Efficacy of a Covalent Microtubule Stabilizer in Taxane-Resistant Ovarian Cancer Models
Abstract
:1. Introduction
2. Results
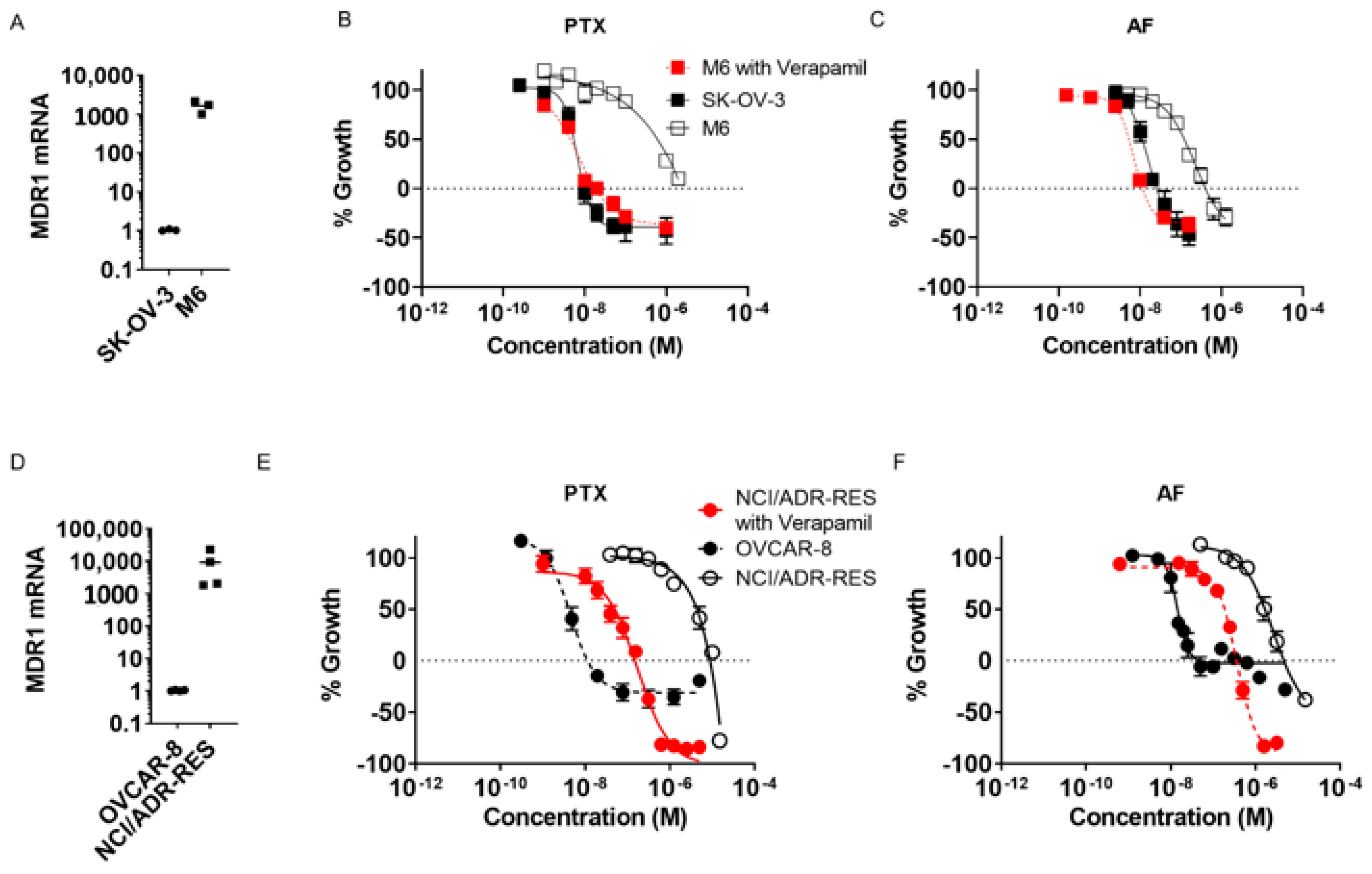
2.1. Taccalonolide AF Retains Efficacy in Taxane-Resistant Ovarian Cancer Models In Vitro
2.2. Taccalonolide AF Has Potent and Persistent Effects in Ovarian Cancer Cell Lines Following Acute Exposure
2.3. Taccalonolide AF Has Antitumor Efficacy against a Taxane-Resistant Ovarian Cancer Flank Xenograft Model
2.4. Taccalonolide AF Inhibits Micrometastasis from an i.p. Disseminated Ovarian Cancer Model
3. Discussion
4. Materials and Methods
4.1. Chemicals Compounds
4.2. Human Ovarian Cancer Cell Lines
4.3. Antiproliferative and Cytotoxicity Assay
4.4. qRT-PCR
4.5. Persistence Assay
4.6. Animal Care and Welfare
4.7. NCI/ADR-RES Flank Xenograft Model
4.8. Disseminated Metastasis In Vivo Taxane-Resistant Ovarian Cancer Model
4.9. Statistics
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Krishnan, V.; Tallapragada, S.; Schaar, B.; Kamat, K.; Chanana, A.M.; Zhang, Y.; Patel, S.; Parkash, V.; Rinker-Schaeffer, C.; Folkins, A.K.; et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun. Biol. 2020, 3, 524. [Google Scholar] [CrossRef]
- Bhusari, P.A.; Khairnar, K.B. Greater Omental Pancake Tumour due to Metastasis of Ovarian Cancer—A Cadaveric Study. J. Clin. Diagn. Res. 2014, 8, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, T.; Siraj, S.; Siraj, A.K.; Azam, S.; Qadri, Z.; Parvathareddy, S.K.; Tulbah, A.; Al-Dayel, F.; AlHusaini, H.; AlOmar, O.; et al. Genetic heterogeneity and evolutionary history of high-grade ovarian carcinoma and matched distant metastases. Br. J. Cancer 2020, 122, 1219–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartnett, J.; Thom, B.; Kline, N. Caregiver Burden in End-Stage Ovarian Cancer. Clin. J. Oncol. Nurs. 2016, 20, 169–173. [Google Scholar] [CrossRef] [Green Version]
- van Stein, R.M.; Aalbers, A.G.J.; Sonke, G.S.; van Driel, W.J. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review. JAMA Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Park, S.J.; Kim, H.S. Rotational intraperitoneal pressurized aerosol chemotherapy in a porcine model. Gland Surg. 2021, 10, 1271–1275. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzi-Barough, L.; Sarookhani, M.R.; Sharifi, M.; Moghbelinejad, S.; Jangjoo, S.; Salehi, R. Molecular mechanisms of drug resistance in ovarian cancer. J. Cell. Physiol. 2018, 233, 4546–4562. [Google Scholar] [CrossRef]
- Kim, S.; Han, Y.; Kim, S.I.; Kim, H.S.; Kim, S.J.; Song, Y.S. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis. Oncol. 2018, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Zou, J.; Fang, Y.; Meng, Y.; Xiao, C.; Fu, J.; Liu, S.; Bai, P.; Yao, Y. Fisetin and polymeric micelles encapsulating fisetin exhibit potent cytotoxic effects towards ovarian cancer cells. BMC Complement. Altern. Med. 2018, 18, 91. [Google Scholar] [CrossRef] [Green Version]
- Giannakakou, P.; Sackett, D.; Fojo, T. Tubulin/microtubules: Still a promising target for new chemotherapeutic agents. J. Natl. Cancer Inst. 2000, 92, 182–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.B.; Tian, Q.; Zhang, J.F.; Xiang, Y. Antitumor effects and molecular mechanisms of action of natural products in ovarian cancer. Oncol. Lett. 2020, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Calderon Iglesias, R.; Ruiz, R.; Aparicio, S.; Crespo, J.; Dzul Lopez, L.; Giampieri, F.; Battino, M. The use of natural compounds for the targeting and chemoprevention of ovarian cancer. Cancer Lett. 2017, 411, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yee, S.S.; Ramachandran, K.; Risinger, A.L. Elucidating target specificity of the taccalonolide covalent microtubule stabilizers employing a combinatorial chemical approach. Nat. Commun. 2020, 11, 654. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, Y.; Li, G.B.; Li, S.A.; Wu, C.; Gigant, B.; Qin, W.; Chen, H.; Wu, Y.; Chen, Q.; et al. Mechanism of microtubule stabilization by taccalonolide AJ. Nat. Commun. 2017, 8, 15787. [Google Scholar] [CrossRef]
- Risinger, A.L.; Li, J.; Bennett, M.J.; Rohena, C.C.; Peng, J.; Schriemer, D.C.; Mooberry, S.L. Taccalonolide binding to tubulin imparts microtubule stability and potent in vivo activity. Cancer Res. 2013, 73, 6780–6792. [Google Scholar] [CrossRef] [Green Version]
- Risinger, A.L.; Li, J.; Du, L.; Benavides, R.; Robles, A.J.; Cichewicz, R.H.; Kuhn, J.G.; Mooberry, S.L. Pharmacokinetic Analysis and in Vivo Antitumor Efficacy of Taccalonolides AF and AJ. J. Nat. Prod. 2017, 80, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Ola, A.R.B.; Risinger, A.L.; Du, L.; Zammiello, C.L.; Peng, J.; Cichewicz, R.H.; Mooberry, S.L. Taccalonolide Microtubule Stabilizers Generated Using Semisynthesis Define the Effects of Mono Acyloxy Moieties at C-7 or C-15 and Disubstitutions at C-7 and C-25. J. Nat. Prod. 2018, 81, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fidalgo, J.A.; Grau, F.; Farinas, L.; Oaknin, A. Systemic treatment of newly diagnosed advanced epithelial ovarian cancer: From chemotherapy to precision medicine. Crit. Rev. Oncol. Hematol. 2020, 158, 103209. [Google Scholar] [CrossRef] [PubMed]
- Markman, M. Intraperitoneal chemotherapy in the management of malignant disease. Expert Rev. Anticancer Ther. 2001, 1, 142–148. [Google Scholar] [CrossRef]
- Dedrick, R.L.; Myers, C.E.; Bungay, P.M.; DeVita, V.T., Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat. Rep. 1978, 62, 1–11. [Google Scholar] [PubMed]
- Balthasar, J.P.; Fung, H.L. Pharmacokinetic and pharmacodynamic optimization of intraperitoneal chemotherapy. Life Sci. 1996, 58, 535–543. [Google Scholar] [CrossRef]
- Jaaback, K.; Johnson, N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [Green Version]
- Hess, L.M.; Benham-Hutchins, M.; Herzog, T.J.; Hsu, C.H.; Malone, D.C.; Skrepnek, G.H.; Slack, M.K.; Alberts, D.S. A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. Int. J. Gynecol. Cancer 2007, 17, 561–570. [Google Scholar] [CrossRef]
- Los, G.; Mutsaers, P.H.; Lenglet, W.J.; Baldew, G.S.; McVie, J.G. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother. Pharmacol. 1990, 25, 389–394. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Perniola, G.; Palaia, I.; Musella, A.; Di Donato, V.; Cascialli, G.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Role of intraperitoneal chemotherapy in ovarian cancer in the platinum-taxane-based era: A meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 136, 64–69. [Google Scholar] [CrossRef]
- Peng, J.; Risinger, A.L.; Li, J.; Mooberry, S.L. Synthetic reactions with rare taccalonolides reveal the value of C-22,23 epoxidation for microtubule stabilizing potency. J. Med. Chem. 2014, 57, 6141–6149. [Google Scholar] [CrossRef]
- Johnatty, S.E.; Beesley, J.; Gao, B.; Chen, X.; Lu, Y.; Law, M.H.; Henderson, M.J.; Russell, A.J.; Hedditch, E.L.; Emmanuel, C.; et al. ABCB1 (MDR1) polymorphisms and ovarian cancer progression and survival: A comprehensive analysis from the Ovarian Cancer Association Consortium and The Cancer Genome Atlas. Gynecol. Oncol. 2013, 131, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, I.S.; He, W.; Yin, L. Understanding of human ATP binding cassette superfamily and novel multidrug resistance modulators to overcome MDR. Biomed. Pharmacother. 2018, 100, 335–348. [Google Scholar] [CrossRef]
- Bender, J.; Fang, J.; Simon, R. A computational study of the inhibition mechanisms of P-glycoprotein mediated paclitaxel efflux by kinase inhibitors. BMC Syst. Biol. 2017, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Risinger, A.L.; Jackson, E.M.; Polin, L.A.; Helms, G.L.; LeBoeuf, D.A.; Joe, P.A.; Hopper-Borge, E.; Luduena, R.F.; Kruh, G.D.; Mooberry, S.L. The taccalonolides: Microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008, 68, 8881–8888. [Google Scholar] [CrossRef] [Green Version]
- Liscovitch, M.; Ravid, D. A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 2007, 245, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Sait, K.H.W.; Alam, Q.; Alam, M.Z.; Anfinan, N.; Wali, A.W.N.; Rasool, M. MDR1 Gene Polymorphisms and Its Association With Expression as a Clinical Relevance in Terms of Response to Chemotherapy and Prognosis in Ovarian Cancer. Front. Genet. 2020, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updat. 2021, 55, 100754. [Google Scholar] [CrossRef]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [Green Version]
- Anglesio, M.S.; Wiegand, K.C.; Melnyk, N.; Chow, C.; Salamanca, C.; Prentice, L.M.; Senz, J.; Yang, W.; Spillman, M.A.; Cochrane, D.R.; et al. Type-specific cell line models for type-specific ovarian cancer research. PLoS ONE 2013, 8, e72162. [Google Scholar] [CrossRef]
- Kloudova, K.; Hromadkova, H.; Partlova, S.; Brtnicky, T.; Rob, L.; Bartunkova, J.; Hensler, M.; Halaska, M.J.; Spisek, R.; Fialova, A. Expression of tumor antigens on primary ovarian cancer cells compared to established ovarian cancer cell lines. Oncotarget 2016, 7, 46120–46126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaufort, C.M.; Helmijr, J.C.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van, I.W.F.; Heine, A.A.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef]
- Kwok, A.L.; Wong, O.G.; Wong, E.S.; Tsun, O.K.; Chan, K.K.; Cheung, A.N. Caution over use of ES2 as a model of ovarian clear cell carcinoma. J. Clin. Pathol. 2014, 67, 921–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudrej, P.; Olbryt, M.; Zembala-Nozynska, E.; Kujawa, K.A.; Cortez, A.J.; Fiszer-Kierzkowska, A.; Piglowski, W.; Nikiel, B.; Glowala-Kosinska, M.; Bartkowska-Chrobok, A.; et al. Establishment and Characterization of the Novel High-Grade Serous Ovarian Cancer Cell Line OVPA8. Int. J. Mol. Sci. 2018, 19, 2080. [Google Scholar] [CrossRef] [Green Version]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Bourgeois, D.L.; Kabarowski, K.A.; Porubsky, V.L.; Kreeger, P.K. High-grade serous ovarian cancer cell lines exhibit heterogeneous responses to growth factor stimulation. Cancer Cell Int. 2015, 15, 112. [Google Scholar] [CrossRef]
- Hallas-Potts, A.; Dawson, J.C.; Herrington, C.S. Ovarian cancer cell lines derived from non-serous carcinomas migrate and invade more aggressively than those derived from high-grade serous carcinomas. Sci. Rep. 2019, 9, 5515. [Google Scholar] [CrossRef] [PubMed]
- De Haven Brandon, A.; Box, G.; Hallsworth, A.; Court, W.; Matthews, N.; Herodek, B.; Arteagabeitia, A.B.; Valenti, M.; Kirkin, V. Identification of ovarian high-grade serous carcinoma cell lines that show estrogen-sensitive growth as xenografts in immunocompromised mice. Sci. Rep. 2020, 10, 10799. [Google Scholar] [CrossRef]
- Haley, J.; Tomar, S.; Pulliam, N.; Xiong, S.; Perkins, S.M.; Karpf, A.R.; Mitra, S.; Nephew, K.P.; Mitra, A.K. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget 2016, 7, 32810–32820. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.K.; Davis, D.A.; Tomar, S.; Roy, L.; Gurler, H.; Xie, J.; Lantvit, D.D.; Cardenas, H.; Fang, F.; Liu, Y.; et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol. Oncol. 2015, 138, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Karagoz, K.; Mehta, G.A.; Khella, C.A.; Khanna, P.; Gatza, M.L. Integrative proteogenomic analyses of human tumours identifies ADNP as a novel oncogenic mediator of cell cycle progression in high-grade serous ovarian cancer with poor prognosis. EBioMedicine 2019, 50, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Emori, M.M.; Papp, E.; MacDuffie, E.; Konecny, G.E.; Velculescu, V.E.; Drapkin, R. Beyond genomics: Critical evaluation of cell line utility for ovarian cancer research. Gynecol. Oncol. 2015, 139, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towle, M.J.; Salvato, K.A.; Wels, B.F.; Aalfs, K.K.; Zheng, W.; Seletsky, B.M.; Zhu, X.; Lewis, B.M.; Kishi, Y.; Yu, M.J.; et al. Eribulin induces irreversible mitotic blockade: Implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res. 2011, 71, 496–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoquist, K.M.; Espinoza, D.; Mileshkin, L.; Ananda, S.; Shannon, C.; Yip, S.; Goh, J.; Bowtell, D.; Harrison, M.; Friedlander, M.L. REZOLVE (ANZGOG-1101): A phase 2 trial of intraperitoneal bevacizumab to treat symptomatic ascites in patients with chemotherapy-resistant, epithelial ovarian cancer. Gynecol. Oncol. 2021. [Google Scholar] [CrossRef]
- Conway, G.D. The Role of Testisin and PAR-2 Signaling in Ovarian Cancer Metastasis. Ph.D. Thesis, University of Maryland, Baltimore, MD, USA, 2019. [Google Scholar]
- Dahn, M.L.; Dean, C.A.; Jo, D.B.; Coyle, K.M.; Marcato, P. Human-specific GAPDH RT-qPCR is an accurate and sensitive method of xenograft metastasis quantification. bioRxiv 2020. [Google Scholar] [CrossRef]
- Steinberga, I.; Jansson, K.; Sorbe, B. Quality Indicators and Survival Outcome in Stage IIIB-IVB Epithelial Ovarian Cancer Treated at a Single Institution. In Vivo 2019, 33, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Sherman-Baust, C.A.; Becker, K.G.; Wood Iii, W.H.; Zhang, Y.; Morin, P.J. Gene expression and pathway analysis of ovarian cancer cells selected for resistance to cisplatin, paclitaxel, or doxorubicin. J. Ovarian Res. 2011, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Balaguer, F.A.; Muhlethaler, T.; Estevez-Gallego, J.; Calvo, E.; Gimenez-Abian, J.F.; Risinger, A.L.; Sorensen, E.J.; Vanderwal, C.D.; Altmann, K.H.; Mooberry, S.L.; et al. Crystal Structure of the Cyclostreptin-Tubulin Adduct: Implications for Tubulin Activation by Taxane-Site Ligands. Int. J. Mol. Sci. 2019, 20, 1392. [Google Scholar] [CrossRef] [Green Version]
- Yaghoubi, S.; Karimi, M.H.; Lotfinia, M.; Gharibi, T.; Mahi-Birjand, M.; Kavi, E.; Hosseini, F.; Sineh Sepehr, K.; Khatami, M.; Bagheri, N.; et al. Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J. Cell Physiol. 2020, 235, 31–64. [Google Scholar] [CrossRef] [PubMed]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, F.; Xie, Z.; Zu, X.; Xu, W.; Jiang, Y. P-Glycoprotein/MDR1 regulates pokemon gene transcription through p53 expression in human breast cancer cells. Int. J. Mol. Sci. 2010, 11, 3039–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cell Line | Paclitaxel (nM) | Relative Resistance | Taccalonolide AF (nM) | Relative Resistance |
|---|---|---|---|---|
| OVCAR-8 | 4.2 ± 0.8 | - | 14 ± 1 | - |
| NCI/ADR-RES | 3655 ± 416 | 870 | 1754 ± 273 | 124 |
| NCI/ADR-RES + Verapamil | 45 ± 15 | 11 | 183 ± 17 | 13 |
| SK-OV-3 | 5.4 ± 0.5 | - | 11 ± 2 | - |
| SK-OV-3-MDR-1-6/6 | 486 ± 193 | 90 | 127 ± 8 | 11 |
| SK-OV-3-MDR-1-6/6 + Verapamil | 4.4 ± 0.4 | 0.8 | 5.3 ± 0.3 | 0.5 |
| Cell Line | Paclitaxel (nM) | Relative Resistance | Taccalonolide AF (nM) | Relative Resistance |
|---|---|---|---|---|
| OVCAR-8 | 11 ± 1 | - | 142 ± 110 | - |
| NCI/ADR-RES | 4812 ± 924 | 458 | 4291 ± 949 | 30 |
| NCI/ADR-RES + Verapamil | 174 ± 11 | 17 | 367 ± 32 | 2.6 |
| SK-OV-3 | 10 ± 1 | - | 27 ± 4 | - |
| SK-OV-3-MDR-1-6/6 | ~1000 | 98 | 412 ± 67 | 15 |
| SK-OV-3-MDR-1-6/6 + Verapamil | 17 ± 1 | 1.6 | 11.4 ± 0.6 | 0.4 |
| Cell Line | Paclitaxel (nM) | Taccalonolide AF (nM) | Ovarian Cancer Cell Line Characterization |
|---|---|---|---|
| ES-2 | 23 ± 11 | 11.2 ± 0.5 | Clear cell [40], likely HGSOC [41,42,43] |
| OV-90 | 5 ± 2 | 41 ± 6 | HGSOC [40,43,44] |
| OVCAR-3 | 1.7 ± 0.3 | 10 ± 3 | HGSOC [40,44,45,46] |
| OVCAR-5 | 10 ± 3 | 24 ± 3 | HGSOC [47,48,49] |
| Kuramochi | 15 ± 3 | 17 ± 4 | HGSOC [46,47,50] |
| JHOS-4 | 4.5 ± 0.7 | 6.4 ± 0.8 | HGSOC [43,50] |
| OVSAHO | 13 ± 6 | 21 ± 4 | HGSOC [46,47,50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, S.S.; Risinger, A.L. Efficacy of a Covalent Microtubule Stabilizer in Taxane-Resistant Ovarian Cancer Models. Molecules 2021, 26, 4077. https://doi.org/10.3390/molecules26134077
Yee SS, Risinger AL. Efficacy of a Covalent Microtubule Stabilizer in Taxane-Resistant Ovarian Cancer Models. Molecules. 2021; 26(13):4077. https://doi.org/10.3390/molecules26134077
Chicago/Turabian StyleYee, Samantha S., and April L. Risinger. 2021. "Efficacy of a Covalent Microtubule Stabilizer in Taxane-Resistant Ovarian Cancer Models" Molecules 26, no. 13: 4077. https://doi.org/10.3390/molecules26134077
APA StyleYee, S. S., & Risinger, A. L. (2021). Efficacy of a Covalent Microtubule Stabilizer in Taxane-Resistant Ovarian Cancer Models. Molecules, 26(13), 4077. https://doi.org/10.3390/molecules26134077






