Assessment of Concentrated Liquid Coffee Acceptance during Storage: Sensory and Physicochemical Perspective
Abstract
:1. Introduction
2. Results and Discussion
2.1. Survival Function
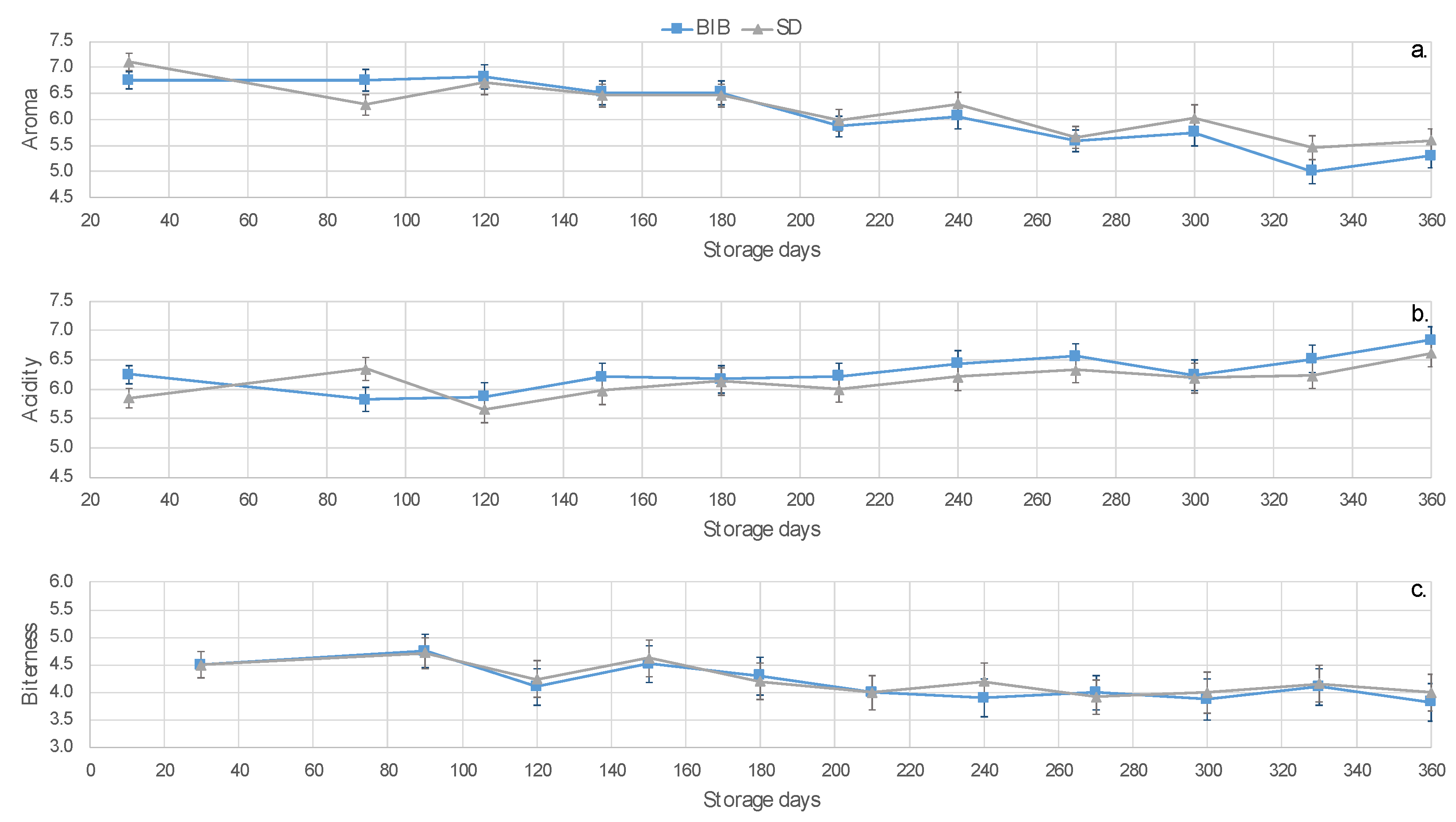
2.2. Sensory Attributes and Acceptability
2.3. Acceptance Modeling
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Concentrated Liquid Coffee (CLC)
3.3. Shelf Life Design
3.4. Coffee Beverages Preparation
3.5. Analytical Methods
3.5.1. Microbiological Analysis
3.5.2. pH and Titratable Acidity
3.5.3. Total Dissolved Solid Content (TDS)
3.5.4. Color
3.5.5. Chlorogenic Acids Content
3.5.6. Carbohydrate Analysis
3.5.7. Fatty Acid Analysis
3.5.8. Organic Acid Analysis
3.5.9. Oxygen and Carbon Dioxide Contents
3.6. Sensory Analysis
3.7. Statistical Analysis
3.7.1. Acceptability Modeling: Survival Function
3.7.2. Sensory QDA: Storage
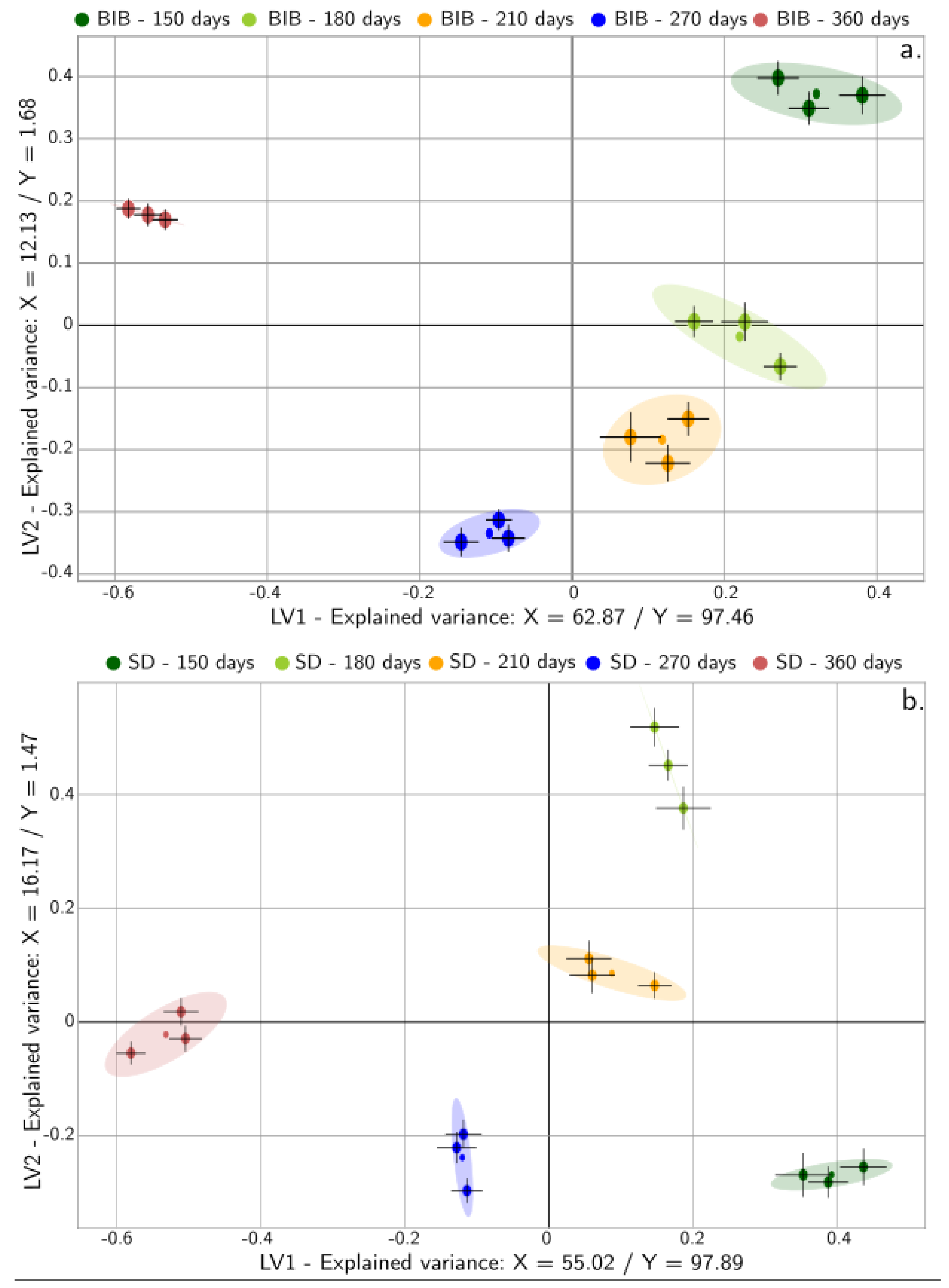
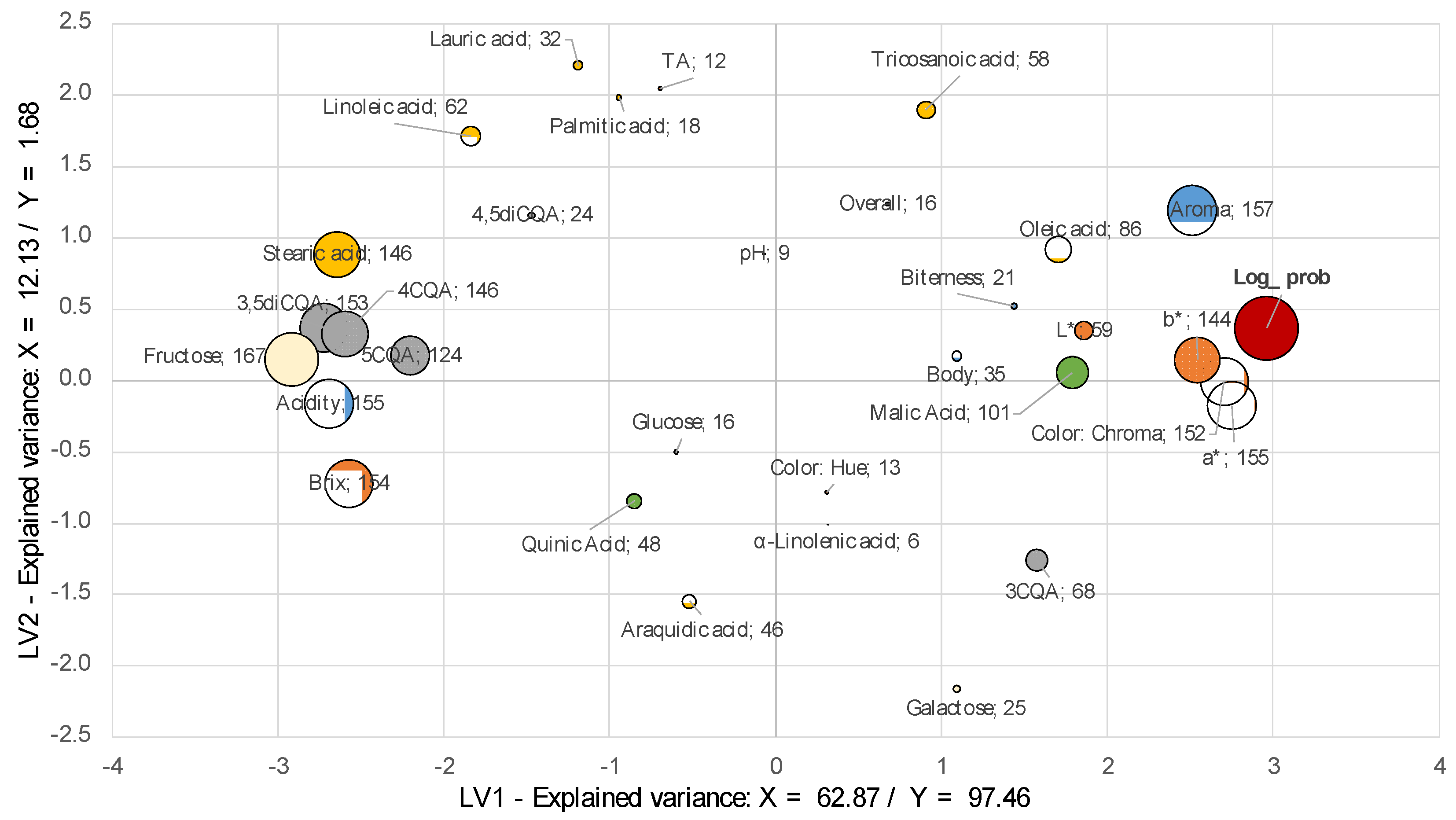
3.7.3. Multivariate Analysis: Physicochemical, Non-Volatile, and Sensory Representation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Global Iced/RTD Coffee Drinks 2019; GlobalData: London, UK, 2019.
- Manzocco, L.; Calligaris, S.; Anese, M.; Nicoli, M.C. The stability and shelf life of coffee products. In The Stability and Shelf Life of Food; Elsevier: London, UK, 2016; pp. 375–398. ISBN 9780081004357. [Google Scholar]
- Manzano, M.; Cocolin, L.; Cragnolini, L.; Nicoli, M.C.; Comi, G.; Language, O. Microbial stability in concentrated coffee brews. Ind. Aliment. 2000, 39, 848–853. [Google Scholar]
- Sopelana, P.; Pérez-Martínez, M.; López-Galilea, I.; de Peña, M.P.; Cid, C. Effect of ultra high temperature (UHT) treatment on coffee brew stability. Food Res. Int. 2013, 50, 682–690. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Investigation of the change in the flavor of a coffee drink during heat processing. J. Agric. Food Chem. 2003, 51, 2674–2678. [Google Scholar] [CrossRef] [PubMed]
- Charles-Bernard, M.; Roberts, D.D.; Kraehenbuehl, K. Interactions between volatile and nonvolatile coffee components. 2. Mechanistic study focused on volatile thiols. J. Agric. Food Chem. 2005, 53, 4426–4433. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Sopelana, P.; De Peña, M.P.; Cid, C. Application of multivariate analysis to the effects of additives on chemical and sensory quality of stored coffee brew. J. Agric. Food Chem. 2008, 56, 11845–11853. [Google Scholar] [CrossRef]
- Manzocco, L.; Nicoli, M.C. Modeling the effect of water activity and storage temperature on chemical stability of coffee brews. J. Agric. Food Chem. 2007, 55, 6521–6526. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Lagazio, C.; Manzocco, L.; Barnabà, M.; Cappuccio, R. Risks and pitfalls of sensory data analysis for shelf life prediction: Data simulation applied to the case of coffee. LWT Food Sci. Technol. 2008, 41, 2070–2078. [Google Scholar] [CrossRef]
- Makri, E.; Tsimogiannis, D.; Dermesonluoglu, E.K.; Taoukisa, P.S. Modeling of Greek coffee aroma loss during storage at different temperatures and water activities. Procedia Food Sci. 2011, 1, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Benković, M.; Tušek, A. Regression models for description of roasted ground coffee powder color change during secondary shelf-life as related to storage conditions and packaging material. Beverages 2018, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Manzocco, L.; Lagazio, C. Coffee brew shelf life modelling by integration of acceptability and quality data. Food Qual. Prefer. 2009, 20, 24–29. [Google Scholar] [CrossRef]
- Man, C.M.D. 15-Shelf-life testing. In Understanding and Measuring the Shelf-Life of Food; Steele, R., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 340–356. ISBN 978-1-85573-732-7. [Google Scholar]
- Nicoli, M.C.; Calligaris, S.; Manzocco, L. Shelf-life testing of coffee and related products: Uncertainties, pitfalls, and perspectives. Food Eng. Rev. 2009, 1, 159–168. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Sopelana, P.; De Peña, M.P.; Cid, C. Effects of refrigeration and oxygen on the coffee brew composition. Eur. Food Res. Technol. 2008, 227, 1633–1640. [Google Scholar] [CrossRef]
- Müller, C.; Hofmann, T. Quantitative studies on the formation of phenol/2-furfurylthiol conjugates in coffee beverages toward the understanding of the molecular mechanisms of coffee aroma staling. J. Agric. Food Chem. 2007, 55, 4095–4102. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Hemmersbach, S.; van’t Slo, G.; Hofmann, T. Synthesis and structure determination of covalent conjugates formed from the sulfury-roasty-smelling 2-furfurylthiol and di- or trihydroxybenzenes and their identification in coffee brew. J. Agric. Food Chem. 2006, 54, 10076–10085. [Google Scholar] [CrossRef]
- Hofmann, T.; Schieberle, P. Chemical interactions between odor-active thiols and melanoidins involved in the aroma staling of coffee beverages. J. Agric. Food Chem. 2002, 50, 319–326. [Google Scholar] [CrossRef]
- Hofmann, T.; Czerny, M.; Calligaris, S.; Schieberle, P. Model studies on the influence of coffee melanoidins on flavor volatiles of coffee beverages. J. Agric. Food Chem. 2001, 49, 2382–2386. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Shelf life extension of sugarcane juice using preservatives and gamma radiation processing. J. Food Sci. 2011, 76, M573–M578. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Ríos-Corripio, M.A.; Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A. Characteristics and uses of novel and conventional preservatives for fruit drinks and beverages. In Preservatives and Preservation Aproaches in Beverages; Grumezescu, A., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 31–67. ISBN 978-0-12-816685-7. [Google Scholar]
- Vara, S.; Karnena, M.K.; Dwarapureddi, B.K. Natural preservatives for nonalcoholic beverages. In Preservatives and Preservation Aproaches in Beverages; Grumezescu, A., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 179–201. ISBN 978-0-12-816685-7. [Google Scholar]
- Asiah, N.; Aqil, M.; Dwiranti, N.S.; David, W.; Ardiansyah, A. Sensory and chemical changes of cold and hot brew arabica coffee at various resting time. Asia Pac. J. Sustain. Agric. Food Energy 2019, 7, 2017–2020. [Google Scholar] [CrossRef]
- Torma, A.; Orbán, C.S.; Bodor, Z.S.; Benedek, C.S. Evaluation of sensory and antioxidant properties of commercial coffee substitutes. Acta Aliment. 2019, 48, 297–305. [Google Scholar] [CrossRef]
- Charles-Bernard, M.; Kraehenbuehl, K.; Rytz, A.; Roberts, D.D. Interactions between volatile and nonvolatile coffee components. 1. Screening of nonvolatile components. J. Agric. Food Chem. 2005, 53, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Schoenauer, S.; Schieberle, P. Structure–odor correlations in homologous series of mercapto furans and mercapto thiophenes synthesized by changing the structural motifs of the key coffee odorant furan-2-ylmethanethiol. J. Agric. Food Chem. 2018, 66, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Millan-Sango, D.; Valdramidis, V.P. Chapter 28—Quantitative assessment of the shelf life of fruit juices. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 557–569. ISBN 978-0-12-802230-6. [Google Scholar]
- Latimer, G.W.; AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Deutsche Institut für Normung. DIN 10767:2015-08. Analysis of Coffee and Coffee Products-Determination of Chlorogenic Acids Content in Roasted Coffee and Soluble Coffee-HPLC Method, 3rd ed.; Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- International Organization for Standardization. Instant Coffee—Determination of Free and Total Carbohydrate Contents-Method Using High Performance Anion-Exchange Chromatography, International Standard, 4th ed.; International Organization for Standardization: Genève, Switzerland, 1995; ISO 11292. [Google Scholar]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Descriptive analysis techniques. In Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-470-67139-9. [Google Scholar]
- Asociación Española de Normalización. UNE.-ISO 6658: Análisis sensorial. In Metodología. Guía General (ISO 6658:2005): Norma Española; AENOR: Madrid, Spain, 2008. [Google Scholar]
- Ballabio, D.; Mauri, A.; Todeschini, R.; Buratti, S. Geographical classification of wine and olive oil by means of classification and influence matrix analysis (CAIMAN). Anal. Chim. Acta 2006, 570, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Hofmann, T.; Schieberle, P.; Grosch, W. Model studies on the oxidative stability of odor-active thiols occurring in food flavors. J. Agric. Food Chem. 1996, 44, 251–255. [Google Scholar] [CrossRef]


| BIB & SD | |||
|---|---|---|---|
| Attributes | Mean Square | F-Value | Pr (>F) |
| Aroma | 35.41 | 57.9 | 0.000 |
| Acidity | 3.20 | 5.2 | 0.024 |
| Bitterness | 3.95 | 6.5 | 0.012 |
| Body | 0.63 | 1.0 | 0.312 |
| Overall | 0.34 | 0.6 | 0.459 |
| Variable | BIB | SD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | VIP | SR | Coefficient | Models | VIP | SR | Coefficient | |||||||
| # Use | Mean | SE | Mean | SE | Mean | SE | # Use | Mean | SE | Mean | SE | Mean | SE | |
| Aroma | 100 | 1.57 | 0.05 | 3.92 | 0.81 | 0.14 | 0.04 | 100 | 1.73 | 0.05 | 3.74 | 1.09 | 0.11 | 0.02 |
| Acidity | 100 | 1.55 | 0.05 | 4.23 | 1.23 | −0.08 | 0.03 | 100 | 1.44 | 0.14 | 1.32 | 0.80 | −0.04 | 0.06 |
| Biterness | 25 | 0.85 | 0.25 | 0.44 | 0.39 | 0.04 | 0.02 | 60 | 0.92 | 0.23 | 0.31 | 0.18 | 0.03 | 0.05 |
| Body | 54 | 0.64 | 0.22 | 0.20 | 0.19 | 0.04 | 0.03 | 19 | 0.62 | 0.28 | 0.15 | 0.14 | 0.02 | 0.02 |
| Overall | 31 | 0.51 | 0.16 | 0.09 | 0.07 | 0.04 | 0.04 | 100 | 1.24 | 0.08 | 0.68 | 0.16 | 0.07 | 0.03 |
| TA | 27 | 0.44 | 0.10 | 0.02 | 0.01 | 0.02 | 0.02 | 51 | 0.32 | 0.06 | 0.01 | 0.01 | 0.04 | 0.01 |
| Brix | 100 | 1.54 | 0.04 | 3.52 | 0.34 | −0.11 | 0.03 | 71 | 0.27 | 0.08 | 0.00 | 0.00 | −0.07 | 0.04 |
| pH | 33 | 0.26 | 0.18 | 0.03 | 0.05 | −0.03 | 0.05 | 26 | 0.82 | 0.10 | 0.22 | 0.07 | −0.05 | 0.02 |
| Color: Chroma | 100 | 1.52 | 0.06 | 4.08 | 1.67 | 0.06 | 0.03 | 100 | 1.46 | 0.07 | 1.27 | 0.23 | 0.09 | 0.01 |
| Color: a* | 100 | 1.55 | 0.07 | 5.41 | 3.94 | 0.07 | 0.03 | 100 | 1.81 | 0.04 | 5.76 | 0.74 | 0.13 | 0.02 |
| Color: b* | 100 | 1.44 | 0.06 | 2.47 | 0.76 | 0.05 | 0.04 | 73 | 1.01 | 0.15 | 0.38 | 0.15 | 0.06 | 0.02 |
| Color: L* | 56 | 1.06 | 0.07 | 0.63 | 0.18 | 0.02 | 0.06 | 48 | 0.65 | 0.20 | 0.13 | 0.09 | 0.04 | 0.02 |
| Color: Hue | 44 | 0.30 | 0.14 | 0.02 | 0.03 | 0.03 | 0.05 | 48 | 0.88 | 0.13 | 0.27 | 0.10 | 0.05 | 0.01 |
| 3CQA | 81 | 0.83 | 0.11 | 0.30 | 0.11 | 0.06 | 0.03 | 34 | 1.03 | 0.05 | 0.37 | 0.05 | −0.03 | 0.02 |
| 3,5diCQA | 100 | 1.53 | 0.04 | 3.67 | 0.68 | −0.07 | 0.02 | 98 | 1.17 | 0.06 | 0.55 | 0.10 | −0.07 | 0.02 |
| 4CQA | 100 | 1.46 | 0.06 | 2.68 | 0.98 | −0.07 | 0.02 | 100 | 1.49 | 0.07 | 1.39 | 0.26 | 0.09 | 0.02 |
| 4,5diCQA | 31 | 0.76 | 0.15 | 0.24 | 0.11 | 0.01 | 0.04 | 78 | 0.76 | 0.09 | 0.18 | 0.05 | −0.07 | 0.02 |
| 5CQA | 100 | 1.24 | 0.07 | 1.10 | 0.31 | −0.05 | 0.02 | 98 | 1.24 | 0.13 | 0.72 | 0.25 | −0.07 | 0.02 |
| Lauric acid | 55 | 0.58 | 0.05 | 0.10 | 0.01 | 0.00 | 0.02 | 38 | 0.14 | 0.05 | 0.00 | 0.00 | 0.00 | 0.01 |
| Palmitic acid | 40 | 0.46 | 0.05 | 0.06 | 0.01 | 0.00 | 0.01 | 59 | 0.92 | 0.03 | 0.27 | 0.01 | 0.07 | 0.01 |
| Stearic acid | 100 | 1.46 | 0.04 | 2.36 | 0.11 | −0.09 | 0.02 | 100 | 1.62 | 0.03 | 2.17 | 0.12 | −0.10 | 0.01 |
| Oleic acid | 82 | 1.05 | 0.04 | 0.57 | 0.05 | 0.08 | 0.02 | 23 | 0.52 | 0.02 | 0.07 | 0.00 | 0.02 | 0.02 |
| Linoleic acid | 65 | 0.95 | 0.02 | 0.41 | 0.02 | −0.04 | 0.01 | 100 | 1.20 | 0.03 | 0.58 | 0.01 | −0.10 | 0.02 |
| α-Linolenic acid | 29 | 0.21 | 0.11 | 0.00 | 0.00 | −0.02 | 0.03 | 64 | 0.23 | 0.03 | 0.01 | 0.00 | 0.01 | 0.02 |
| Araquidic acid | 95 | 0.49 | 0.04 | 0.06 | 0.00 | −0.06 | 0.02 | 96 | 0.55 | 0.04 | 0.07 | 0.01 | −0.08 | 0.02 |
| Tricosanoic acid | 82 | 0.71 | 0.04 | 0.17 | 0.01 | 0.08 | 0.02 | 83 | 0.80 | 0.03 | 0.19 | 0.01 | 0.07 | 0.02 |
| Fructose | 100 | 1.67 | 0.04 | 12.03 | 1.02 | −0.12 | 0.03 | 100 | 1.88 | 0.04 | 11.78 | 0.74 | −0.14 | 0.01 |
| Galactose | 46 | 0.54 | 0.04 | 0.08 | 0.01 | 0.01 | 0.02 | 58 | 0.53 | 0.03 | 0.07 | 0.00 | −0.04 | 0.01 |
| Glucose | 40 | 0.39 | 0.03 | 0.05 | 0.01 | −0.03 | 0.02 | 40 | 0.71 | 0.02 | 0.13 | 0.00 | −0.03 | 0.01 |
| Malic acid | 96 | 1.05 | 0.03 | 0.56 | 0.03 | 0.08 | 0.02 | 100 | 1.16 | 0.03 | 0.53 | 0.01 | 0.09 | 0.01 |
| Quinic acid | 82 | 0.58 | 0.03 | 0.11 | 0.01 | −0.06 | 0.02 | 47 | 0.26 | 0.08 | 0.01 | 0.00 | 0.01 | 0.01 |
| BIB | SD | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| RMSE Train | 0.106 | 0.043 | 0.067 | 0.024 |
| R2 Train | 0.996 | 0.005 | 0.999 | 0.001 |
| RMSE Test | 0.102 | 0.039 | 0.064 | 0.012 |
| R2 Test | 0.987 | 0.010 | 0.992 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero, M.; Velásquez, S.; Zapata, J.; López, C.; Cisneros-Zevallos, L. Assessment of Concentrated Liquid Coffee Acceptance during Storage: Sensory and Physicochemical Perspective. Molecules 2021, 26, 3545. https://doi.org/10.3390/molecules26123545
Quintero M, Velásquez S, Zapata J, López C, Cisneros-Zevallos L. Assessment of Concentrated Liquid Coffee Acceptance during Storage: Sensory and Physicochemical Perspective. Molecules. 2021; 26(12):3545. https://doi.org/10.3390/molecules26123545
Chicago/Turabian StyleQuintero, Mónica, Sebastián Velásquez, Julián Zapata, Carlos López, and Luis Cisneros-Zevallos. 2021. "Assessment of Concentrated Liquid Coffee Acceptance during Storage: Sensory and Physicochemical Perspective" Molecules 26, no. 12: 3545. https://doi.org/10.3390/molecules26123545
APA StyleQuintero, M., Velásquez, S., Zapata, J., López, C., & Cisneros-Zevallos, L. (2021). Assessment of Concentrated Liquid Coffee Acceptance during Storage: Sensory and Physicochemical Perspective. Molecules, 26(12), 3545. https://doi.org/10.3390/molecules26123545







