Daptomycin: A Novel Macrocyclic Antibiotic as a Chiral Selector in an Organic Polymer Monolithic Capillary for the Enantioselective Analysis of a Set of Pharmaceuticals
Abstract
1. Introduction
2. Results
2.1. Preparation and Characterization of the Monoliths
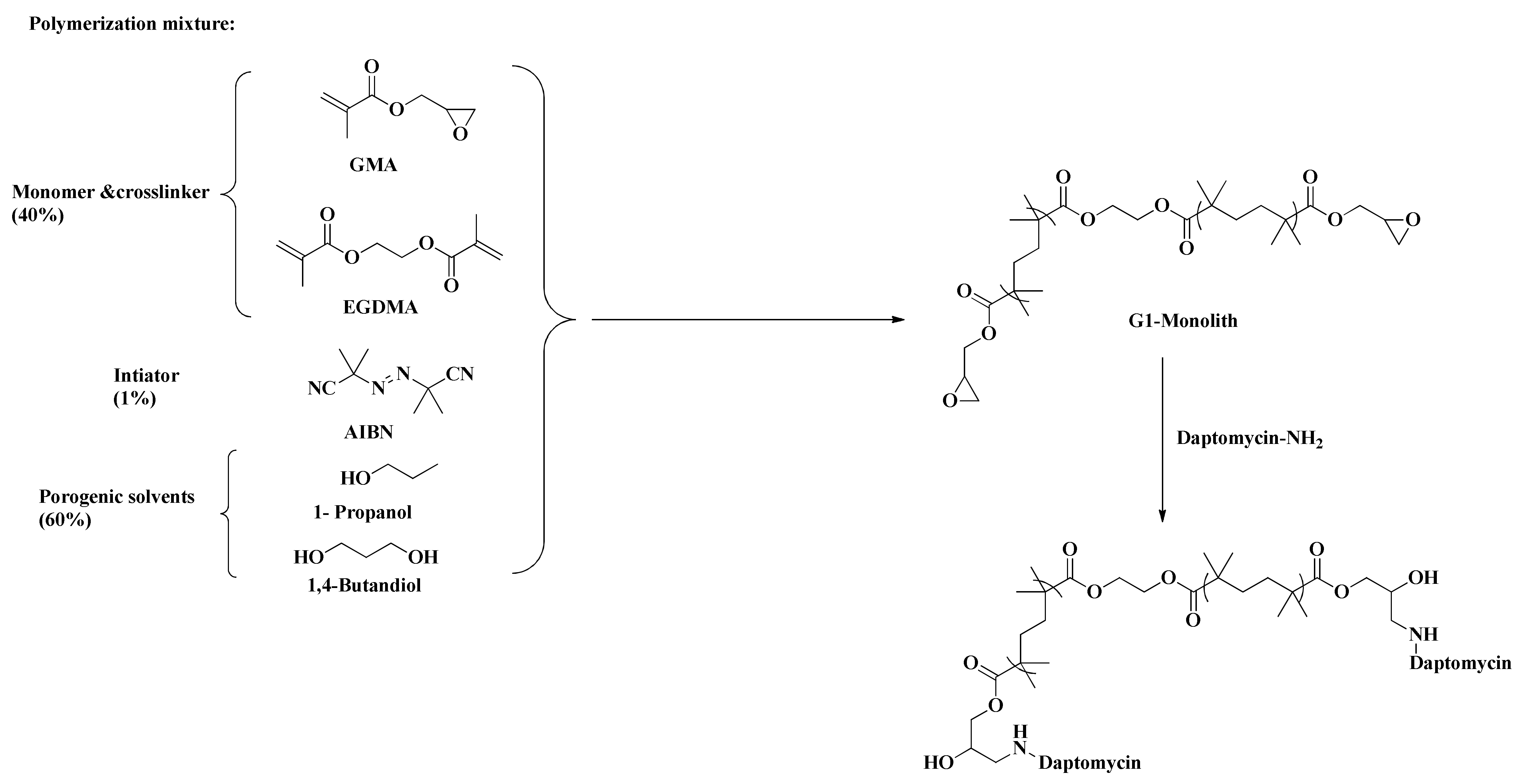
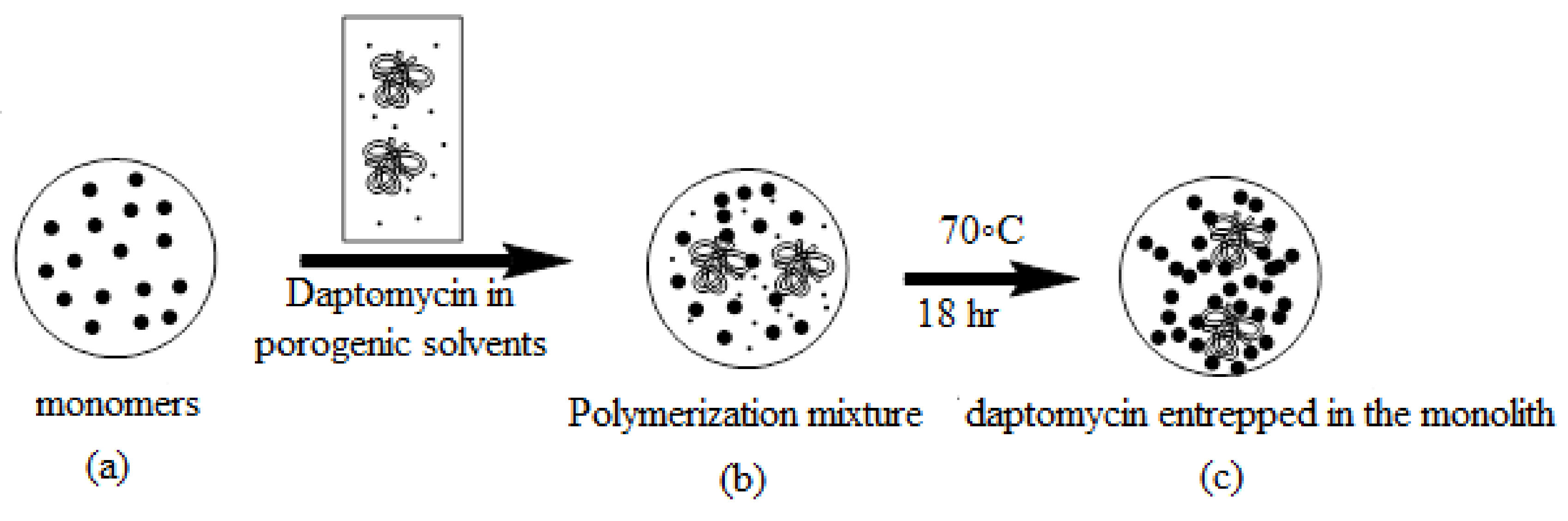
2.1.1. Preparation of the Monoliths
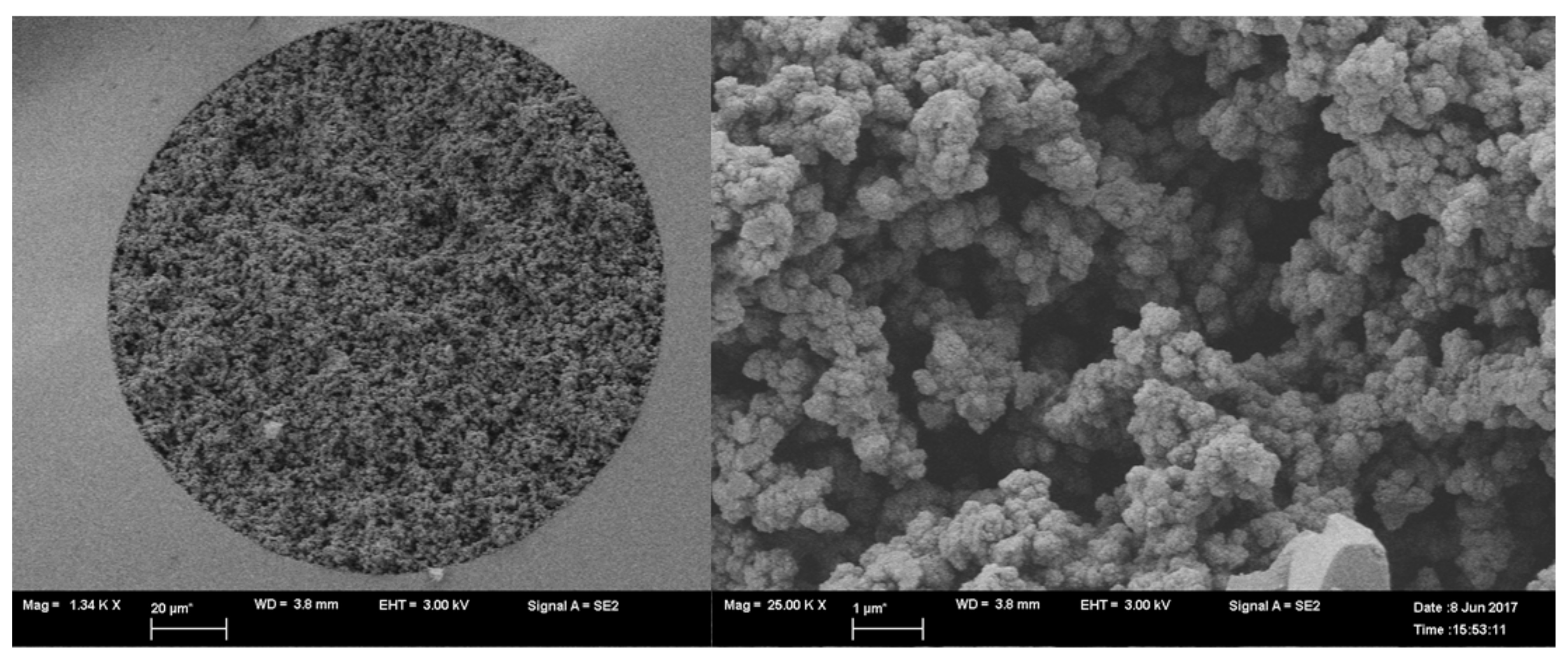
2.1.2. Imaging by SEM and Studying the Surface Characteristics of the Monoliths
2.1.3. Calculation of Total Porosity
2.1.4. Mechanical Stability
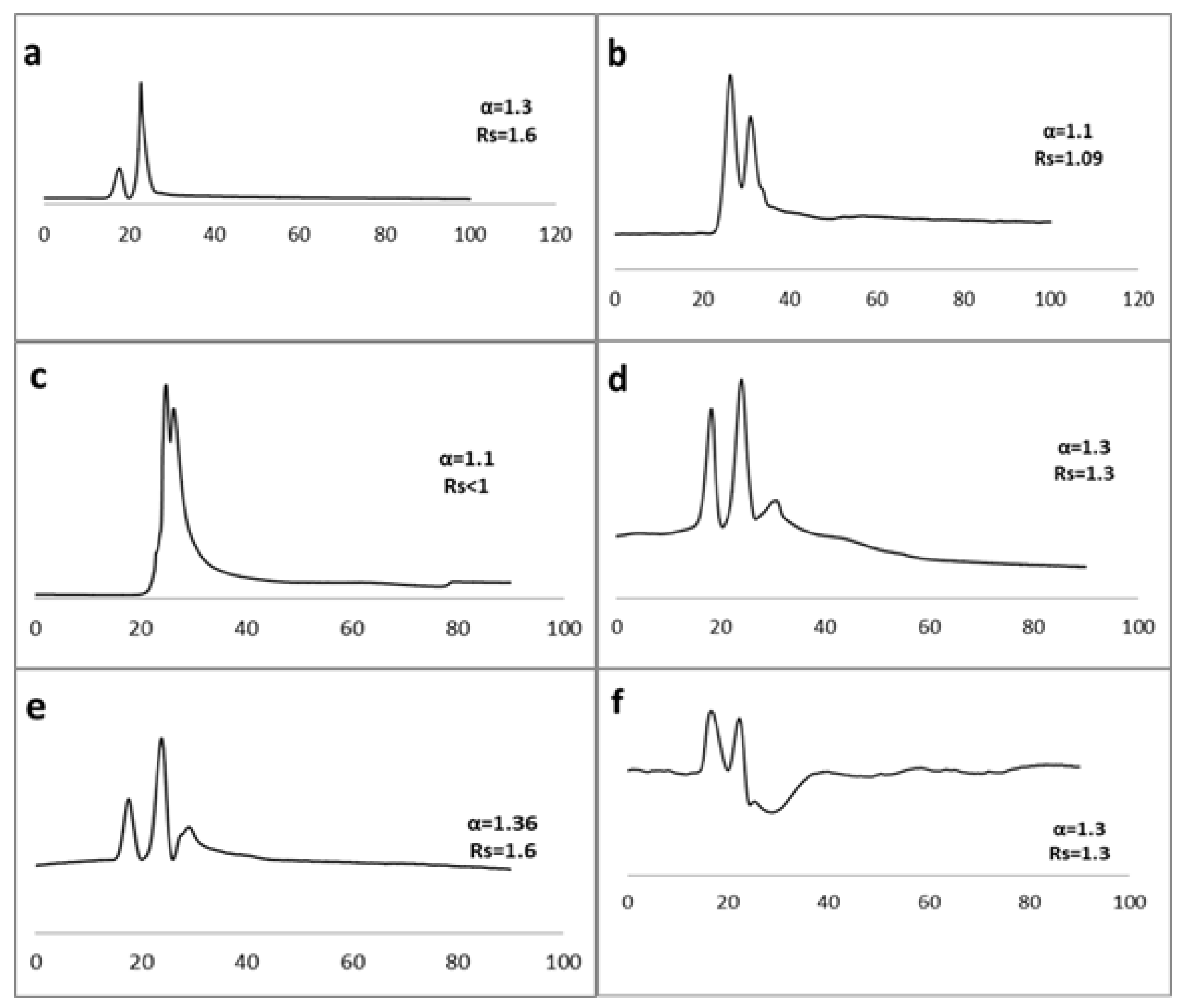
2.2. Enantioseparation of Racemic Pharmaceutical Drugs
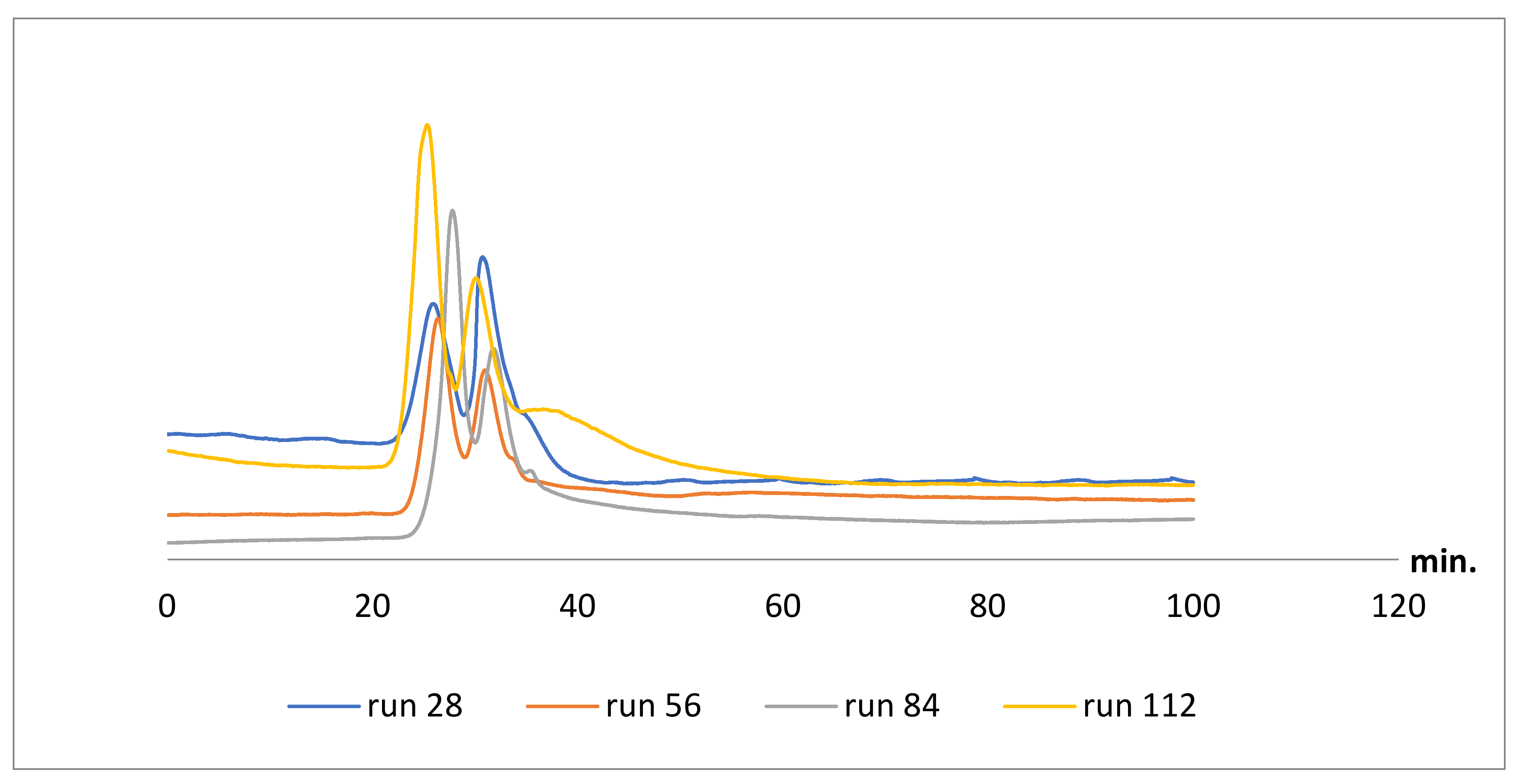
2.3. Columns Repeatability
3. Discussion
4. Experimental
4.1. Reagents and Materials
4.2. Functioning and Characterization of the Monolithic Capillaries
4.2.1. Activation of the Empty Fused Silica Capillaries
4.2.2. Preparation of Immobilized Daptomycin-Based Monolithic Capillary
4.2.3. Preparation of Daptomycin Functionalized Monomer (Encapsulation)
4.2.4. Imaging the Prepared Monoliths by SEM
4.3. Preparation of Stock Solutions and Samples
4.4. HPLC Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fouad, A.; Shaykoon, M.S.A.; Ibrahim, S.M.; El-Adl, S.M.; Ghanem, A. Colistin Sulfate Chiral Stationary Phase for the Enantioselective Separation of Pharmaceuticals Using Organic Polymer Monolithic Capillary Chromatography. Molecules 2019, 24, 833. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.W.; Nair, U.B. Capillary electrophoretic enantioseparations using macrocyclic antibiotics as chiral selectors. Electrophoresis 1997, 18, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Iii, A.B.F. Chiral separations using the macrocyclic antibiotics: A review. J. Chromatogr. A 2001, 906, 73–89. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Maia, A.S.; Cass, Q.; Tiritan, M.E. Enantioseparation of chiral pharmaceuticals in biomedical and environmental analyses by liquid chromatography: An overview. J. Chromatogr. B 2014, 968, 8–21. [Google Scholar] [CrossRef]
- Staroverov, S.; Kuznetsov, M.; Nesterenko, P.; Vasiarov, G.; Katrukha, G.; Fedorova, G. New chiral stationary phase with macrocyclic glycopeptide antibiotic eremomycin chemically bonded to silica. J. Chromatogr. A 2006, 1108, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Petrusevska, K.; Kuznetsov, M.A.; Gedicke, K.; Meshko, V.; Staroverov, S.M.; Seidel-Morgenstern, A. Chromatographic enantioseparation of amino acids using a new chiral stationary phase based on a macrocyclic glycopeptide antibiotic. J. Sep. Sci. 2006, 29, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Qiu, H.X.; Staroverov, S.M.; Kuznestov, M.A.; Armstrong, D.W. Chiral Recognition with Macrocyclic Glycopeptides: Mechanisms and Applications. In Chiral Recognition in Separation Methods; Springer: Berlin/Heidelberg, Germany, 2010; pp. 203–222. [Google Scholar]
- Zhang, L.; Gedicke, K.; Kuznetsov, M.; Staroverov, S.; Seidel-Morgenstern, A. Application of an eremomycin-chiral stationary phase for the separation of dl-methionine using simulated moving bed technology. J. Chromatogr. A 2007, 1162, 90–96. [Google Scholar] [CrossRef]
- Kuznetsov, M.A.; Nesterenko, P.N.; Vasiyarov, G.G.; Staroverov, S.M. Sorbents with immobilized glycopeptide antibiotics for separating optical isomers by high-performance liquid chromatography. Appl. Biochem. Microbiol. 2006, 42, 536–544. [Google Scholar] [CrossRef]
- Gause, G.F.; Brazhnikova, M.G.; Lomakina, N.N.; Berdnikova, T.F.; Fedorova, G.B.; Tokareva, N.L.; Borisova, V.N.; Batta, G.Y. Eremomycin-new glycopeptide antibiotic: Chemical properties and structure. J. Antibiot. 1989, 42, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Harvanová, M.; Gondová, T. New enantioselective LC method development and validation for the assay of modafinil. J. Pharm. Biomed. Anal. 2017, 138, 267–271. [Google Scholar] [CrossRef]
- Fedorova, I.A.; Shapovalova, E.N.; Shpigun, O.A.; Staroverov, S.M. Bovine serum albumin adsorbed on eremomycin and grafted on silica as new mixed-binary chiral sorbent for improved enantioseparation of drugs. J. Food Drug Anal. 2016, 24, 848–854. [Google Scholar] [CrossRef]
- Ilisz, I.; Grecsó, N.; Forró, E.; Fülöp, F.; Armstrong, D.W.; Péter, A. High-performance liquid chromatographic separation of paclitaxel intermediate phenylisoserine derivatives on macrocyclic glycopeptide and cyclofructan-based chiral stationary phases. J. Pharm. Biomed. Anal. 2015, 114, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Hroboňová, K.; Lehotay, J.; Čižmárik, J. HPLC Enantioseparation of Phenylcarbamic Acid Derivatives by Using Macrocyclic Chiral Stationary Phases. Nova Biotechnol. Chim. 2016, 15, 12–22. [Google Scholar] [CrossRef]
- Maia, A.S.; Castro, P.M.; Tiritan, M.E. Integrated liquid chromatography method in enantioselective studies: Biodegradation of ofloxacin by an activated sludge consortium. J. Chromatogr. B 2016, 1029–1030, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.A.; Shapovalova, E.N.; Shpigun, O.A. Separation of β-blocker and amino acid enantiomers on a mixed chiral sorbent modified with macrocyclic antibiotics eremomycin and vancomycin. J. Anal. Chem. 2017, 72, 76–82. [Google Scholar] [CrossRef]
- Miao, V.; Coëffet-LeGal, M.-F.; Brian, P.; Brost, R.; Penn, J.; Whiting, A.; Martin, S.; Ford, R.; Parr, I.; Bouchard, M.; et al. Daptomycin biosynthesis in Streptomyces roseosporus: Cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology 2005, 151, 1507–1523. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef]
- Pogliano, N.; Silverman, J.A. Daptomycin-Mediated Reorganization of Membrane Architecture Causes Mislocalization of Essential Cell Division Proteins. J. Bacteriol. 2012, 194, 4494–4504. [Google Scholar] [CrossRef]
- Baltz, R.H. Daptomycin: Mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 2009, 13, 144–151. [Google Scholar] [CrossRef]
- Fouad, A.; Marzouk, A.A.; Ibrahim, S.M.; Sobhy, M.; Ghanem, A. Functionalized polymer monoliths with carbamylated amylose for the enantioselective reversed phase nano-liquid chromatographic separation of a set of racemic pharmaceuticals. J. Chromatogr. A. 2017, 1515, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Schaller, D.; Hilder, E.F.; Haddad, P.R. Separation of antidepressants by capillary electrophoresis with in-line solid-phase extraction using a novel monolithic adsorbent. Anal. Chim. Acta 2006, 556, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Pittler, E.; Schmid, M.G. Enantioseparation of dansyl amino acids by HPLC on a monolithic column dynamically coated with a vancomycin derivative. Biomed. Chromatogr. 2010, 24, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Claude Guillaume, Y.; André, C. Fast enantioseparation by HPLC on a modified carbon nanotube monolithic stationary phase with a pyrenyl aminoglycoside derivative. Talanta 2013, 115, 418–421. [Google Scholar] [CrossRef]
- Hsieh, M.-L.; Chau, L.-K.; Hon, Y.-S. Single-step approach for fabrication of vancomycin-bonded silica monolith as chiral stationary phase. J. Chromatogr. A 2014, 1358, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.; Ibrahim, D.; Adly, F.G.; Ghanem, A. An insight into chiral monolithic stationary phases for enantioselective high-performance liquid chromatography applications. J. Sep. Sci. 2019, 42, 2303–2340. [Google Scholar] [CrossRef]
- Fouad, A.; Ghanem, A. Immobilized Chiral Selectors on Monolithic High-Performance Liquid Chromatography Columns. In Advances in Chromatography; Taylor & Francis group: London, UK, 2017; pp. 111–167. [Google Scholar]
- Mallik, R.; Jiang, T.; Hage, D.S. High-Performance Affinity Monolith Chromatography: Development and Evaluation of Human Serum Albumin Columns. Anal. Chem. 2004, 76, 7013–7022. [Google Scholar] [CrossRef]
- Křvenková, J.; Bílková, Z.; Foret, F. Chararacterization of a monolithic immobilized trypsin microreactor with on-line coupling to ESI-MS. J. Sep. Sci. 2005, 28, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Adly, F.G.; Sokerik, Y.; Antwi, N.Y.; Shenashen, M.A.; El-Safty, S.A. Trimethyl- β-cyclodextrin-encapsulated monolithic capillary columns: Preparation, characterization and chiral nano-LC application. Talanta 2017, 169, 239–248. [Google Scholar] [CrossRef]
- Yusuf, K.; Aqel, A.; ALOthman, Z.; Badjah-Hadj-Ahmed, A.Y. Preparation and characterization of alkyl methacrylate-based monolithic columns for capillary gas chromatography applications. J. Chromatogr. A 2013, 1301, 200–208. [Google Scholar] [CrossRef]
- Gusev, I.; Huang, X.; Horváth, C. Capillary columns with in situ formed porous monolithic packing for micro high-performance liquid chromatography and capillary electrochromatography. J. Chromatogr. A 1999, 855, 273–290. [Google Scholar] [CrossRef]
- Bragg, W.; Shamsi, S.A. A novel positively charged achiral co-monomer for β-cyclodextrin monolithic stationary phase: Improved chiral separation of acidic compounds using capillary electrochromatography coupled to mass spectrometry. J. Chromatogr. A 2012, 1267, 144–155. [Google Scholar] [CrossRef]
- Urban, J.; Jandera, P. Recent advances in the design of organic polymer monoliths for reversed-phase and hydrophilic interaction chromatography separations of small molecules. Anal. Bioanal. Chem. 2012, 405, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Staňková, M.; Škeříková, V.; Urban, J. Cross-linker effects on the separation efficiency on (poly) methacrylate capillary monolithic columns. Part, I. Reversed-phase liquid chromatography. J. Chromatogr. A 2013, 1274, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xie, C.; Yu, Z.; Kong, L.; Ye, M.; Zou, H. Monolithic enantiomer-selective stationary phases for capillary electrochromatography. J. Sep. Sci. 2006, 29, 1332–1343. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Kubota, T.; Ikai, T.; Yamamoto, C.; Kamigaito, M.; Tanaka, N.; Nakanishi, K.; Okamoto, Y. High-performance liquid chromatographic enantioseparations on capillary columns containing crosslinked polysaccharide phenylcarbamate derivatives attached to monolithic silica. J. Sep. Sci. 2006, 29, 1988–1995. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, M.; Wu, R.; Dong, J.; Ou, J.; Zou, H. Preparation of Perphenylcarbamoylated β-Cyclodextrin-silica Hybrid Monolithic Column with “One-Pot” Approach for Enantioseparation by Capillary Liquid Chromatography. Anal. Chem. 2011, 83, 3616–3622. [Google Scholar] [CrossRef]
- Dermaux, A.; Lynen, F.; Sandra, P. Chiral Capillary Electrochromatography on a Vancomycin Stationary Phase. J. High. Resolut. Chromatogr. 1998, 21, 575–576. [Google Scholar] [CrossRef]
- Karlsson, C.; Karlsson, L.; Armstrong, D.W.; Owens, P.K. Evaluation of a vancomycin chiral stationary phase in capillary electrochromatography using polar organic and reversed-phase modes. Anal. Chem. 2000, 72, 4394–4401. [Google Scholar] [CrossRef] [PubMed]
- Desiderio, C.; Aturki, Z.; Fanali, S. Use of vancomycin silica stationary phase in packed capillary electrochromatography I. Enantiomer separation of basic compounds. Electrophoresis 2001, 22, 535–543. [Google Scholar] [CrossRef]
- Fanali, S.; Catarcini, P.; Quaglia, M.G. Use of vancomycin silica stationary phase in packed capillary electrochromatography: III. Enantiomeric separation of basic compounds with the polar organic mobile phase. Electrophoresis 2002, 23, 477–485. [Google Scholar] [CrossRef]
- Karlsson, C.; Wikström, H.; Armstrong, D.W.; Owens, P.K. Enantioselective reversed-phase and non-aqueous capillary electrochromatography using a teicoplanin chiral stationary phase. J. Chromatogr. A 2000, 897, 349–363. [Google Scholar] [CrossRef]
- Wikström, H.; Svensson, L.; Torstensson, A.; Owens, P. Immobilisation and evaluation of a vancomycin chiral stationary phase for capillary electrochromatography. J. Chromatogr. A 2000, 869, 395–409. [Google Scholar] [CrossRef]
- Si Ahmed, K.; Tazerouti, F.; Badjah-Hadj-Ahmed, A.Y.; Meklati, B.Y. Preparation and chromatographic properties of a multimodal chiral stationary phase based on phenyl-carbamate-propyl-β-CD for HPLC. J. Sep. Sci. 2007, 30, 2025–2036. [Google Scholar] [CrossRef]




| Column | εT (%) |
|---|---|
| D1 | 21.75 ± (1.6) |
| D2 | 31.25 ± (1.13) |
| D3 | 33.1 ± (1.07) |
| Column | Nitrogen (% w/w) |
|---|---|
| G | 1.8 |
| D3 | 2.15 |
| Column D2 | ||||
|---|---|---|---|---|
| Phase | Mobile Phase | Drug | Separation Factor (α) | Resolution (Rs) |
| Reversed Phase | ACN:water 35:65 | Fluribiprofen 21 | 1.4 | 1.8 |
| Trifloroethanol 41 | 1.3 | 1.1 | ||
| Pindolol 7 | 1.3 | 1.45 | ||
| Atenolol 3 | 1.1 | <1 | ||
| Naftopidil 8 | 1.3 | 1.3 | ||
| Ampicillin 25 | 1.1 | <1 | ||
| 6 hydroxy flavanone 38 | 1.2 | <1 | ||
| ACN:water 20:80 | Trifloroethanol 41 | 1.37 | 1.5 | |
| Flavanone 39 | 1.3 | 1.3 | ||
| Atenolol 3 | 1.1 | <1 | ||
| Ampicillin 25 | 1.3 | 1.1 | ||
| Pindolol 7 | 1.1 | 1 | ||
| ACN:water 10:90 | Flavanone 39 | 1.3 | 1.4 | |
| Ibuprofen 23 | 1.3 | 1.4 | ||
| Indooprofen 22 | 1.3 | 1.75 | ||
| Atenolol 3 | 1.3 | 1.45 | ||
| Aminoglutethimide 33 | 1.59 | 1 | ||
| Naftopidil 8 | 1.27 | 1.5 | ||
| 1-Indanol 49 | 1.1 | <1 | ||
| Pindolol 7 | 1.4 | 1.9 | ||
| Acebutolol 6 | 1.26 | 1.3 | ||
| Normatenphrine 30 | 1.3 | 1.27 | ||
| 4-hydroxymandelic acid 44 | 1.34 | 1.6 | ||
| ACN:water 5:95 | Atenolol 3 | 1.2 | 1.17 | |
| Aminoglutethimide 33 | 1.4 | 1.6 | ||
| Ampicillin 25 | 1.3 | 1.2 | ||
| Pindolol 7 | 1.3 | 1.6 | ||
| Column D3 | ||||
|---|---|---|---|---|
| Phase | Mobile Phase | Drug | Separation Factor (α) | Resolution (Rs) |
| Reversed Phase | Methanol:water 40:60 | Hexaconazole | 1.14 | 1 |
| Aminoglutethimide 33 | 1.4 | <1 | ||
| Methanol:water 5:95 | O-methoxy mandelic acid 43 | 1.6 | 1.5 | |
| Aminoglutethimide 33 | 1.4 | 1.8 | ||
| Methanol:water 3:97, 1% TFA | Metoprolol 5 | 1.4 | 1.3 | |
| Atenolol 3 | 1.17 | <1 | ||
| ACN:water 35:65 | Fluribiprofen 21 | 1.25 | 1.5 | |
| Propranolol 4 | 1.1 | 1.1 | ||
| Flavanone 39 | 1.2 | 1.44 | ||
| Ibuprofen 23 | 1.8 | 2.1 | ||
| Pindolol 7 | 1.3 | 1.5 | ||
| Naftopidil 8 | 1.3 | 1.23 | ||
| Normatenphrine 30 | 1.16 | 1.25 | ||
| ACN:water 20:80 | Flavanone 39 | 1.2 | 1.3 | |
| Atenolol 3 | 1.1 | <1 | ||
| Tocainide 11 | 1.9 | 1 | ||
| pindolol 7 | 1.17 | 1.09 | ||
| Acebutolol 6 | 1.1 | <1 | ||
| 1-Indanol 49 | 1.36 | 1.55 | ||
| 4-hydroxymandelic acid 43 | 1.3 | 1.55 | ||
| Cizoliritin carbinol 19 | 1.3 | 1.3 | ||
| Atenolol 3 | 1.14 | <1 | ||
| Ibuprofen 23 | 1.3 | 1.4 | ||
| Naftopidil 8 | 1.3 | 1.55 | ||
| Ampicillin 25 | 1.3 | 1.45 | ||
| Pindolol 7 | 1.3 | 2 | ||
| Acebutolol 6 | 1.3 | 1.6 | ||
| ACN:water 5:95 | Carprofen 15 | 1.2 | 1.38 | |
| Metoprolol 5 | 1.1 | <1 | ||
| 1-In danol 49 | 1.36 | 1.64 | ||
| Normatenphrine 30 | 1.3 | 1.3 | ||
| 4-hydroxymandelic acid 43 | 1.3 | 1.3 | ||
| Cizoliritin carbinol 19 | 1.29 | 1.5 | ||
| Ibuprofen 23 | 1.2 | 1.6 | ||
| Pindolol 7 | 1.28 | 1.3 | ||
| ACN:water 5:95, 1% TFA | Thalidomide 40 | 1.29 | 1.27 | |
| Ampicillin 25 | 1.3 | 1.3 | ||
| Normatenphrine 30 | 1.4 | 1.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouad, A.; Marzouk, A.A.; Shaykoon, M.S.A.; Ibrahim, S.M.; El-Adl, S.M.; Ghanem, A. Daptomycin: A Novel Macrocyclic Antibiotic as a Chiral Selector in an Organic Polymer Monolithic Capillary for the Enantioselective Analysis of a Set of Pharmaceuticals. Molecules 2021, 26, 3527. https://doi.org/10.3390/molecules26123527
Fouad A, Marzouk AA, Shaykoon MSA, Ibrahim SM, El-Adl SM, Ghanem A. Daptomycin: A Novel Macrocyclic Antibiotic as a Chiral Selector in an Organic Polymer Monolithic Capillary for the Enantioselective Analysis of a Set of Pharmaceuticals. Molecules. 2021; 26(12):3527. https://doi.org/10.3390/molecules26123527
Chicago/Turabian StyleFouad, Ali, Adel A. Marzouk, Montaser Sh. A. Shaykoon, Samy M. Ibrahim, Sobhy M. El-Adl, and Ashraf Ghanem. 2021. "Daptomycin: A Novel Macrocyclic Antibiotic as a Chiral Selector in an Organic Polymer Monolithic Capillary for the Enantioselective Analysis of a Set of Pharmaceuticals" Molecules 26, no. 12: 3527. https://doi.org/10.3390/molecules26123527
APA StyleFouad, A., Marzouk, A. A., Shaykoon, M. S. A., Ibrahim, S. M., El-Adl, S. M., & Ghanem, A. (2021). Daptomycin: A Novel Macrocyclic Antibiotic as a Chiral Selector in an Organic Polymer Monolithic Capillary for the Enantioselective Analysis of a Set of Pharmaceuticals. Molecules, 26(12), 3527. https://doi.org/10.3390/molecules26123527








