Clitorienolactones and Isoflavonoids of Clitorea ternatea Roots Alleviate Stress-Like Symptoms in a Reserpine-Induced Zebrafish Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. UHPLC–DAD–MS/MS Profiling of C. ternatea Crude Root Extract in Negative Ionization Mode
2.1.1. Clitorienolactones
2.1.2. Flavonoids
2.1.3. Identification of Amino Acids and Carboxylic Acids
2.2. UHPLC–DAD–MS/MS (Positive Ionization Mode) Profile of Clitorea ternatea Crude Root Extract
2.3. Metabolite Composition of the Ethyl Acetate and 50% MeOH Fractions
2.4. Effect on Reserpine-Induced Stress in Zebrafish
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Material
3.3. Solvent Extraction of Dried Roots
3.4. Fractionation of Crude Extract
3.5. UHPLC–DAD–MS/MS Analysis
3.6. Zebrafish and Maintenance
3.7. Induction of Stress
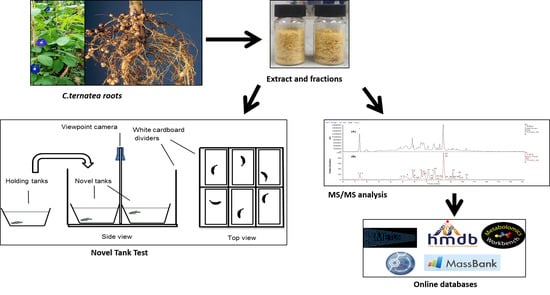
3.8. Behavioral Assay: Novel Tank Test
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Paris, J. The Mistreatment of Major Depressive Disorder. Can. J. Psychiatry 2014, 59, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Guven, N.; Dietis, N. Stress-based animal models of depression: Do we actually know what we are doing? Brain Res. 2016, 1652, 30–42. [Google Scholar] [CrossRef]
- Gutman, D.; Nemeroff, C. The Hand Book of Stress Science: Biology, Psychology and Health, 1st ed.; Springer Publishing Company: New York, NY, USA, 2011; pp. 345–357. [Google Scholar]
- Czéh, B.; Michaelis, T.; Watanabe, T.; Frahm, J.; De Biurrun, G.; Van Kampen, M.; Bartolomucci, A.; Fuchs, E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA 2001, 98, 12796–12801. [Google Scholar] [CrossRef]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, T.M.; Wen, X.Y.; Foster, J.A.; Kennedy, S.H. Zebrafish Models of Major Depressive Disorders. J. Neurosci. Res. 2016, 94, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Schechter, L.; Ring, R.; Beyer, C.; Hughes, Z.; Khawaja, X.; Malberg, J.; Rosenzweig-Lipson, S. Innovative approaches for the development of antidepressant drugs: Current and future strategies. NeuroRX 2005, 2, 590–611. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Mottram, P. A comparison of side effects of selective serotonin reuptake inhibitors and tricyclic antidepressants in older depressed patients: A meta-analysis. Int. J. Geriatr. Psychiatry 2004, 19, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D. Popular Herbal and Natural Remedies Used in Psychiatry. FOCUS 2018, 16, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Doss, A.; Nandagopalan, V. Antibacterial Studies on Leaves of Clitoria ternatea Linn. A High Potential Medicinal Plant. Int. J. Appl. Biol. Pharmaceut. Tech. 2011, 2, 453–456. [Google Scholar]
- Doyle, J.; Luckow, M. The Rest of the Iceberg. Legume Diversity and Evolution in a Phylogenetic Context. Plant Physiol. 2003, 131, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Ohal, C.; Shroff, S.; Bhutada, R.; Somani, R.; Kasture, V.; Kasture, S. Clitorea ternatea and the CNS. Pharmacol. Biochem. Behav. 2003, 75, 529–536. [Google Scholar] [CrossRef]
- Mukherjee, P.; Kumar, V.; Mal, M.; Houghton, P. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Margret, A.; Begum, T.; Parthasarathy, S.; Suvaithenamudhan, S. A Strategy to Employ Clitorea ternatea as a Prospective Brain Drug Confronting Monoamine Oxidase (MAO) Against Neurodegenerative Diseases and Depression. Nat. Prod. Bioprospecting 2015, 5, 293–306. [Google Scholar] [CrossRef]
- Parvathi, M.; Ravishankar, K. Evaluation of Antidepressant, Motor Coordination and Locomotor Activities of Ethanolic Root Extract of Clitorea ternatea. J. Nat. Remedies 2013, 13, 19–24. [Google Scholar]
- Parimaladevi, B.; Boominathan, R.; Mandal, S. Evaluation of antipyretic potential of Clitorea ternatea L. extract in rats. Phytomedicine 2004, 11, 323–326. [Google Scholar] [CrossRef]
- Parimaladevi, B.; Boominathan, R.; Mandal, S. Anti-inflammatory, analgesic and antipyretic properties of Clitorea ternatea root. Fitoterapia 2003, 74, 345–349. [Google Scholar]
- Rai, K.; Murthy, K.; Karanth, K.; Nalini, K.; Rao, M.; Srinivasan, K. Clitorea ternatea Root Extract Enhances Acetylcholine Content in Rat Hippocampus. Fitoterapia 2002, 73, 685–689. [Google Scholar] [CrossRef]
- Taranalli, A.; Cheeramkuzhy, T. Influence of Clitorea ternatea Extracts on Memory and Central Cholinergic Activity in Rats. Pharm. Biol. 2000, 38, 51–56. [Google Scholar] [CrossRef]
- Vasisht, K.; Dhobi, M.; Khullar, S.; Mandal, S.; Karan, M. Norneolignans from the roots of Clitorea ternatea L. Tetrahedron Lett. 2016, 57, 1758–1762. [Google Scholar] [CrossRef]
- Rajagopalan, N. Free amino acids and amides in legume root nodules. Curr. Sci. 1964, 33, 454–456. [Google Scholar]
- Benerjee, S.K.; Chakravarti, R.N. Taraxerol from Clitorea ternatea linn. Bull. Calcutta Sch. Trop. Med. 1964, 11, 106–107. [Google Scholar]
- Kumar, V.; Mukherjee, K.; Kumar, S.; Mal, M.; Mukherjee, P. Validation of HPTLC method for the analysis of taraxerol in Clitorea ternatea. Phytochem. Anal. 2007, 19, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Berté, T.E.; Dalmagro, A.P.; Zimath, P.L.; Gonçalves, A.E.; Meyre-Silva, C.; Bürger, C.; Weber, C.J.; Dos Santos, D.A.; Cechinel-Filho, V.; De Souza, M.M. Taraxerol as a possible therapeutic agent on memory impairments and Alzheimer’s disease: Effects against scopolamine and streptozotocin-induced cognitive dysfunctions. Steroids 2018, 132, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Yang, E.; Neelkantan, N.; Mikhaylova, A.; Arnold, R.; Poudel, M.; Stewart, A.; Kalueff, A. Developing ‘integrative’ zebrafish models of behavioral and metabolic disorders. Behav. Brain Res. 2013, 256, 172–187. [Google Scholar] [CrossRef]
- Stewart, A.; Nguyen, M.; Wong, K.; Poudel, M.; Kalueff, A. Developing zebrafish models of autism spectrum disorder (ASD). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 50, 27–36. [Google Scholar] [CrossRef]
- Kalueff, A.; Echevarria, D.; Stewart, A. Gaining translational momentum: More zebrafish models for neuroscience research. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Piraud, M.; Vianey-Saban, C.; Petritis, K.; Elfakir, C.; Steghens, J.; Morla, A.; Bouchu, D. ESI-MS/MS analysis of underivatised amino acids: A new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun. Mass Spectrom. 2003, 17, 1297–1311. [Google Scholar] [CrossRef]
- Smith, C.; Maille, G.; Want, E.; Qin, C.; Trauger, S.; Brandon, T.; Custodio, D.; Abagyan, R.; Siuzdak, G. METLIN. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Kang, J.; Hick, L.; Price, W. A fragmentation study of isoflavones in negative electrospray ionization by MSn ion trap mass spectrometry and triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 857–868. [Google Scholar] [CrossRef]
- Rijke, E.D.; Out, P.; Niessen, W.; Ariese, F.; Gooijer, C.; Brinkman, U. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef]
- Wähälä, K.; Rasku, S.; Parikka, K. Deuterated phytoestrogen flavonoids and isoflavonoids for quantitation. J. Chromatogr. B 2002, 777, 111–122. [Google Scholar] [CrossRef]
- March, R.; Miao, X.; Metcalfe, C.; Stobiecki, M.; Marczak, L. A fragmentation study of an isoflavone glycoside, genistein-7-O-glucoside, using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 232, 171–183. [Google Scholar] [CrossRef]
- Ablajan, K. A study of characteristic fragmentation of isoflavonoids by using negative ion ESI-MSn. J. Mass Spectrom. 2010, 46, 77–84. [Google Scholar] [CrossRef]
- Eklund, P.; Backman, M.; Kronberg, L.; Smeds, A.; Sjöholm, R. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2007, 43, 97–107. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Optimization of a liquid chromatography method based on simultaneous electrospray ionization mass spectrometric and ultraviolet photodiode array detection for analysis of flavonoid glycosides. Rapid Commun. Mass Spectrom. 2002, 16, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Claeys, M. Characterization and differentiation of diglycosyl flavonoids by positive ion fast atom bombardment and tandem mass spectrometry. Biol. Mass Spectrom. 1994, 23, 406–416. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.; Han, F.; Yan, S.; Hua, Y.; Akio, K. Rapid HPLC method for determination of 12 isoflavone components in soybean seeds. Agric. Sci. China 2011, 10, 70–77. [Google Scholar] [CrossRef]
- Prasain, J.K.; Jones, K.; Kirk, M.; Wilson, L.; Smith-Johnson, M.; Weaver, C.; Barnes, S. Profiling and Quantification of Isoflavonoids in Kudzu Dietary Supplements by High-Performance Liquid Chromatography and Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 4213–4218. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Choi, S.-S.; Song, M.J.; Kim, O.-B.; Kim, Y. Fragmentation patterns of protonated amino acids formed by atmospheric pressure chemical ionization. Rapid Commun. Mass Spectrom. 2012, 27, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Adam, B. Methods for the Analysis of Underivatized Amino Acids by LC/MS. Available online: https://www.agilent.com/cs/library/applications/5991-8582EN_HILIC_Underivatized_Amino_Acid_application.pdf (accessed on 9 November 2020).
- Takano, Y.; Yoshito, C.; Naohiko, O. LC/ESI-MS Analysis of Underivatized Amino Acids and Mass Spectrum. Res. Org. Geochem. 2015, 31, 1–17. [Google Scholar]
- Zakaria, F.; Akhtar, M.T.; Wan Ibrahim, W.N.; Abu Bakar, N.; Muhammad, A.; Shohaimi, S.; Maulidiani, M.; Ahmad, H.; Ismail, I.S.; Shaari, K. Perturbations in Amino Acid Metabolism in Reserpine-treated Zebrafish Brain Detected by 1H NMR-Based Metabolomics. Zebrafish 2020, 18, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Kyzar, E.; Stewart, A.; Landsman, S.; Collins, C.; Gebhardt, M.; Robinson, K.; Kalueff, A. Behavioral effects of bidirectional modulators of brain monoamines reserpine and d-amphetamine in zebrafish. Brain Res. 2013, 1527, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Ullmann, J.F.; Norton, W.H.; Parker, M.O.; Brennan, C.H.; Gerlai, R.; Kalueff, A.V. Molecular psychiatry of zebrafish. Mol. Psychiatry 2015, 20, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Haneul, C.; Chang-Joong, L.; Jiseon, C.; Jinsoo, H.; Yunkyoung, L. Anxiolytic effects of an acetylcholinesterase inhibitor, physostigmine, in the adult zebrafish. Anim. Cells Syst. 2012, 16, 198–206. [Google Scholar]
- Yong-seok, C.; Chang-Joong, L.; Yeon-Hwa, K. MK-801-induced learning impairments reversed by physostigmine and nicotine in zebrafish. Anim. Cells Syst. 2011, 15, 115–121. [Google Scholar]
- McCloskey, M.; Young, T.; Anderson, S. Research Article: The influence of acetylcholinesterase on anxiety- and depression-like behaviors in fluoxetine-treated male mice. BIOS 2017, 88, 29–38. [Google Scholar] [CrossRef]
- Mineur, Y.; Obayemi, A.; Wigestrand, M.; Fote, G.; Calarco, C.; Li, A.; Picciotto, M. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Saricicek, A.; Esterlis, I.; Maloney, K.; Mineur, Y.; Ruf, B.; Muralidharan, A.; Chen, J.; Cosgrove, K.; Kerestes, R.; Ghose, S.; et al. Persistent β2*-Nicotinic Acetylcholinergic Receptor Dysfunction in Major Depressive Disorder. Am. J. Psychiatry 2012, 169, 851–859. [Google Scholar] [CrossRef]
- Suarez-Lopez, J.; Hood, N.; Suárez-Torres, J.; Gahagan, S.; Gunnar, M.; López-Paredes, D. Associations of acetylcholinesterase activity with depression and anxiety symptoms among adolescents growing up near pesticide spray sites. Int. J. Hyg. Environ. Health 2019, 222, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Janowsky, D.; Davis, J.; El-Yousef, M.; Sekerke, H. A Cholinergic-Adrenergic Hypothesis of Mania and Depression. Lancet 1972, 300, 632–635. [Google Scholar] [CrossRef]
- Mori-Okamoto, J.; Otawara-Hamamoto, Y.; Yamato, H.; Yoshimura, H. Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. J. Ethnopharmacol. 2004, 92, 93–101. [Google Scholar] [CrossRef]
- Kageyama, A.; Sakakibara, H.; Zhou, W.; Yoshioka, M.; Ohsumi, M.; Shimoi, K.; Yokogoshi, H. Genistein Regulated Serotonergic Activity in the Hippocampus of Ovariectomized Rats under Forced Swimming Stress. Biosci. Biotechnol. Biochem. 2010, 74, 2005–2010. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.; Hong, S.; Lee, K.; Lee, M.; Hwang, B.; Ro, J. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch. Pharmacal Res. 2005, 28, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Saroya, R.; Smith, R.; Seymour, C.; Mothersill, C. Injection of Reserpine into Zebrafish, Prevents Fish to Fish Communication of Radiation-induced Bystander Signals: Confirmation In-vivo of a Role for Serotonin in the Mechanism. Dose Response 2010, 8, 317–330. [Google Scholar] [CrossRef]
- Kinkel, M.; Eames, S.; Philipson, L.; Prince, V. Intraperitoneal Injection into Adult Zebrafish. J. Vis. Exp. 2010, 42, e2126. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.; Gebhardt, M.; Stewart, A.; Cachat, J.; Brimmer, M.; Chawla, J.; Craddock, C.; Kyzar, E.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]





| No | Retention Time (RT) (min) | Parent Ion Experimental (m/z) | Parent Ion Theoretical (m/z) | Error (ppm) | MS/MS Fragment Ions (Intensity, %) | Tentative Identification | Molecular Formula | Source |
|---|---|---|---|---|---|---|---|---|
| Amino Acids and Carboxylic Acids | ||||||||
| 1 | 1.24 | 154.0605 | 154.0615 | –6.49 | 154 (100), 137 (42), 110 (15) | Histidine | C6H9N3O2 | [30] |
| 2 | 1.31 | 173.1027 | 173.1038 | −6.35 | 131 (100) | Arginine | C6H14N4O2 | [30] |
| 3 | 1.36 | 131.0445 | 131.0456 | −8.39 | 131 (19), 114 (100), 113 (62), 95 (14), 88 (32), 72 (15), 70 (40), 58 (10) | Asparagine | C4H8N2O3 | [31] |
| 4 | 4.82 | 203.0811 | 203.0820 | −4.43 | 203 (72), 159 (20), 142 (28), 116 (100) | Tryptophan | C11H12N2O2 | [30,32] |
| 15 | 7.38 | 187.0959 | 187.0970 | −5.87 | 187 (47), 125 (100), 97 (8), 57 (3) | Azelaic acid | C9H16O4 | [30,32] |
| Clitorienolactones | ||||||||
| 17 | 7.59 | 297.0754 | 297.0763 | −3.03 | 297 (20), 253 (100), 191 (5) 159 (10), 133 (20), 119 (34), 109 (23), 93 (20) | Clitorienolactone D | C17H14O5 | [22] |
| 18 | 7.66 | 327.0858 | 327.0868 | −3.06 | 327 (26), 283 (67), 267 (100), 161 (11), 159 (14), 109 (6) | Clitorienolactone C | C18H16O6 | [22] |
| 23 | 8.41 | 311.0910 | 311.0919 | −2.89 | 311 (32), 205 (74), 191 (10), 190 (100), 161 (9) | Clitorienolactone B | C18H16O5 | [22] |
| 24 | 8.47 | 341.1013 | 341.1025 | −3.51 | 341 (42), 205 (68), 191 (7), 190 (100), 161 (7) | Clitorienolactone A | C19H18O6 | [22] |
| Clitorienolactone Glycosides | ||||||||
| 5 | 5.29 | 621.1796 | 621.1819 | −3.70 | 621 (6), Y1−: 459 (25), Y0−: 297 (19), 253 (100) | Clitorienolactone D 4-O-dihexoside | C29H34O15 | - |
| 6 | 5.84 | 651.1931 | 651.1925 | 0.92 | 651 (5), Y1−: 489 (35), Y0−: 327 (35), 283 (100), 268 (54) | Clitorienolactone C 4-O-dihexoside | C30H36O16 | - |
| 7 | 5.85 | 635.1950 | 635.1976 | −4.09 | Y1−: 473 (31), Y0−: 311 (100), 205 (79), 190 (20) | Clitorienolactone B 4-O-dihexoside | C30H36O15 | - |
| 8 | 5.95 | 665.2057 | 665.2081 | −3.61 | Y1−: 503 (33), Y0−: 341 (100), 205 (65),190 (16) | Clitorienolactone A 4-O-dihexoside | C31H38O16 | - |
| 11 | 6.53 | 459.1278 | 459.1291 | −2.83 | 459 (8), Y0−: 297 (43), 253 (100),109 (8) | Clitorienolactone D 4-O-hexoside | C23H24O10 | - |
| 12 | 6.63 | 489.1383 | 489.1396 | −2.66 | 489 (6), Y0−: 327 (83), 283 (100), 268 (67) | Clitorienolactone C 4-O-hexoside | C24H26O11 | - |
| 13 | 6.85 | 503.1537 | 503.1553 | −3.18 | Y0−: 341 (100), 205 (80), 190 (52), 161 (4) | Clitorienolactone A 4-O-hexoside | C25H28O11 | - |
| 14 | 7.15 | 473.1429 | 473.1447 | −3.8 | 473 (18), Y0−: 311 (96), 205 (100), 190 (49), 161 (5) | Clitorienolactone B 4-O-hexoside | C24H26O10 | - |
| Flavonoids | ||||||||
| 19 | 7.89 | 253.0493 | 253.0500 | −2.76 | 253 (2), 224 (10), 223 (38), 208 (32), 196 (8), 195 (41), 180 (23), 167 (8), 135 (8), 133 (36), 132 (81), 117 (8), 91 (100) | Daidzein * | C15H10O4 | [33,34] |
| 20 | 8.01 | 297.0753 | 297.0763 | −3.36 | 297 (38), 282 (100), 267 (10), 254 (30), 239 (28), 195 (4) | 3′,4′-dimethoxyflavonol | C17H14O5 | [30] |
| 21 | 8.04 | 283.0598 | 283.0606 | −2.82 | 268 (100), 267 (2), 240 (6), 239 (27), 212 (1), 211 (7), 184 (3), 148 (2), 135 (1), | Glycitein | C16H12O5 | [33,35] |
| 22 | 8.18 | 299.0546 | 299.0555 | −3.01 | 299 (24), 284 (25), 271 (13), 256 (100), 255 (6) | Diosmetin | C16H12O6 | - |
| 25 | 8.76 | 269.0444 | 269.0450 | −2.23 | 269 (100), 241 (7), 224 (3), 213 (1), 197 (4), 183 (5), 135 (10), 133 (4) | Genistein * | C15H10O5 | [33,36] |
| 26 | 9.11 | 313.0704 | 313.0712 | −2.55 | 313 (93), 298 (100), 283 (34), 270 (14), 255 (19),239 (6), 226 (83), 211 (43) | Luteolin-3′,4′-dimethyl ether | C17H14O6 | [30] |
| 27 | 9.19 | 309.0752 | 309.0763 | −3.56 | 309 (100), 294 (25), 266 (29), 250 (10), 249 (30), 148 (13) | Hoslundal | C18H14O5 | - |
| 28 | 9.34 | 339.0855 | 339.0868 | −3.83 | 339 (24), 324 (14), 310 (16), 309 (100), 281 (13), 253 (8), 209 (5) | Ambanol | C19H16O6 | - |
| 29 | 9.46 | 267.0651 | 267.0657 | −2.25 | 252 (100), 224 (3), 223 (55), 208 (11), 195 (17), 135 (1), 132 (24), 91 (2) | Formononetin * | C16H12O4 | [33] |
| 30 | 9.77 | 353.1013 | 353.1025 | −3.40 | 353 (100), 338 (40), 323 (71), 308 (32), 293 (17), 279 (73) | Ambonone | C20H18O6 | - |
| 31 | 9.98 | 323.0911 | 323.0919 | −2.47 | 323 (100), 308 (62), 293 (61), 265 (32), 249 (26), 237 (16), 221 (4), 145 (14) | Neoraunone | C19H16O5 | - |
| 32 | 10.34 | 353.1014 | 353.1025 | −3.11 | 353 (100), 338 (33), 323 (73), 308 (38), 293 (14), 279 (69) | Ambonone isomer | C20H18O6 | - |
| Flavonoid Glycosides | ||||||||
| 9 | 6.05 | 431.0963 | 431.0978 | −3.48 | 431 (52), Y0−: 269.0446 (26), [Y0−H]−•: 268 (100), 241 (1), 224(4), 135 (1) | Genistin | C21H20O10 | [37] |
| 10 | 6.25 | 415.1017 | 415.1029 | −2.89 | 415 (26), Y0−: 253.0494 (32), [Y0−H]−•: 252 (100), 223 (1) | Daidzin | C21H20O9 | [38] |
| 16 | 7.45 | 445.1124 | 445.1134 | −2.25 | Y0−: 283.0612 (100), 268 (8), 267 (1), 255 (16), 240 (2), 239 (2), 133 (40) | Glycitin | C22H22O10 | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngadni, M.A.; Akhtar, M.T.; Ismail, I.S.; Norazhar, A.I.; Lee, S.Y.; Maulidiani, M.; Shaari, K. Clitorienolactones and Isoflavonoids of Clitorea ternatea Roots Alleviate Stress-Like Symptoms in a Reserpine-Induced Zebrafish Model. Molecules 2021, 26, 4137. https://doi.org/10.3390/molecules26144137
Ngadni MA, Akhtar MT, Ismail IS, Norazhar AI, Lee SY, Maulidiani M, Shaari K. Clitorienolactones and Isoflavonoids of Clitorea ternatea Roots Alleviate Stress-Like Symptoms in a Reserpine-Induced Zebrafish Model. Molecules. 2021; 26(14):4137. https://doi.org/10.3390/molecules26144137
Chicago/Turabian StyleNgadni, Muhammad Afiq, Muhammad Tayyab Akhtar, Intan Safinar Ismail, Anis Irfan Norazhar, Soo Yee Lee, Maulidiani Maulidiani, and Khozirah Shaari. 2021. "Clitorienolactones and Isoflavonoids of Clitorea ternatea Roots Alleviate Stress-Like Symptoms in a Reserpine-Induced Zebrafish Model" Molecules 26, no. 14: 4137. https://doi.org/10.3390/molecules26144137
APA StyleNgadni, M. A., Akhtar, M. T., Ismail, I. S., Norazhar, A. I., Lee, S. Y., Maulidiani, M., & Shaari, K. (2021). Clitorienolactones and Isoflavonoids of Clitorea ternatea Roots Alleviate Stress-Like Symptoms in a Reserpine-Induced Zebrafish Model. Molecules, 26(14), 4137. https://doi.org/10.3390/molecules26144137