; Tsogoeva, S.B. Synthesis of Artemisinin-Derived Dimers, Trimers and Dendrimers: Investigation of Their Antimalarial and Antiviral Activities Including Putative Mechanisms of Action. Chemistry 2018, 24, 8103–8113. [Google Scholar] [CrossRef]
- Moore, E.M.; Lockwood, D.N. Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 151–158. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Ali, N. Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects. Expert Rev. Anti-Infect. Ther. 2013, 11, 79–98. [Google Scholar] [CrossRef]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- Danesh-Bahreini, M.A.; Shokri, J.; Samiei, A.; Kamali-Sarvestani, E.; Barzegar-Jalali, M.; Mohammadi-Samani, S. Nanovaccine for leishmaniasis: Preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int. J. Nanomed. 2011, 6, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, E.D.; Liang, Y.; Shelite, T.R.; Walker, D.H.; Melby, P.C.; Soong, L. Permissive and protective roles for neutrophils in leishmaniasis. Clin. Exp. Immunol. 2015, 182, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Guerra, J.A.; Prestes, S.R.; Silveira, H.; Coelho, L.I.; Gama, P.; Moura, A.; Amato, V.; Barbosa, M.; Ferreira, L.C. Mucosal Leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. Plos Negl. Trop. Dis. 2011, 5, e980. [Google Scholar] [CrossRef] [Green Version]
- Mohamed-Ahmed, A.H.; Brocchini, S.; Croft, S.L. Recent advances in development of amphotericin B formulations for the treatment of visceral leishmaniasis. Curr. Opin. Infect. Dis. 2012, 25, 695–702. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Balasegaram, M.; Meheus, F.; Alvar, J.; Lynen, L.; Boelaert, M. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 2010, 10, 184–194. [Google Scholar] [CrossRef]
- Ritmeijer, K.; ter Horst, R.; Chane, S.; Aderie, E.M.; Piening, T.; Collin, S.M.; Davidson, R.N. Limited effectiveness of high-dose liposomal amphotericin B (AmBisome) for treatment of visceral leishmaniasis in an Ethiopian population with high HIV prevalence. Clin. Infect. Dis. 2011, 53, e152–e158. [Google Scholar] [CrossRef] [Green Version]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.; Nakamura, C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 2011, 15, e525–e532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, J.P.; Almeida, T.F.; Petersen, A.L.; Guedes, C.E.; Mota, M.S.; Lima, J.G.; Palma, L.C.; Buck, G.A.; Krieger, M.A.; Probst, C.M.; et al. Proteomic analysis reveals differentially expressed proteins in macrophages infected with Leishmania amazonensis or Leishmania major. Microbes Infect. 2013, 15, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, K.; Verma, A.K.; Mishra, P.R.; Jain, N.K. Characterization and evaluation of amphotericin B loaded MDP conjugated poly(propylene imine) dendrimers. Nanomedicine 2015, 11, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Daftarian, P.M.; Stone, G.W.; Kovalski, L.; Kumar, M.; Vosoughi, A.; Urbieta, M.; Blackwelder, P.; Dikici, E.; Serafini, P.; Duffort, S.; et al. A targeted and adjuvanted nanocarrier lowers the effective dose of liposomal amphotericin B and enhances adaptive immunity in murine cutaneous leishmaniasis. J. Infect. Dis. 2013, 208, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.; Verma, A.K.; Mishra, P.R.; Jain, N.K. Surface-engineered dendrimeric nanoconjugates for macrophage-targeted delivery of amphotericin B: Formulation development and in vitro and in vivo evaluation. Antimicrob. Agents Chemother. 2015, 59, 2479–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fomovska, A.; Wood, R.D.; Mui, E.; Dubey, J.P.; Ferreira, L.R.; Hickman, M.R.; Lee, P.J.; Leed, S.E.; Auschwitz, J.M.; Welsh, W.J.; et al. Salicylanilide inhibitors of Toxoplasma gondii. J. Med. Chem. 2012, 55, 8375–8391. [Google Scholar] [CrossRef] [Green Version]
- Behnke, M.S.; Wootton, J.C.; Lehmann, M.M.; Radke, J.B.; Lucas, O.; Nawas, J.; Sibley, L.D.; White, M.W. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS ONE 2010, 5, e12354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Augagneur, Y.; Wesolowski, D.; Tae, H.S.; Altman, S.; Ben Mamoun, C. Gene selective mRNA cleavage inhibits the development of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2012, 109, 6235–6240. [Google Scholar] [CrossRef] [Green Version]
- Lai, B.S.; Witola, W.H.; El Bissati, K.; Zhou, Y.; Mui, E.; Fomovska, A.; McLeod, R. Molecular target validation, antimicrobial delivery, and potential treatment of Toxoplasma gondii infections. Proc. Natl. Acad. Sci. USA 2012, 109, 14182–14187. [Google Scholar] [CrossRef] [Green Version]
- Prieto, M.J.; Bacigalupe, D.; Pardini, O.; Amalvy, J.I.; Venturini, C.; Morilla, M.J.; Romero, E.L. Nanomolar cationic dendrimeric sulfadiazine as potential antitoxoplasmic agent. Int. J. Pharm. 2006, 326, 160–168. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef] [Green Version]
- Gothwal, A.; Kesharwani, P.; Gupta, U.; Khan, I.; Cairul Iqbal Mohd Amin, M.; Banerjee, S.; K Iyer, A. Dendrimers as an effective nanocarrier in cardiovascular disease. Curr. Pharm. Des. 2015, 21, 4519–4526. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Amin, M.C.I.M.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Thakur, S.; Kesharwani, P.; Tekade, R.K.; Jain, N.K. Impact of pegylation on biopharmaceutical properties of dendrimers. Polymer 2015, 59, 67–92. [Google Scholar] [CrossRef]
- Bhadra, D.; Bhadra, S.; Jain, N. PEGylated peptide-based dendritic nanoparticulate systems for delivery of artemether. J. Drug Deliv. Sci. Technol. 2005, 15, 65–73. [Google Scholar] [CrossRef]
- Asthana, A.; Chauhan, A.S.; Diwan, P.V.; Jain, N.K. Poly (amidoamine)(PAMAM) dendritic nanostructures for controlled sitespecific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech 2005, 6, E536–E542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.; Meijer, E.; Paulus, W.; Duncan, R. Dendrimers:: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent cancer targeting potential of poly (propyleneimine) dendrimer. Biomaterials 2014, 35, 5539–5548. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Tekade, R.K.; Gajbhiye, V.; Jain, K.; Jain, N.K. Cancer targeting potential of some ligand-anchored poly (propylene imine) dendrimers: A comparison. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent safety and efficacy of folic acid conjugated dendrimer based anticancer drug formulations. Pharm. Res. 2015, 32, 1438–1450. [Google Scholar] [CrossRef]
- Tajarobi, F.; El-Sayed, M.; Rege, B.; Polli, J.; Ghandehari, H. Transport of poly amidoamine dendrimers across Madin–Darby canine kidney cells. Int. J. Pharm. 2001, 215, 263–267. [Google Scholar] [CrossRef]
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interaction of poly (amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconjug. Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef]
- Stasko, N.A.; Johnson, C.B.; Schoenfisch, M.H.; Johnson, T.A.; Holmuhamedov, E.L. Cytotoxicity of polypropylenimine dendrimer conjugates on cultured endothelial cells. Biomacromolecules 2007, 8, 3853–3859. [Google Scholar] [CrossRef]
- Hong, S.; Rattan, R.; Majoros, I.J.; Mullen, D.G.; Peters, J.L.; Shi, X.; Bielinska, A.U.; Blanco, L.; Orr, B.G.; Baker, J.R., Jr.; et al. The role of ganglioside GM1 in cellular internalization mechanisms of poly (amidoamine) dendrimers. Bioconjug. Chem. 2009, 20, 1503–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.-J.; Sen, S.; Pearson, R.M.; Uddin, S.; Král, P.; Hong, S. Poly (ethylene glycol) corona chain length controls end-group-dependent cell interactions of dendron micelles. Macromolecules 2014, 47, 6911–6918. [Google Scholar] [CrossRef]
- Pearson, R.M.; Patra, N.; Hsu, H.-J.; Uddin, S.; Král, P.; Hong, S. Positively charged dendron micelles display negligible cellular interactions. ACS Macro Lett. 2013, 2, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Munro, N.; Srinageshwar, B.; Shalabi, F.; Florendo, M.; Otero, P.; Thompson, C.; Kippe, J.; Malkowski, C.; Climie, S.; Stewart, A.N.; et al. A novel approach to label bone marrow-derived mesenchymal stem cells with mixed-surface PAMAM dendrimers. Stem Cell Res. Ther. 2019, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules 2018, 23, 2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. PAMAM Dendrimers Cross the Blood-Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 628. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Kawamoto, S.; Saga, T.; Sato, N.; Hiraga, A.; Konishi, J.; Togashi, K.; Brechbiel, M.W. Micro-MR angiography of normal and intratumoral vessels in mice using dedicated intravascular MR contrast agents with high generation of polyamidoamine dendrimer core: Reference to pharmacokinetic properties of dendrimer-based MR contrast agents. J. Magn. Reson. Imaging 2001, 14, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunoqrot, S.; Bae, J.W.; Pearson, R.M.; Shyu, K.; Liu, Y.; Kim, D.-H.; Hong, S. Temporal control over cellular targeting through hybridization of folate-targeted dendrimers and PEG-PLA nanoparticles. Biomacromolecules 2012, 13, 1223–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunoqrot, S.; Bae, J.W.; Jin, S.-E.; Pearson, R.M.; Liu, Y.; Hong, S. Kinetically controlled cellular interactions of polymer− polymer and polymer− liposome nanohybrid systems. Bioconjug. Chem. 2011, 22, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunoqrot, S.; Liu, Y.; Kim, D.-H.; Hong, S. In vitro evaluation of dendrimer–polymer hybrid nanoparticles on their controlled cellular targeting kinetics. Mol. Pharm. 2013, 10, 2157–2166. [Google Scholar] [CrossRef] [Green Version]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613.e7. [Google Scholar] [CrossRef] [Green Version]
- Hershfield, M.S.; Ganson, N.J.; Kelly, S.J.; Scarlett, E.L.; Jaggers, D.A.; Sundy, J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis. Res. Ther. 2014, 16, R63. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.-H.; Zimmerman, S.C. Orthogonality in organic, polymer, and supramolecular chemistry: From Merrifield to click chemistry. Chem. Commun. 2013, 49, 1679–1695. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Vidal, F.; Guzman, L. Dendrimer nanocarriers drug action: Perspective for neuronal pharmacology. Neural Regen. Res. 2015, 10, 1029–1031. [Google Scholar]
- De Stefano, D.; Carnuccio, R.; Maiuri, M.C. Nanomaterials toxicity and cell death modalities. J. Drug Deliv. 2012, 2012, 167896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, T.; Bühler, S.; Bartenschlager, R. Chronic viral hepatitis and its association with liver cancer. Biol. Chem. 2017, 398, 817–837. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef]
- Kanda, T.; Yajima, M.; Ikuta, K. Epstein-Barr virus strain variation and cancer. Cancer Sci. 2019, 110, 1132–1139. [Google Scholar] [CrossRef]
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Iezzoni, J.C.; Gaffey, M.J.; Weiss, L.M. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am. J. Clin. Pathol. 1995, 103, 308–315. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Pelucchi, C.; Negri, E.; López-Carrillo, L.; Tsugane, S.; Hidaka, A.; Shigueaki Hamada, G.; Hernández-Ramírez, R.U.; López-Cervantes, M.; Malekzadeh, R.; et al. Exploring the interactions between Hp infection and other risk factors of gastric cancer: A pooled analysis in the Stomach cancer Pooling (StoP) Project. Int. J. Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Li, K.; Wen, S.; Liu, H.; Peng, C.; Cai, H.; Shen, M.; Zhang, G.; Shi, X. Targeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2013, 34, 5200–5209. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Z.; Xu, X.; Luo, Y.; Peng, C.; Shen, M.; Shi, X. (99m)Tc-Labeled Multifunctional Low-Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted SPECT/CT Dual-Mode Imaging of Tumors. ACS Appl. Mater. Interfaces 2016, 8, 19883–19891. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, L.; Zhao, Q.; Li, D.; Liu, C.; Yu, Z.; Shen, M.; Majoral, J.P.; Mignani, S.; Zhao, J.; et al. A promising dual mode SPECT/CT imaging platform based on (99m)Tc-labeled multifunctional dendrimer-entrapped gold nanoparticles. J. Mater. Chem. B 2017, 5, 3810–3815. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, S.; Zhu, M.; Li, D.; Xing, Y.; Shen, M.; Shi, X.; Zhao, J. (99m)Tc-labelled multifunctional polyethylenimine-entrapped gold nanoparticles for dual mode SPECT and CT imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Wang, R.; Chen, F.; Zhao, L.; Wang, P.; Li, X.; Bányai, I.; Ouyang, Q.; Shi, X.; Shen, M. (99m)Tc-Labeled RGD-Polyethylenimine Conjugates with Entrapped Gold Nanoparticles in the Cavities for Dual-Mode SPECT/CT Imaging of Hepatic Carcinoma. ACS Appl. Mater. Interfaces 2018, 10, 6146–6154. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Wang, X.; Guo, X.; Zhang, X.; Qi, Y.; Shen, Y.M. Radiosynthesis, biodistribution and micro-SPECT imaging study of dendrimer-avidin conjugate. Bioorg. Med. Chem. 2011, 19, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Xu, X.; Zhang, X.; Zhu, H.; Huang, L.; Qi, Y.; Shen, Y.M. Synthesis, biodistribution, and microsingle photon emission computed tomography (SPECT) imaging study of technetium-99m labeled PEGylated dendrimer poly(amidoamine) (PAMAM)-folic acid conjugates. J. Med. Chem. 2010, 53, 3262–3272. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davis, J.J. Multimodality and nanoparticles in medical imaging. Dalton Trans. 2011, 40, 6087–6103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Xing, Y.; Peng, J.; Zheng, Z.; Tang, W.; Sun, Y.; Xu, C.; Peng, F. Chest CT for detecting COVID-19: A systematic review and meta-analysis of diagnostic accuracy. Eur. Radiol. 2020, 30, 5720–5727. [Google Scholar] [CrossRef]
- Tenda, E.D.; Yulianti, M.; Asaf, M.M.; Yunus, R.E.; Septiyanti, W.; Wulani, V.; Pitoyo, C.W.; Rumende, C.M.; Setiati, S. The Importance of Chest CT Scan in COVID-19. Acta Med. Indones. 2020, 52, 68–73. [Google Scholar] [PubMed]
- Yordanov, A.T.; Lodder, A.L.; Woller, E.K.; Cloninger, M.J.; Patronas, N.; Milenic, D.; Brechbiel, M.W. Novel iodinated dendritic nanoparticles for computed tomography (CT) imaging. Nano Lett. 2002, 2, 595–599. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, L.; Wen, S.; Tang, Y.; Shen, M.; Zhang, G.; Shi, X. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2014, 35, 7635–7646. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, F.; Xiong, Z.; Shen, M.; Shi, X. Dendrimer-entrapped gold nanoparticles modified with RGD peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surf. B Biointerfaces 2015, 133, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhu, J.; Shen, M.; Chen, X.; Baker, J.R.; Wang, S.H.; Zhang, G.; Shi, X. Targeted cancer cell inhibition using multifunctional dendrimer-entrapped gold nanoparticles. MedChemComm 2013, 4, 1001–1005. [Google Scholar] [CrossRef]
- Britton, M.M. Magnetic resonance imaging of chemistry. Chem. Soc. Rev. 2010, 39, 4036–4043. [Google Scholar] [CrossRef] [PubMed]
- Ghai, A.; Singh, B.; Panwar Hazari, P.; Schultz, M.K.; Parmar, A.; Kumar, P.; Sharma, S.; Dhawan, D.; Kumar Mishra, A. Radiolabeling optimization and characterization of (68)Ga labeled DOTA-polyamido-amine dendrimer conjugate—Animal biodistribution and PET imaging results. Appl. Radiat. Isot. 2015, 105, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.W.; Baek, H.; Mahakian, L.M.; Kusunose, J.; Hamzah, J.; Ruoslahti, E.; Ferrara, K.W. (64)Cu-labeled LyP-1-dendrimer for PET-CT imaging of atherosclerotic plaque. Bioconjug. Chem. 2014, 25, 231–239. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Cheng, Y.; Xiong, Z.; Tang, Y.; Guo, L.; Shi, X.; Zhao, J. Chlorotoxin-Conjugated Multifunctional Dendrimers Labeled with Radionuclide 131I for Single Photon Emission Computed Tomography Imaging and Radiotherapy of Gliomas. ACS Appl. Mater. Interfaces 2015, 7, 19798–19808. [Google Scholar] [CrossRef]
- Darvish Mohamadi, T.; Amanlou, M.; Ghalandarlaki, N.; Mehravi, B.; Shafiee Ardestani, M.; Yaghmaei, P. Gd(3+)-DTPA-Meglumine-Anionic Linear Globular Dendrimer G1: Novel Nanosized Low Toxic Tumor Molecular MR Imaging Agent. ISRN Pharm. 2013, 2013, 378452. [Google Scholar] [CrossRef]
- Mustafa, R.; Zhou, B.; Yang, J.; Zheng, L.; Zhang, G.; Shi, X. Dendrimer-functionalized LAPONITE® nanodisks loaded with gadolinium for T 1-weighted MR imaging applications. RSC Adv. 2016, 6, 95112–95119. [Google Scholar] [CrossRef]
- Dear, J.W.; Kobayashi, H.; Jo, S.K.; Holly, M.K.; Hu, X.; Yuen, P.S.; Brechbiel, M.W.; Star, R.A. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int. 2005, 67, 2159–2167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lloveras, V.; Pulido, D.; Liko, F.; Pinto, L.F.; Albericio, F.; Royo, M.; Vidal-Gancedo, J. Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI. Pharmaceutics 2020, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawamoto, S.; Star, R.A.; Waldmann, T.A.; Tagaya, Y.; Brechbiel, M.W. Micro-magnetic resonance lymphangiography in mice using a novel dendrimer-based magnetic resonance imaging contrast agent. Cancer Res. 2003, 63, 271–276. [Google Scholar] [PubMed]
- Yang, J.; Luo, Y.; Xu, Y.; Li, J.; Zhang, Z.; Wang, H.; Shen, M.; Shi, X.; Zhang, G. Conjugation of iron oxide nanoparticles with RGD-modified dendrimers for targeted tumor MR imaging. ACS Appl. Mater. Interfaces 2015, 7, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Gumiel, A.; Sepúlveda-Crespo, D.; Jiménez, J.L.; Gómez, R.; Muñoz-Fernández, M.; de la Mata, F.J. Dendronized magnetic nanoparticles for HIV-1 capture and rapid diagnostic. Colloids Surf. B Biointerfaces 2019, 181, 360–368. [Google Scholar] [CrossRef]
- Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Park, J.A.; Kim, H.K.; Kim, J.H.; Jeong, S.W.; Jung, J.C.; Lee, G.H.; Lee, J.; Chang, Y.; Kim, T.J. Gold nanoparticles functionalized by gadolinium-DTPA conjugate of cysteine as a multimodal bioimaging agent. Bioorg. Med. Chem. Lett. 2010, 20, 2287–2291. [Google Scholar] [CrossRef]
- Kim, D.; Yu, M.K.; Lee, T.S.; Park, J.J.; Jeong, Y.Y.; Jon, S. Amphiphilic polymer-coated hybrid nanoparticles as CT/MRI dual contrast agents. Nanotechnology 2011, 22, 155101. [Google Scholar] [CrossRef]
- van Schooneveld, M.M.; Cormode, D.P.; Koole, R.; van Wijngaarden, J.T.; Calcagno, C.; Skajaa, T.; Hilhorst, J.; ’t Hart, D.C.; Fayad, Z.A.; Mulder, W.J.; et al. A fluorescent, paramagnetic and PEGylated gold/silica nanoparticle for MRI, CT and fluorescence imaging. Contrast Media Mol. Imaging 2010, 5, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagit, A.; Soenke, B.; Johannes, B.; Shlomo, M. Synthesis and characterization of dual modality (CT/MRI) core-shell microparticles for embolization purposes. Biomacromolecules 2010, 11, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, K.; Li, J.; Wen, S.; Chen, Q.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Dendrimer-Assisted Formation of Fe3O4/Au Nanocomposite Particles for Targeted Dual Mode CT/MR Imaging of Tumors. Small 2015, 11, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, H.; Liu, H.; Wen, S.; Peng, C.; Shen, M.; Zhang, G.; Shi, X. Multifunctional dendrimer-entrapped gold nanoparticles modified with RGD peptide for targeted computed tomography/magnetic resonance dual-modal imaging of tumors. Anal. Chem. 2015, 87, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.N.M.; Alvares, R.D.; Oakden, W.; Chaudhary, R.; Hill, M.L.; Pichaandi, J.; Mo, G.C.; Yip, C.; Macdonald, P.M.; Stanisz, G.J. Polymer-stabilized lanthanide fluoride nanoparticle aggregates as contrast agents for magnetic resonance imaging and computed tomography. Chem. Mater. 2010, 22, 4728–4739. [Google Scholar] [CrossRef]
- Regino, C.A.; Walbridge, S.; Bernardo, M.; Wong, K.J.; Johnson, D.; Lonser, R.; Oldfield, E.H.; Choyke, P.L.; Brechbiel, M.W. A dual CT-MR dendrimer contrast agent as a surrogate marker for convection-enhanced delivery of intracerebral macromolecular therapeutic agents. Contrast Media Mol. Imaging 2008, 3, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Li, K.; Cai, H.; Chen, Q.; Shen, M.; Huang, Y.; Peng, C.; Hou, W.; Zhu, M.; Zhang, G.; et al. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef]
- Li, N.; Jin, Y.; Xue, L.Z.; Li, P.Y.; Yan, D.Y.; Zhu, X.Y. 188Re-Labeled hyperbranched polysulfonamine as a robust tool for targeted cancer diagnosis and radioimmunotherapy. Chin. J. Polym. Sci. 2013, 3, 530–540. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Liu, J.; Zhu, X.; Yan, D. Self-assembly of hyperbranched polymers and its biomedical applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Pang, Y.; Huang, W.; Qiu, F.; Jiang, X.; Zhu, X.; Yan, D.; Chen, Q. Photoluminescent hyperbranched poly(amido amine) containing β-cyclodextrin as a nonviral gene delivery vector. Bioconjug. Chem. 2011, 22, 1162–1170. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Y.; Wang, X.; Jeong, E.K.; Parker, D.L.; Lu, Z.R. In Vivo evaluation of a PAMAM-cystamine-(Gd-DO3A) conjugate as a biodegradable macromolecular MRI contrast agent. Exp. Biol. Med. 2007, 232, 1081–1089. [Google Scholar] [CrossRef]
- You, S.; Jung, H.Y.; Lee, C.; Choe, Y.H.; Heo, J.Y.; Gang, G.T.; Byun, S.K.; Kim, W.K.; Lee, C.H.; Kim, D.E.; et al. High-performance dendritic contrast agents for X-ray computed tomography imaging using potent tetraiodobenzene derivatives. J. Control. Release 2016, 226, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, L.; Peng, C.; Guo, R.; Shen, M.; Shi, X.; Zhang, G. Computed tomography imaging of cancer cells using acetylated dendrimer-entrapped gold nanoparticles. Biomaterials 2011, 32, 2979–2988. [Google Scholar] [CrossRef]
- Filippi, M.; Catanzaro, V.; Patrucco, D.; Botta, M.; Tei, L.; Terreno, E. First in vivo MRI study on theranostic dendrimersomes. J. Control. Release 2017, 248, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Guzmán Merino, A.; Fraile-Martínez, O.; Recio-Ruiz, J.; Pekarek, L.; Guijarro, L.G.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; García-Gallego, S. Dendrimers and Dendritic Materials: From Laboratory to Medical Practice in Infectious Diseases. Pharmaceutics 2020, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.J.; Huong, D.T.; Han, J.H.; Kim, J.Y.; Lee, W.J.; Shin, H.J.; Han, E.T.; Park, H. Performance of coumarin-derived dendrimer-based fluorescence-linked immunosorbent assay (FLISA) to detect malaria antigen. Malar. J. 2014, 13, 266. [Google Scholar] [CrossRef] [Green Version]
- Markwalter, C.F.; Corstjens, P.; Mammoser, C.M.; Camps, G.; van Dam, G.J.; Wright, D.W. Poly(amidoamine)-coated magnetic particles for enhanced detection of Schistosoma circulating anodic antigen in endemic urine samples. Analyst 2018, 144, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Biomedical Applications of Dendrimers | Advantages of Using Dendrimers | Examples |
|---|---|---|
| Contrasting factor for magnetic resonance imaging |
| Gadomer 17. |
| Contrast agent containing chelates of gadolinium ions based on lysine dendrimers [53] | ||
Drug carrier:
|
| Niclosamide. |
| An antiparasitic drug, almost insoluble in water—after encapsulation in PAMAM dendrimers. Solubility increased. Enables the drug release to be controlled [55] | ||
| Fluorouracil (5FU). An anticancer drug, combined with a different generation of PAMAM dendrimer inhibited E6 and E7 oncogene activity. Showed slower drug release, lower toxicity and greater tumor accumulation [56] | ||
| Ketoprofen (a non-steroidal anti-inflammatory drug). A conjugate with the PAMAM dendrimer was better soluble in water, blood concentration was increased, and action was prolonged [57] | ||
| G4 PAMAM with tetracycline [58] | ||
| G4 PAMAM dendrimers linked with Trastuzumab (HER2 antibody) for breast tumor targeted delivery [59] | ||
| Gene therapy (non-viral gene transporters) |
| SuperFect® (Qiagen, Hilden, Germany). Acommercially available transfection PAMAM agent based on dendrimers [60] |
| G3 PPI dendrimer with 1,4-diaminobutane as core combined with plasmid DNA [30] | ||
| siRNA and doxorubicin codelivery system for the treatment of multidrug resistance cancers [61] | ||
| Dendrimer vaccines |
| Vaccine against malaria. Built of MAP (multiple antigenic peptide) dendrimers [62] |
| Vaccine against Ebola virus. A DNA containing vaccine based on 4 polyamidoamine dendrimers (PAMAM G4) [63] | ||
| Antiviral and antibacterial drugs with a dendrimeric structure |
| Sialodendrimers. Inhibition of hemagglutination of human erythrocytes caused by the influenza virus [64] |
| VivaGel® (Starpharma Ltd., Melbourne, Australia). Based on polylysine (G4) dendrimers. Protection against HIV infection [65] | ||
| Treatment of neurodegenerative diseases (Alzheimer’s, Parkinson’s, prion diseases) |
| PAMAM G3, G4 and G5 dendrimers. Inhibition of the formation of amyloid deposits. Characterized by the ability to degrade existing aggregates [66] |
| Anti-inflammatory molecules |
| 35% PEGylated G4 PAMAM and 45% PEGylated G6 PAMAM dendrimers—increased uptake. Negligible acute or chronic histotoxicity [67] |
| Phosphor dendrimers coated with bisphosphonate residues (ABP) as above.They enhance the proliferation of NK cells. | ||
| PAMAM dendrimers show anti-inflammatory activity in vivo using models representing acute and chronic inflammatory response [68] |
| Virus | Dendrimer Type | Payload | References |
|---|---|---|---|
| Hepatitis C | Carbosilane dendrimers | Sofosbuvir | [124] |
| PETIM | siRNA | [125] | |
| HPV (Cervical cancer) | Peptide dendrimers | siRNA | [126] |
| Doxorubicin | [127] | ||
| FMDV (Foot-and-mouth disease virus) | Peptide dendrimer | Cyclic disulfide epitope | [128] |
| RVS (Respiratory Syncytial Virus) | RFI-641 | [129] |
| Imaging Technique | Advantages | Limitations |
|---|---|---|
| SPECT | Highly branched architecture, adequate spatial cavities and tremendous functional terminals [247], prolonged circulation time, ease of conjugation with drugs and active targeting agents [248,249,250], low cytotoxicity and efficient cell entry capability [247] | The apparent accumulation in the liver and spleen [247].High cost of their complex preparation methods that usually involve multistep syntheses and their toxicity [245,251,252]. |
| CT | Biocompatibility [253], high contrast enhancement in the blood-pool and effectively extended their blood half-lives, preventing undesirable long-term accumulation in vivo and attaining reproducibility for their syntheses and properties [252]. | |
| MR | Rapid blood clearance and lower liver uptake [251], high longitudinal relaxivity and consistent contrast enhancement [254] | |
| CT/MR | Biocompatibility, monodispersity and in vivo stability, particle size controllability, realization of multimodal imaging [245] and high payload [243]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Parveen, F.; Torchilin, V. Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases. Molecules 2021, 26, 3304. https://doi.org/10.3390/molecules26113304
Filipczak N, Yalamarty SSK, Li X, Parveen F, Torchilin V. Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases. Molecules. 2021; 26(11):3304. https://doi.org/10.3390/molecules26113304
Chicago/Turabian StyleFilipczak, Nina, Satya Siva Kishan Yalamarty, Xiang Li, Farzana Parveen, and Vladimir Torchilin. 2021. "Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases" Molecules 26, no. 11: 3304. https://doi.org/10.3390/molecules26113304
APA StyleFilipczak, N., Yalamarty, S. S. K., Li, X., Parveen, F., & Torchilin, V. (2021). Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases. Molecules, 26(11), 3304. https://doi.org/10.3390/molecules26113304







