Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors
Abstract
1. Introduction
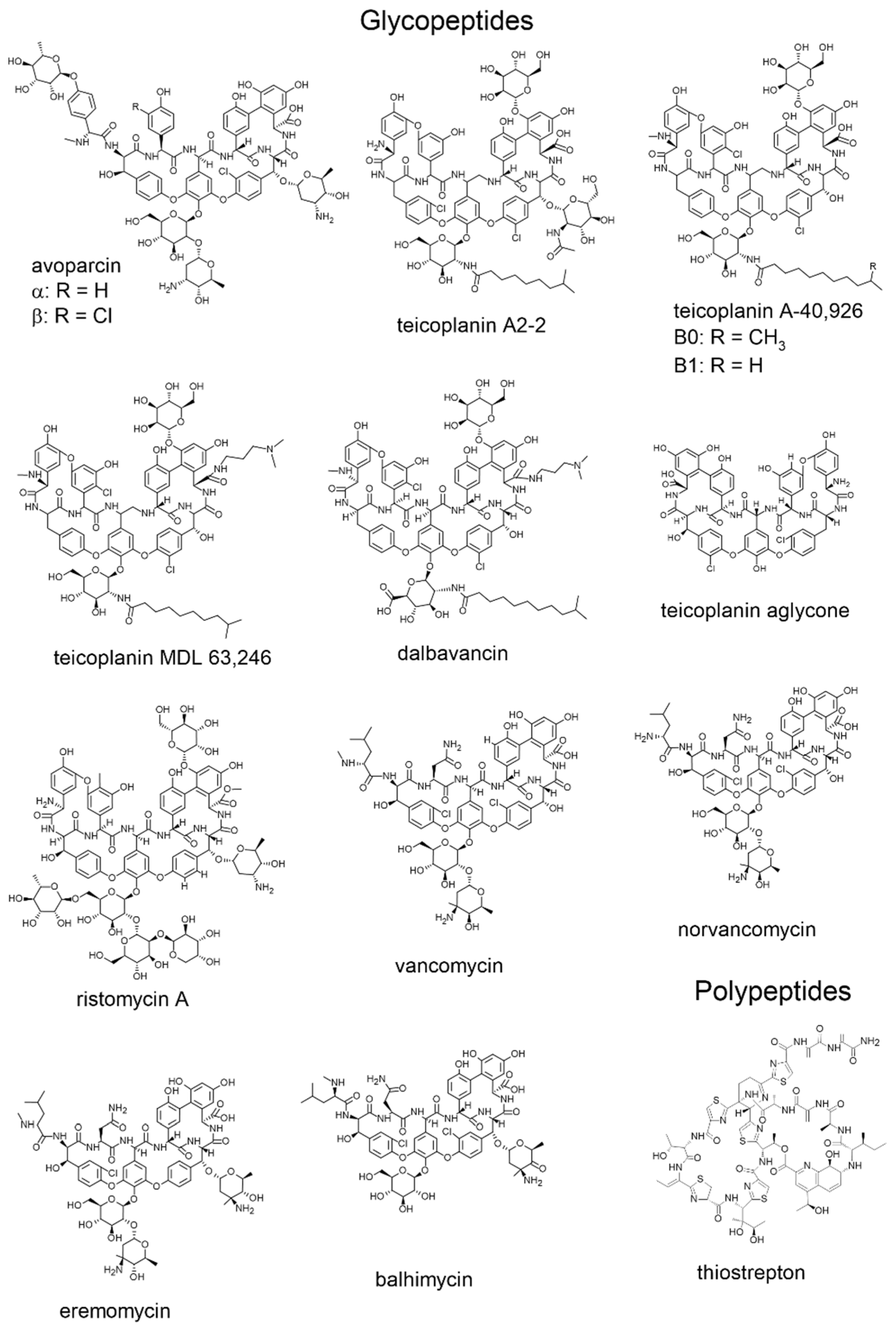
2. Structural Characterization of the Most Important Antibiotics
2.1. Vancomycin
2.2. Ristocetin A
2.3. Teicoplanin and Teicoplanin Aglycon
- it may block access to the inside of the basket,
- it may inhibit the possible interactions with the two phenolic and one alcoholic hydroxyl groups of the aglycon, through which the three sugar moieties are linked in the case of native teicoplanin,
- the alcoholic hydroxyl, ether, and amide groups of the sugar moiety as well as the nonyl chain may provide additional interactions.
3. Retention Mechanism
4. Recent Applications of Different Macrocyclic Antibiotic-Based CSPs
4.1. High-Performance Liquid Chromatographic Enantioseparation of Stereoisomers of Different Analytes on Vancomycin-Based CSPs
4.2. High-Performance Liquid Chromatographic Enantioseparation of Stereoisomers of Different Analytes on Teicoplanin, Teicoplanin Aglycon, Vancomycin, and Ristocetin A-Based CSPs
4.3. Enantioseparations Achieved with Macrocyclic Glycopeptides Bonded on Ultra-High-Performance Particles
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Scriba, G.K.E. Chiral recognition in separation sciences. Part II: Macrocyclic glycopeptide, donor-acceptor, ion-exchange, ligand-exchange and micellar selectors. TrAC Trends Anal. Chem. 2019, 119, 115628. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral recognition in separation sciences. Part I: Polysaccharide and cyclodextrin selectors. TrAC Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral recognition in separation science—An update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. Chiral Separations. Methods and Protocols. In Methods in Molecular Biology, 1985; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2019; ISBN 9781493994373. [Google Scholar]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Chankvetadze, B. Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. TrAC Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Mangelings, D.; Eeltink, S.; Heyden, Y.V. Recent developments in liquid and supercritical fluid chromatographic enantioseparations. In Handbook of Analytical Separations, 2nd ed.; Valkó, K.l., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2020; Volume 8, ISBN 9780444640703. [Google Scholar]
- Ilisz, I.; Berkecz, R.; Péter, A. HPLC separation of amino acid enantiomers and small peptides on macrocyclic antibiotic-based chiral stationary phases: A review. J. Sep. Sci. 2006, 29, 1305–1321. [Google Scholar] [CrossRef]
- Ilisz, I.; Berkecz, R.; Péter, A. Retention mechanism of high-performance liquid chromatographic enantioseparation on macrocyclic glycopeptide-based chiral stationary phases. J. Chromatogr. A 2009, 1216, 1845–1860. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Pataj, Z.; Aranyi, A.; Péter, A. Macrocyclic antibiotic selectors in direct HPLC enantioseparations. Sep. Purif. Rev. 2012, 41, 207–249. [Google Scholar] [CrossRef]
- Shapovalova, E.N.; Fedorova, I.A.; Anan’eva, I.A.; Shpigun, O.A. Macrocyclic antibiotics as chiral selectors in high-performance liquid chromatography and capillary electrophoresis. J. Anal. Chem. 2018, 73, 1064–1075. [Google Scholar] [CrossRef]
- Cardoso, P.A.; César, I.C. Chiral Method Development Strategies for HPLC using macrocyclic glycopeptide-based stationary phases. Chromatographia 2018, 81, 841–850. [Google Scholar] [CrossRef]
- Ilisz, I.; Orosz, T.; Péter, A. High-performance liquid chromatography enantioseparations using macrocyclic glycopeptide-based chiral stationary phases—An Overview. In Chiral Separations: Methods and Protocols; Scriba, G.K.E., Ed.; Humana Press: New York, NY, USA, 2019; pp. 201–237. ISBN 978-1-4939-9437-3. [Google Scholar]
- Armstrong, D.W.; Zhou, Y. Use of a macrocyclic antibiotic as the chiral selector for enantiomeric separations by TLC. J. Liq. Chromatogr. 1994, 17, 1695–1707. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Tang, Y.; Chen, S.; Bagwlll, C.; Chen, J. Macrocyclic antibiotics as a new class. Anal. Chem. 1994, 66, 1473–1484. [Google Scholar] [CrossRef]
- Patel, D.C.; Breitbach, Z.S.; Wahab, M.F.; Barhate, C.L.; Armstrong, D.W. Gone in seconds: Praxis, performance, and peculiarities of ultrafast chiral liquid chromatography with superficially porous particles. Anal. Chem. 2015, 87, 9137–9148. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.C.; Wahab, M.F.; O’Haver, T.C.; Armstrong, D.W. Separations at the speed of sensors. Anal. Chem. 2018, 90, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Ciogli, A.; Ismail, O.H.; Mazzoccanti, G.; Villani, C.; Gasparrini, F. Enantioselective ultra high performance liquid and supercritical fluid chromatography: The race to the shortest chromatogram. J. Sep. Sci. 2018, 41, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Felletti, S.; De Luca, C.; Pasti, L.; Marchetti, N.; Costa, V.; Gasparrini, F.; Cavazzini, A.; Catani, M. The way to ultrafast, high-throughput enantioseparations of bioactive compounds in liquid and supercritical fluid chromatography. Molecules 2018, 23, 2709. [Google Scholar] [CrossRef] [PubMed]
- Bezhitashvili, L.; Bardavelidze, A.; Mskhiladze, A.; Gumustas, M.; Ozkan, S.A.; Volonterio, A.; Farkas, T.; Chankvetadze, B. Application of cellulose 3,5-dichlorophenylcarbamate covalently immobilized on superficially porous silica for the separation of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2018, 1571, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Khundadze, N.; Pantsulaia, S.; Fanali, C.; Farkas, T.; Chankvetadze, B. On our way to sub-second separations of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2018, 1572, 37–43. [Google Scholar] [CrossRef]
- Best, G.K.; Best, N.H.; Durham, N.N. Chromatographic separation of the vancomycin complex. Antimicrob. Agents Chemother. (Bethesda) 1968, 8, 115–119. [Google Scholar]
- Jordan, D.C. Antibiotics. In Antibiotics; Gottlieb, D., Shaw, P., Eds.; Springer: New York, NY, USA, 1967; p. 84. [Google Scholar]
- Barna, J.C.J.; Williams, D.H.; Stone, D.J.M.; Leung, T.W.C.; Doddrell, D.M. Structure elucidation of the teicoplanin antibiotics. J. Am. Chem. Soc. 1984, 106, 4895. [Google Scholar] [CrossRef]
- Berthod, A.; Chen, X.; Kullman, J.P.; Armstrong, D.W.; Gasparrini, F.; D’Acquarica, I.; Villani, C.; Carotti, A. Role of the carbohydrate moieties in chiral recognition on teicoplanin-based LC stationary phases. Anal. Chem. 2000, 72, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Wang, Y.; Wang, L.; Zhao, L.; Pan, L.; Cheng, M.; Li, F. Determination of trantinterol enantiomers in human plasma by high-performance liquid chromatography-tandem mass spectrometry using vancomycin chiral stationary phase and solid phase extraction and stereoselective pharmacokinetic application. Chirality 2015, 27, 327–331. [Google Scholar] [CrossRef]
- Wang, T.; Shen, B.; Shi, Y.; Xiang, P.; Yu, Z. Chiral separation and determination of R/S-methamphetamine and its metabolite R/S-amphetamine in urine using LC-MS/MS. Forensic Sci. Int. 2015, 246, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.; McBriar, T.; He, P.; Beavis, A. Chiral determination and assay of optical isomers in clandestine drug laboratory samples using LC-MSMS. Anal. Methods 2017, 9, 3380–3387. [Google Scholar] [CrossRef]
- Gherdaoui, D.; Bekdouche, H.; Zerkout, S.; Fegas, R.; Righezza, M. Chiral separation of ketoprofen on an achiral NH2 column by HPLC using vancomycin as chiral mobile phase additive. J. Iran. Chem. Soc. 2016, 13, 2319–2323. [Google Scholar] [CrossRef]
- Abdollahpour, A.; Heydari, R.; Shamsipur, M. Two Synthetic Methods for Preparation of chiral stationary phases using crystalline degradation products of vancomycin: Column performance for enantioseparation of acidic and basic drugs. AAPS PharmSciTech 2017, 18, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.A.; Shapovalova, E.N.; Shpigun, O.A. Separation of β-blocker and amino acid enantiomers on a mixed chiral sorbent modified with macrocyclic antibiotics eremomycin and vancomycin. J. Anal. Chem. 2017, 72, 76–82. [Google Scholar] [CrossRef]
- Anan’eva, I.A.; Polyakova, Y.A.; Shapovalova, E.N.; Mazhuga, A.G.; Shpigun, O.A. Separation of β-blocker enantiomers on silica modified with gold nanoparticles with immobilized macrocyclic antibiotic vancomicin. J. Anal. Chem. 2018, 73, 152–159. [Google Scholar] [CrossRef]
- Shahnani, M.; Sefidbakht, Y.; Maghari, S.; Mehdi, A.; Rezadoost, H.; Ghassempour, A. Enantioseparation of mandelic acid on vancomycin column: Experimental and docking study. Chirality 2020, 32, 1289–1298. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Cravo, S.; Palmeira, A.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M.; Fernandes, C. Enantiomeric resolution and docking studies of chiral xanthonic derivatives on chirobiotic columns. Molecules 2018, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Gogolishvili, O.S.; Reshetova, E.N. Chromatographic enantioseparation and adsorption thermodynamics of hydroxy acids and their derivatives on antibiotic-based chiral stationary phases as affected by eluent pH. Chromatographia 2021, 84, 53–73. [Google Scholar] [CrossRef]
- Dixit, S.; Park, J.H. Enantioseparation of basic chiral drugs on a carbamoylated erythromycin-zirconia hybrid monolith using capillary electrochromatography. J. Chromatogr. A 2015, 1416, 129–136. [Google Scholar] [CrossRef]
- Dixit, S.; Lee, I.S.; Park, J.H. Carbamoylated azithromycin incorporated zirconia hybrid monolith for enantioseparation of acidic chiral drugs using non-aqueous capillary electrochromatography. J. Chromatogr. A 2017, 1507, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Valleix, A.; Tizon, V.; Leonce, E.; Caussignac, C.; Armstrong, D.W. Retention and selectivity of teicoplanin stationary phases after copper complexation and isotopic exchange. Anal. Chem. 2001, 73, 5499–5508. [Google Scholar] [CrossRef]
- Ilisz, I.; Grecsó, N.; Forró, E.; Fülöp, F.; Armstrong, D.W.; Péter, A. High-performance liquid chromatographic separation of paclitaxel intermediate phenylisoserine derivatives on macrocyclic glycopeptide and cyclofructan-based chiral stationary phases. J. Pharm. Biomed. Anal. 2015, 114, 312–320. [Google Scholar] [CrossRef]
- Deáková, Z.; Ďuračková, Z.; Armstrong, D.W.; Lehotay, J. Separation of enantiomers of selected sulfur-containing amino acids by using serially coupled achiral-chiral Columns. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 789–794. [Google Scholar] [CrossRef]
- Deáková, Z.; Durăcková, Z.; Armstrong, D.W.; Lehotay, J. Two-dimensional high performance liquid chromatography for determination of homocysteine, methionine and cysteine enantiomers in human serum. J. Chromatogr. A 2015, 1408, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bystrická, Z.; Bystrický, R.; Lehotay, J. Thermodynamic study of HPLC enantioseparations of some sulfur-containing amino acids on teicoplanin columns in ion-pairing reversed-phase mode. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 775–781. [Google Scholar] [CrossRef]
- Shu, Y.; Lang, J.C.; Breitbach, Z.S.; Qiu, H.; Smuts, J.P.; Kiyono-Shimobe, M.; Yasuda, M.; Armstrong, D.W. Separation of therapeutic peptides with cyclofructan and glycopeptide based columns in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2015, 1390, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Orosz, T.; Grecsó, N.; Lajkó, G.; Szakonyi, Z.; Fülöp, F.; Armstrong, D.W.; Ilisz, I.; Péter, A. Liquid chromatographic enantioseparation of carbocyclic β-amino acids possessing limonene skeleton on macrocyclic glycopeptide-based chiral stationary phases. J. Pharm. Biomed. Anal. 2017, 145, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lomenova, A.; Hroboňová, K. Comparison of HPLC separation of phenylalanine enantiomers on different types of chiral stationary phases. Food Anal. Methods 2018, 11, 3314–3323. [Google Scholar] [CrossRef]
- Maia, A.S.; Castro, P.M.L.; Tiritan, M.E. Integrated liquid chromatography method in enantioselective studies: Biodegradation of ofloxacin by an activated sludge consortium. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1029–1030, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Huong, N.L.; Hoang, N.H.; Hong, S.Y.; Sohng, J.K.; Yoon, Y.J.; Park, J.W. Characterization of fortimicin aminoglycoside profiles produced from Micromonospora olivasterospora DSM 43868 by high-performance liquid chromatography-electrospray ionization-ion trap-mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Dolzan, M.D.; Shu, Y.; Smuts, J.P.; Petersen, H.; Ellegaard, P.; Micke, G.A.; Armstrong, D.W.; Breitbach, Z.S. Enantiomeric separation of citalopram analogues by HPLC using macrocyclic glycopeptide and cyclodextrin based chiral stationary phases. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 154–160. [Google Scholar] [CrossRef]
- Harvanová, M.; Gondová, T. New enantioselective LC method development and validation for the assay of modafinil. J. Pharm. Biomed. Anal. 2017, 138, 267–271. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Kasprzyk-Hordern, B. Simultaneous enantiomeric analysis of pharmacologically active compounds in environmental samples by chiral LC-MS/MS with a macrocyclic antibiotic stationary phase. J. Mass Spectrom. 2017, 52, 94–108. [Google Scholar] [CrossRef]
- Feder-Kubis, J.; Flieger, J.; Tatarczak-Michalewska, M.; Płazińska, A.; Madejska, A.; Swatko-Ossor, M. Renewable sources from plants as the starting material for designing new terpene chiral ionic liquids used for the chromatographic separation of acidic enantiomers. RSC Adv. 2017, 7, 32344–32356. [Google Scholar] [CrossRef]
- Flieger, J.; Feder-Kubis, J.; Tatarczak-Michalewska, M.; Płazińska, A.; Madejska, A.; Swatko-Ossor, M. Natural terpene derivatives as new structural task-specific ionic liquids to enhance the enantiorecognition of acidic enantiomers on teicoplanin-based stationary phase by high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 2374–2381. [Google Scholar] [CrossRef]
- Shapovalova, E.N.; Fedorova, I.A.; Priporova, A.A.; Ananieva, I.A.; Shpigun, O.A. Determination of the enantiomeric purity of albuterol on sorbents modified by macrocyclic antibiotics. Moscow Univ. Chem. Bull. 2017, 72, 56–62. [Google Scholar] [CrossRef]
- Foroughbakhshfasaei, M.; Szabó, Z.I.; Mirzahosseini, A.; Horváth, P.; Tóth, G. Enantiomeric quality control of R-Tofisopam by HPLC using polysaccharide-type chiral stationary phases in polar organic mode. Electrophoresis 2018, 39, 2566–2574. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Asnin, L. Chiral separation and modeling of quinolones on teicoplanin macrocyclic glycopeptide antibiotics CSP. Chirality 2018, 30, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Suhail, M.; Al-Othman, Z.A.; Alwarthan, A.; Aboul-Enein, H.Y. Enantiomeric resolution of multiple chiral centres racemates by capillary electrophoresis. Biomed. Chromatogr. 2016, 30, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, L.; Pucciarini, L.; Regazzoni, L.; Gilardoni, E.; Carini, M.; Vistoli, G.; Aldini, G.; Sardella, R. Direct HPLC separation of carnosine enantiomers with two chiral stationary phases based on penicillamine and teicoplanin derivatives. J. Sep. Sci. 2018, 41, 1240–1246. [Google Scholar] [CrossRef]
- Sardella, R.; Ianni, F.; Cossignani, L.; Aldini, G.; Carotti, A. Binding modes identification through molecular dynamic simulations: A case study with carnosine enantiomers and the Teicoplanin A2-2-based chiral stationary phase. J. Sep. Sci. 2020, 43, 1728–1736. [Google Scholar] [CrossRef]
- Asnin, L.D.; Boteva, A.A.; Krasnykh, O.P.; Stepanova, M.V.; Ali, I. Unusual van Deemter plots of optical isomers on a chiral brush-type liquid chromatography column. J. Chromatogr. A 2019, 1592, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.A.; Foroughbakhshfasaei, M.; Fiser, B.; Horváth, P.; Kiss, E.; Sekkoum, K.; Gyéresi, Á.; Hancu, G.; Noszál, B.; Szabó, Z.I.; et al. Reversed-phase HPLC enantioseparation of pantoprazole using a teicoplanin aglycone stationary phase—Determination of the enantiomer elution order using HPLC-CD analyses. Chirality 2020, 32, 158–167. [Google Scholar] [CrossRef]
- Reshetova, E.N.; Kopchenova, M.V.; Vozisov, S.E.; Vasyanin, A.N.; Asnin, L.D. Enantioselective retention mechanisms of dipeptides on antibiotic-based chiral stationary phases: Leucyl-leucine, glycyl-leucine, and leucyl-glycine as case studies. J. Chromatogr. A 2019, 1602, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Asnin, L.D.; Kopchenova, M.V.; Vozisov, S.E.; Klochkova, M.A.; Klimova, Y.A. Enantioselective retention mechanisms of dipeptides on antibiotic-based chiral stationary phases. II. Effect of the methanol content in the mobile phase. J. Chromatogr. A 2020, 1626, 461371. [Google Scholar] [CrossRef]
- Klimova, Y.A.; Asnin, L.D. Enantioselective adsorption dynamics of leucyl-leucine in a Chirobiotic R column. J. Chromatogr. A 2021, 1635, 461771. [Google Scholar] [CrossRef] [PubMed]
- Kopchenova, M.V.; Stepanova, M.V.; Asnin, L.D. Unusual difference in enantioselectivity of two chiral stationary phases with grafted antibiotic Ristocetin A. Chromatographia 2021, 84, 307–311. [Google Scholar] [CrossRef]
- Sánchez-Hernández, L.; Bernal, J.L.; Del Nozal, M.J.; Toribio, L. Chiral analysis of aromatic amino acids in food supplements using subcritical fluid chromatography and Chirobiotic T2 column. J. Supercrit. Fluids 2016, 107, 519–525. [Google Scholar] [CrossRef]
- Khater, S.; West, C. Characterization of three macrocyclic glycopeptide stationary phases in supercritical fluid chromatography. J. Chromatogr. A 2019, 1604, 460485. [Google Scholar] [CrossRef]
- Barhate, C.L.; Wahab, M.F.; Breitbach, Z.S.; Bell, D.S.; Armstrong, D.W. High efficiency, narrow particle size distribution, sub-2 μm based macrocyclic glycopeptide chiral stationary phases in HPLC and SFC. Anal. Chim. Acta 2015, 898, 128–137. [Google Scholar] [CrossRef]
- Min, Y.; Sui, Z.; Liang, Z.; Zhang, L.; Zhang, Y. Teicoplanin bonded sub-2 μm superficially porous particles for enantioseparation of native amino acids. J. Pharm. Biomed. Anal. 2015, 114, 247–253. [Google Scholar] [CrossRef]
- Wahab, M.F.; Wimalasinghe, R.M.; Wang, Y.; Barhate, C.L.; Patel, D.C.; Armstrong, D.W. Salient sub-second separations. Anal. Chem. 2016, 88, 8821–8826. [Google Scholar] [CrossRef] [PubMed]
- Barhate, C.L.; Breitbach, Z.S.; Pinto, E.C.; Regalado, E.L.; Welch, C.J.; Armstrong, D.W. Ultrafast separation of fluorinated and desfluorinated pharmaceuticals using highly efficient and selective chiral selectors bonded to superficially porous particles. J. Chromatogr. A 2015, 1426, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wimalasinghe, R.M.; Breitbach, Z.S.; Lee, J.T.; Armstrong, D.W. Separation of peptides on superficially porous particle based macrocyclic glycopeptide liquid chromatography stationary phases: Consideration of fast separations. Anal. Bioanal. Chem. 2017, 409, 2437–2447. [Google Scholar] [CrossRef]
- Hellinghausen, G.; Lee, J.T.; Weatherly, C.A.; Lopez, D.A.; Armstrong, D.W. Evaluation of nicotine in tobacco-free-nicotine commercial products. Drug Test. Anal. 2017, 9, 944–948. [Google Scholar] [CrossRef]
- Hellinghausen, G.; Roy, D.; Wang, Y.; Lee, J.T.; Lopez, D.A.; Weatherly, C.A.; Armstrong, D.W. A comprehensive methodology for the chiral separation of 40 tobacco alkaloids and their carcinogenic E/Z-(R,S)-tobacco-specific nitrosamine metabolites. Talanta 2018, 181, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Ciogli, A.; Villani, C.; De Martino, M.; Pierini, M.; Cavazzini, A.; Bell, D.S.; Gasparrini, F. Ultra-fast high-efficiency enantioseparations by means of a teicoplanin-based chiral stationary phase made on sub-2 μm totally porous silica particles of narrow size distribution. J. Chromatogr. A 2016, 1427, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Antonelli, M.; Ciogli, A.; Villani, C.; Cavazzini, A.; Catani, M.; Felletti, S.; Bell, D.S.; Gasparrini, F. Future perspectives in high efficient and ultrafast chiral liquid chromatography through zwitterionic teicoplanin-based 2-μm superficially porous particles. J. Chromatogr. A 2017, 1520, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Antonelli, M.; Ciogli, A.; De Martino, M.; Catani, M.; Villani, C.; Cavazzini, A.; Ye, M.; Bell, D.S.; Gasparrini, F. Direct analysis of chiral active pharmaceutical ingredients and their counterions by ultra high performance liquid chromatography with macrocyclic glycopeptide-based chiral stationary phases. J. Chromatogr. A 2018, 1576, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Barhate, C.L.; Regalado, E.L.; Contrella, N.D.; Lee, J.; Jo, J.; Makarov, A.A.; Armstrong, D.W.; Welch, C.J. Ultrafast chiral chromatography as the second dimension in two-dimensional liquid chromatography experiments. Anal. Chem. 2017, 89, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Barhate, C.L.; Lopez, D.A.; Makarov, A.A.; Bu, X.; Morris, W.J.; Lekhal, A.; Hartman, R.; Armstrong, D.W.; Regalado, E.L. Macrocyclic glycopeptide chiral selectors bonded to core-shell particles enables enantiopurity analysis of the entire verubecestat synthetic route. J. Chromatogr. A 2018, 1539, 87–92. [Google Scholar] [CrossRef]
- Hellinghausen, G.; Roy, D.; Lee, J.T.; Wang, Y.; Weatherly, C.A.; Lopez, D.A.; Nguyen, K.A.; Armstrong, J.D.; Armstrong, D.W. Effective methodologies for enantiomeric separations of 150 pharmacology and toxicology related 1° 2° and 3° amines with core-shell chiral stationary phases. J. Pharm. Biomed. Anal. 2018, 155, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Hellinghausen, G.; Lopez, D.A.; Lee, J.T.; Wang, Y.; Weatherly, C.A.; Portillo, A.E.; Berthod, A.; Armstrong, D.W. Evaluation of the Edman degradation product of vancomycin bonded to core-shell particles as a new HPLC chiral stationary phase. Chirality 2018, 30, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Hellinghausen, G.; Readel, E.R.; Wahab, M.F.; Lee, J.T.; Lopez, D.A.; Weatherly, C.A.; Armstrong, D.W. Mass spectrometry-compatible enantiomeric separations of 100 pesticides using core-shell chiral stationary phases and evaluation of iterative curve fitting models for overlapping peaks. Chromatographia 2019, 82, 221–233. [Google Scholar] [CrossRef]
- Kenari, M.E.; Putman, J.I.; Singh, R.P.; Fulton, B.B.; Phan, H.; Haimour, R.K.; Tse, K.; Berthod, A.; Lovely, C.J.; Armstrong, D.W. Enantiomeric separation of new chiral azole compounds. Molecules 2021, 26, 213. [Google Scholar] [CrossRef]
- Roy, D.; Armstrong, D.W. Fast super/subcritical fluid chromatographic enantioseparations on superficially porous particles bonded with broad selectivity chiral selectors relative to fully porous particles. J. Chromatogr. A 2019, 1605, 360339. [Google Scholar] [CrossRef]
- Roy, D.; Wahab, M.F.; Berger, T.A.; Armstrong, D.W. Ramifications and insights on the role of water in chiral sub/supercritical fluid chromatography. Anal. Chem. 2019, 91, 14672–14680. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Wahab, M.F.; Talebi, M.; Armstrong, D.W. Replacing methanol with azeotropic ethanol as the co-solvent for improved chiral separations with supercritical fluid chromatography (SFC). Green Chem. 2020, 22, 1249–1257. [Google Scholar] [CrossRef]
- Folprechtová, D.; Kozlov, O.; Armstrong, D.W.; Schmid, M.G.; Kalíková, K.; Tesařová, E. Enantioselective potential of teicoplanin- and vancomycin-based superficially porous particles-packed columns for supercritical fluid chromatography. J. Chromatogr. A 2020, 1612, 460687. [Google Scholar] [CrossRef] [PubMed]

| Properties | Ansamycins | Glycopeptides | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rifampicin | Rifamycin B | Rifamycin SV | Avoparcin | Teicoplanin A2–2 | Teicoplanin A-40,926 | Teicoplanin MDL 63,246 | Dalbavancin | ||
| Molecular weight | 823 | 756 | 698 | α = 1908 β = 1943 | 1877 | B0 = 1732 B1 = 1718 | 1789 | 1817 | |
| Hydrophobic tail | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | |
| Number of … | asymmetric centers | 9 | 9 | 9 | 32 | 23 | B0 = 19 B1 = 18 | 18 | 18 |
| macrocycles | 1 | 1 | 1 | 3 | 4 | 4 | 4 | 4 | |
| aromatic rings | 2 | 2 | 2 | 7 | 7 | 7 | 7 | 7 | |
| sugar moieties | 0 | 0 | 0 | 5 | 3 | 2 | 2 | 2 | |
| hydroxy groups | 5 | 5 | 5 | 16 | 14 | 11 | 12 | 11 | |
| primary amines | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | |
| secondary amines | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | |
| amido groups | 1 | 1 | 1 | 6 | 8 | 7 | 8 | 8 | |
| carboxylic groups | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 1 | |
| methoxy groups | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| methyl esters | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Produced by | Amycolatopsis rifamycinica | Nocardia mediterranei | Nocardia mediterranei | Streptomyces candidus | Actinoplanes teichomycetius | Nonomuraea ATCC 39727 | Synthetic compound | Synthetic compound | |
| Properties | Glycopeptides | Polypeptides | ||||||
|---|---|---|---|---|---|---|---|---|
| Teicoplanin Aglycone | Ristomycin Ristocetin A | Vancomycin | Nor-Vancomycin | Eremomycin | Balhimycin | Thiostrepton | ||
| Molecular weight | 1197 | 2066 | 1449 | 1435 | 1558 | 1446 | 1665 | |
| Hydrophobic tail | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Number of … | asymmetric centers | 8 | 38 | 18 | 18 | 22 | 17 | 17 |
| macrocycles | 4 | 4 | 3 | 3 | 3 | 3 | 2 | |
| aromatic rings | 7 | 7 | 5 | 5 | 5 | 5 | 1 | |
| sugar moieties | 0 | 6 | 2 | 2 | 3 | 2 | 0 | |
| hydroxy groups | 7 | 21 | 9 | 9 | 9 | 8 | 5 | |
| primary amines | 1 | 2 | 1 | 2 | 3 | 1 | 0 | |
| secondary amines | 0 | 0 | 1 | 0 | 0 | 1 | 1 | |
| amido groups | 6 | 6 | 7 | 7 | 7 | 7 | 11 | |
| carboxylic groups | 1 | 0 | 1 | 1 | 1 | 1 | 0 | |
| methoxy groups | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| methyl esters | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Produced by | Synthetic compound | Nocardia lurida | Streptomyces orientalis | Streptomyces orientalis | Amycolatopsis orientalis | Amycolatopsis balhimycina | Streptomyces azureus | |
| Analytes | Selectors | Column’s Trade Mark | The Most Effective Mobile Phases (v/v/v) | Mode | Reference |
|---|---|---|---|---|---|
| trantinterol | Vancomycin | Chirobiotic V | MeOH/MeCN/AcOH/NH4OH 80/20/0.02/0.01 or 60/40/0.02/0.01 | PIM | [26] |
| amphetamine, metamphetamine | Vancomycin | Chirobiotic V Chirobiotic V2 | MeOH/AcOH/NH4OH 100/0.1/0.02 | PIM | [27] |
| amphetamine, metamphetamine, methylenedioxyamphetamine, methorphan, methylenedioxymetamphetamine, ephedrine, pseudoephedrine | Vancomycin | Chirobiotic V2 | MeOH/0.04% NH4TFA | PIM | [28] |
| ketoprofen | Vancomycin | chiral mobile phase additive | 0.05 M KH2PO4 (pH 6.0)/2-propanol 50/50/ | RPM | [29] |
| amlodipin, atropine, baclofen, ibuprofen, mandelic acid, Phe | Vancomycin degradation product | tailor-made | 0.1% NH4TFA in MeOH aq. TEAA (pH 6.5)/MeOH (85/15) 20 mM aq. sodium citrate (pH 6.3)/THF | PIM RPM RPM | [30] |
| metoprolol, pindolol, alprenolol, oxprenolol, labetolol, atenolol Trp, Phe, DOPA, Met, Glu, Ala, Nva, Val, Lys, arg, Ser | immobilized mixed Eremomycin and Vancomycin on silica | tailor-made | 0.1% aq.TEAA (pH 4.5)/MeOH/MeCN (5/20/75) MeCN/aq. AcOH (97/3) | RPM | [31] |
| Β-blockers: nadolol, atenolol, metoprolol, alprenolol, oxprenolol, pindolol | Vancomycin on gold nanoparticles | tailor-made | 25 mM potassium phosphate (pH 4)/MeCN (96/4) 25 or 50 mM ammonium acetate (pH 4)/MeCN (96/4) | RPM | [32] |
| mandelic acid, propranolol | Vancomycin | tailor-made | n-heptane/2-propanol (90/10) containing 0.4% TFA | NPM | [33] |
| chiral xanthone derivatives | Vancomycin, Teicoplanin, Teicoplanin aglycone, Ristocetin A | Chirobiotic V Chirobiotic T Chirobiotic TAG Chirobiotic R | n-hexane(EtOH or n-hexane/2-PrOH aq. TEAA (pH 4.2)/MeOH; NH4OAc (pH 6)/MeOH 100% MeOH, 100% EtOH or 100% 2-PrOH MeOH(AcOH/TEA) | NPM RPM POM PIM | [34] |
| nine aromatic hydroxy acids | Eremomycin Ristomycin Teicoplanin | Nautilus-E Nautilus-R Chirobiotic T | aq. NH4OAc (pH 3.3–6.3)/EtOH (60/40) | RPM | [35] |
| ketoprofen, flurbiprofen, suprofen, carprofen, ibuprofen, warfarin | Clindamycin phosphate (CLIP) and Erythromycin incorporated to zircona hybrid monolith | CLIP-ZHM ERY-ZHM | MeOH/MeCN (20/80) containing 300 mM AcOH and 10 mM TEA | CEC | [36] |
| atenolol, chlorphenamine, esmelol, nefopam, propranolol | azithro-mycin lactobionate, clindamycin phosphate | tailor-made | freshly dissolved azithro-mycin lactobionate and clindamycin phosphate in phosphate buffer (20 mM) adjusted to specific pH with sodium hydroxide | CEC | [37] |
| Group of Racemates | Racemates | Selector | Column’s Trade Mark | The Most Effective Mobile Phase (v/v/v) | Mode | Reference |
|---|---|---|---|---|---|---|
| Amino acids amino acid analogs | phenylisoserine derivatives | Teicoplanin Teicoplanin aglycone, Vancomycin, Vancomycin aglycone | Chirobiotic T Chirobiotic TAG Chirobiotic V Chirobiotic VAG | 0.1% TEAA (pH 4.1)/MeOH (50/50) | RPM | [39] |
| Met, Cys, homo-Cys | Teicoplanin Teicoplanin aglycone | Chirobiotic T Chirobiotic TAG | 25 mM aq. phosphate buffer/1 mM aq. octanesulfonic acid (pH 2.7)/MeCN/MeOH (94/3/3) | RPM | [40,41,42] | |
| therapeutic peptides | Teicoplanin, Vancomycin | Chirobiotic T Chirobiotic V | 20 mM aq. NH4OAc (pH 4.1)/MeCN (5/95) 0.1% aq. TEAA/MeOH (90/10) | HILIC RPM | [43] | |
| carbocyclic β-amino acids possessing limonene skeleton | Teicoplanin Teicoplanin aglycone Ristocetin A | Chirobiotic T Chirobiotic TAG Chirobiotic R | MeOH/AcOH/TEA (100/0.01/0.01) and (100/0.1/0.1) 0.1% aq. TEAA/MeOH (90/10) | PIM RPM | [44] | |
| Phe | Teicoplanin Ristocetin A | Chirobiotic T Chirobiotic R | MeCN/H2O (75/25) MeCN/H2O (60/40) | RPM | [45] | |
| Drugs | ofloxacin | Teicoplanin Teicoplanin aglycon Ristocetin A | Chirobiotic T Chirobiotic TAG Chirobiotic R | 0.45% aq. TEAA (pH 3.6)/EtOH (20/80) 0.45% aq. TEAA (pH 3.6)/EtOH (80/20) | RPM | [46] |
| epimeric mixtures of fortimicin aminoglycosides | Teicoplanin | Chirobiotic T | 10 mM ammonium formate/MeOH | PIM | [47] | |
| citalopram analogs | Teicoplanin Teicoplanin aglycone, Vancomycin, Ristocetin A | Chirobiotic T Chirobiotic TAG Chirobiotic V Chirobiotic V2 Chirobiotic R | 0.1% aq. TEAA (pH 4.1)/MeOH 0.1% aq. TEAA | RPM PIM | [48] | |
| modafanil | Teicoplanin | Chirobiotic T | MeOH/TEA (100/0.05) | PIM | [49] | |
| Drugs | ibuprofen, carboxyibuprofen, 2-hydroxy ibuprofen, chloramphenicol, ifosfamide, indoprofen, ketoprofen, naproxen, praziquantel | Teicoplanin | Chirobiotic T | aq. 10 mM NH4OAc (pH 4.2)/MeOH (70/30) | RPM | [50] |
| Drugs Peptides | mandelic acid, vanylmandelic acid, phenyllactic acid | Teicoplanin + ionic liquids | Chirobiotic T | MeOH/H2O + borneol or fenchol-based ionic liquids | RPM | [51,52] |
| albuterol | Teicoplanin aglycon Eremomycin | Chirobiotic TAG Nautilus-E | MeOH/MeCN/TEA/AcOH (90/10/0.05/0.05) MeOH/MeCN/TEA/AcOH (80/20/0.075/0.025) | PIM | [53] | |
| tofisopam | Teicoplanin Teicoplanin aglycone, | Chirobiotic T Chirobiotic TAG | 0.1% TEAA (pH 4.1)/MeOH | RPM | [54] | |
| primaquine, tafenoquine, flumequine, lomefloxacine, ofloxacin, qunacrine | Teicoplanin | Chirobiotic T | MeOH/MeCN/water/TEA (70/10/20/0.01); (60/30/10/0.1) and (50/30/20/01) | PIM | [55] | |
| primaquine, quinacrine, tafenoquine | Ristocetin A | Chirobiotic R | MeOH/MeCN/water/TEA (70/10/20/0.1); (60/30/10/0.1) | PIM | [56] | |
| carnosine | Teicoplanin | Chirobiotic T | aq. formic acid/MeOH (80/20–20/80), pHa 3.1–3.8 20 mM ammonium formate/MeOH (40/60), pHa 4.5 | RPM | [57,58] | |
| pyrroloquinolo-ne analogs | Ristocetin A | Nautilus-R | water/MeCN (65/35) | RPM | [59] | |
| pantoprazole | Teicoplanin aglycon Teicoplanin Ristocetin A Vancomycin | Chirobiotic TAG Chirobiotic T Chirobiotic R Chirobiotic V | aq. 20 mM NH4OAc/MeOH (40/60) | RPM | [60] | |
| Leu–Leu, Gly–Leu, Leu–Gly | Teicoplanin Ristocetin A | Chirobiotic T Chirobiotic R | aq. 0.097 M AcOH + 0.003 M NH4OAc (pH 3.85)/MeCN aq. 0.003 M AcOH + 0.097 M NH4OAc (pH 6.80)/MeCN | RPM | [61] | |
| Peptides Amino acids | Ala–Ala, Leu–Leu, Gly–Leu, Leu–Gly | Ristocetin A | Chirobiotic R | aq. 0.0002 M NH4OAc/MeOH (100/0–10/90) | RPM | [62] |
| Leu–Leu, Gly–Gly | Ristocetin A | Chirobiotic R | aq. 0.0002 M NH4OAc/MeOH (90/10) | RPM | [63] | |
| Ala–Ala, Gly–Leu, Leu–Gly | Ristocetin A Ristocetin A | Chirobiotic R Nautilus-R | aq. 100 mM NH4OAc/MeOH (60/40) | RPM | [64] | |
| Phe, Tyr, Trp | Teicoplanin | Chirobiotic T2 | CO2/(MeOH/water) 60/(90/10) | SFC | [65] | |
| Miscellenous | 67 racemates: amino acids, β-blockers, profens, pesticides, etc. | Teicoplanin Teicoplanin aglycon Vancomycin | Chirobiotic T Chirobiotic TAG Chirobiotic V2 | CO2/MeOH 90/10; CO2/MeOH (90/10) + 0.1% formic acid or diethylamine in CO2 n-heptane/EtOH (90/10) | SFC NPM | [66] |
| Racemates | Selector | Column Characteristics Trade Mark Particle Size | The Most Effective Mobile Phase (v/v/v) | Mode | Reference |
|---|---|---|---|---|---|
| 60 pairs of enantiomers of: amino acids, peptides, primary amines, β-blockers, thalidomide, nicardipine, proglumide, coumachlor, warfarin, mianserin, etc. | Teicoplanin Teicoplanin aglycone, Vancomycin | TeicoShell, SPP, 2.7-μm TagShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm | water/MeOH (99/1–10/90) aq. 1.0% TEAA (pH 4.1)/MeCN (80/20) MeOH/AcOH/TEA (100/0.15/0.05) | RPM NPM PIM | [16] |
| amino acids, β-blockers, oxazolidinones, mandelic acid, coumachlor, proglumide, thalidomide, warfarin, mianserin | Teicoplanin Teicoplanin aglycone, Vancomycin | Titan-T, FPP-NPSD, 1.9-μm Titan-TAG, FPP-NPSD, 1.9-μm Titan-V, FPP-NPSD, 1.9-μm | n-heptane/EtOH (80/20) water/MeOH (40/60–20/80) 0.1% TEAA (pH 4.1)/MeCN (80/20) 100% MeOH MeOH/MeCN/AcOH/TEA (45/55/0.3/0.2) and (40/60/0.3/0.2) CO2/MeOH/TFA/TEA (71/29/0.1/0.1) or (60/40/0.1/0.1) | NPM RPM POM PIM SFC | [67] |
| Met, Val, Leu, Ala, Nval, Nleu | Teicoplanin | SPP, sub-2-μm | EtOH/water (80/20) and (90/10) MeOH/water (90/10) | RPM | [68] |
| DNPyr–Leu, DNPyr–Nval, N-acetyl-Ala, N-3,5-DNB–Leu, 4-methyl-5-phenyl-2-oxazolidinone | Teicoplanin | SPP, 2.7-μm | 100% MeOH aq. 20 mM NH4HCOO/MeOH (40/60) or (30/70) aq. 5 mM NH4HCOO/MeOH/MeCN (40//40/20) | POM RPM PIM | [69] |
| fluorinated, desfluorinated analytes: ofloxacin, cipro-floxacin, ezitimibe, paro-xetine, voriconazole, aprepitant, atorvastatin | Teicoplanin Vancomycin | TeicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm | MeOH/MeCN/TFA/TEA (10/90/0.3/0.2) MeOH/MeCN/TFA/TEA (50/958/0.3/0.2) | PIM | [70] |
| enkephalin, bradykinin, vasopressin, LHRH peptides triptic digest of equine apomyoglobin | Teicoplanin Teicoplanin aglycone | TeicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm | 2.5–50 mM NH4HCOO (pH 3.2)/MeCN/ (65/35, 30/70) 50 mM NH4HCOO (pH 3.2)/MeOH (50/50) 50 mM NH4HCOO (pH 3.2)/THF (90/10) or (80/20) | RPM | [71] |
| nicotine | modified teicoplanin | NicoShell, SPP, 2.7-μm | 0.1% ammoniumtrifluoro acetate in MeOH | RPM | [72] |
| tobacco alkaloids synthetic tobacco deri-vatives tobacco metabolites (E/Z)-tobacco-nitrosamines | Teicoplanin Teicoplanin (modified) Vancomycin | TeicoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm | 0.025–0.5 wt% HCOONH4 in MeOH MeOH/MeCN/AcOH/NH4OH aq. 16 mM HCOONH4/MeOH aq. 16 mM HCOONH4/EtOH aq. 16 mM HCOONH4/MeCN | PIM POM RPM | [73] |
| N-protected amino acids, α-aryloxy acids, herbicides, anti-inflammantory agents | Teicoplanin | zwitterionic phases UHPC-Titan120-Tzwitt, FPP 1.9-μm | 20 mM aq. NH4OAc/MeOH (15/85) 20 mM aq. NH4OAc/MeCN (15/85) MeOH/MeCN/AcOH/TEA (40/60/0.055/0.03) n-hexane/EtOH (70/30) | RPM HILIC PIM NPM | [74] |
| 2-(4-chloro-phenoxy)-propionic acid d,l-proglumide, danzyl-d,l-Met, Fmoc-d,l-Glu, Z-d,l-Met | Teicoplanin | zwitterionic phases UHPC-Halo-Tzwitt, SPP-2.0-μm UHPC-Halo-Tzwitt,, SPP-2.7-μm UHPC-Titan-Tzwitt, FPP-1.9-μm | aq. 20 mM HCOONH4 (pH 7.5)/MeCN (15/85) | HILIC | [75] |
| haloxyfop, ketolarac, ketoprofen, indoprofen, flunoxaprofen, naproxen, suprofen, ibuprofen, Fmoc-, Boc-, Z-, danzyl-amino acids | Teicoplanin Vancomycin | zwitterionic phases UHPCTitan120-Tzwitt, FPP-1.9-μm UHPC-Titan120-Vzwitt, FPP-1.9-μm anion exchangers UHPC-Titan120-TCOOH, FPP-1.9-μm UHPC- Titan120-VCOOH, FPP-1.9-μm | aq. 10 mM HCOONH4 (pH 6.5)/MeOH (15/85) aq. 20 mM HCOONH4 (pH 6.5)/MeOH (15/85) aq. 15 mM CH3OONH4/MeCN 15/85 aq. 15 mM CH3OONH4 (pH 5.5)/MeCN (40/60) aq. 15 mM CH3OONH4 (pH 5.5)/MeOH (40/60) | RPM HILIC | [76] |
| 4-, 6-, 7-, 8- and 10-hydroxywarfarins, 4-phenylacetic acid, 2-, 3-, 4-, 6-fluorophenyl-acetic acid, 2,4-, 3,5-difluoro-phenylacetic acid | Teicoplanin Vancomycin | 2D-LC Teico-FPP, 1.9-μm Vanco-FPP, 1.9-μm | aq. 5% H3PO4/MeCN 95/5 | RPM | [77] |
| intermediates of verubecestat synthesis | Teicoplanin Teicoplanin (modified) | TeicoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | aq. 0.1% H3PO4/MeCN (70/30) aq. 1.0–2.0% TEAA/MeOH (70/30) aq. 1.0–2.0% TEAA/MeCN (40/60) | RPM | [78] |
| 150 primary-, secondary- and tertiary-amines | Vancomycin Teicoplanin (modified) | VancoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | aq. NH4TFA/MeOH; aq. HCOONH4/MeOH; aq. HCOONH4/MeCN; aq. HCOONH4/EtOH; MeOH/AcOH/NH4OH; MeOH/MeCN/AcOH/TEA; | RPM PIM | [79] |
| amines, amino acids and derivatives; non-steroidal anti-inflammantory drugs, pesticides, nicotine and metabolites | Vancomycin (EDP) Vancomycin Teicoplanin Teicoplanin (modified) | EDP, SPP, 2.7-μm VancoShell, SPP, 2.7-μm TeicoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | 0.1 wt% HCOONH4 in MeOH MeOH/MeCN/AcOH/TEA (40/60/0.3/0.2) aq. 16 mM HCOONH4 (pH 3.6) (30/70) n-hexane/EtOH/TFA/TEA (70/30/0.3/0.2) | PIM POM RPM NPM | [80] |
| 100 pesticides: pyrethroids, fungicides, organophosphates, acylanilides, herbicides, rodenticides, etc. | Teicoplanin Vancomycin Teicoplanin (modified) | TeicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | 0.025–0.5 wt% HCOONH4 in MeOH aq. 16 mM HCOONH4 (pH 3.6)/MeOH (90/10–30/70) aq. 16 mM HCOONH4 (pH 3.6)/MeCN (90/10–20/80) n-hexane/EtOH/TFA/TEA (70/30/0.3/0.2) | PIM RPM NPM | [81] |
| azole compounds: oxazols, thiazols | Teicoplanin Teicoplanin aglycon Vancomycin Teicoplanin (modified) | TeicoShell, SPP, 2.7-μm TagShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | aq. 20 mM HCOONH4 (pH 3–4)/MeOH (85/15–50/50) aq. 16 mM HCOONH4 (pH 3–6)/MeCN (85/15–20/80) MeOH/EtOH (25/75; 50/50) | RPM POM | [82] |
| 100 chiral analytes: amines, derivatized amino acids, nicotine and metabolites, β-blockers, pesticides, drugs etc., | Teicoplanin Vancomycin Teicoplanin (modified) | TeicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | 0.1% TEA in MeOH/CO2 (25/75) 0.1 wt% HCOONH4 in MeOH/CO2 (25/75) 0.1% TFA in MEOH/CO2 (25/75) 0.1% TEA + 0.1–0.3% TFA in MeOH/CO2 (25/75) | SFC | [83] |
| cis-4,5-diphenyl-2-oxazo-lidinone, 2-(4-chloro-phenoxy)propionic acid, fluoxetine, nicardipine, bupivacaine, roglumide | Teicoplanin Vancomycin Teicoplanin (modified) | TeicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | MeOH/CO2 (5/95) 0.1% TEA + 0.1% TFA in MeOH/CO2 (20/80; 15/85) 0.1% TEA + 0.1 % TFA+ 6% water in MeOH/CO2 (20/80) 0.1 wt% HCOONH4 in MeOH/CO2 (20/80) | SFC | [84] |
| phytoalexins, substituted tryptophanes, ketamine derivatives | Teicoplanin Vancomycin | TeicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm | 0.05–0.1% DEA, TEA, TFA or 2-propylamine in MeOH, EtOH, 2-PrOH or 1-PrOH/CO2 (20/80; 60/40; 5/95) | SFC | [85] |
| cis-4,5-diphenyl-2-oxazolidinone, chlorthalidone, 5,5-diphenyl-4-benzyl-2-oxazolidinone, nicotine, bupivacaine, prilocaine, tranylcypromine, amphetamine, venlafaxine, tryptophan, 1,2,2-triphenylethylamine, 2-chloro-indan-1-ylamine, disopyramide, tetramisole, fenoprofen | Teicoplanin Vancomycin Teicoplanin (modified) | TeicoShell, SPP, 2.7-μm VancoShell, SPP, 2.7-μm NicoShell, SPP, 2.7-μm | “190” EtOH/CO2 (20/80; 25/75) 0.1% TEA in “190” EtOH/CO2 (40/60; 20/80) 0.1% TEA + 0.1% TFA in “190” EtOH/CO2 (20/80; 25/75) 0.2% TEA + 0.3% TFA in “190” EtOH/CO2 (25/75; 20/80; 15/85) | SFC | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkecz, R.; Tanács, D.; Péter, A.; Ilisz, I. Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors. Molecules 2021, 26, 3380. https://doi.org/10.3390/molecules26113380
Berkecz R, Tanács D, Péter A, Ilisz I. Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors. Molecules. 2021; 26(11):3380. https://doi.org/10.3390/molecules26113380
Chicago/Turabian StyleBerkecz, Róbert, Dániel Tanács, Antal Péter, and István Ilisz. 2021. "Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors" Molecules 26, no. 11: 3380. https://doi.org/10.3390/molecules26113380
APA StyleBerkecz, R., Tanács, D., Péter, A., & Ilisz, I. (2021). Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors. Molecules, 26(11), 3380. https://doi.org/10.3390/molecules26113380






