Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases
Abstract
:1. Introduction
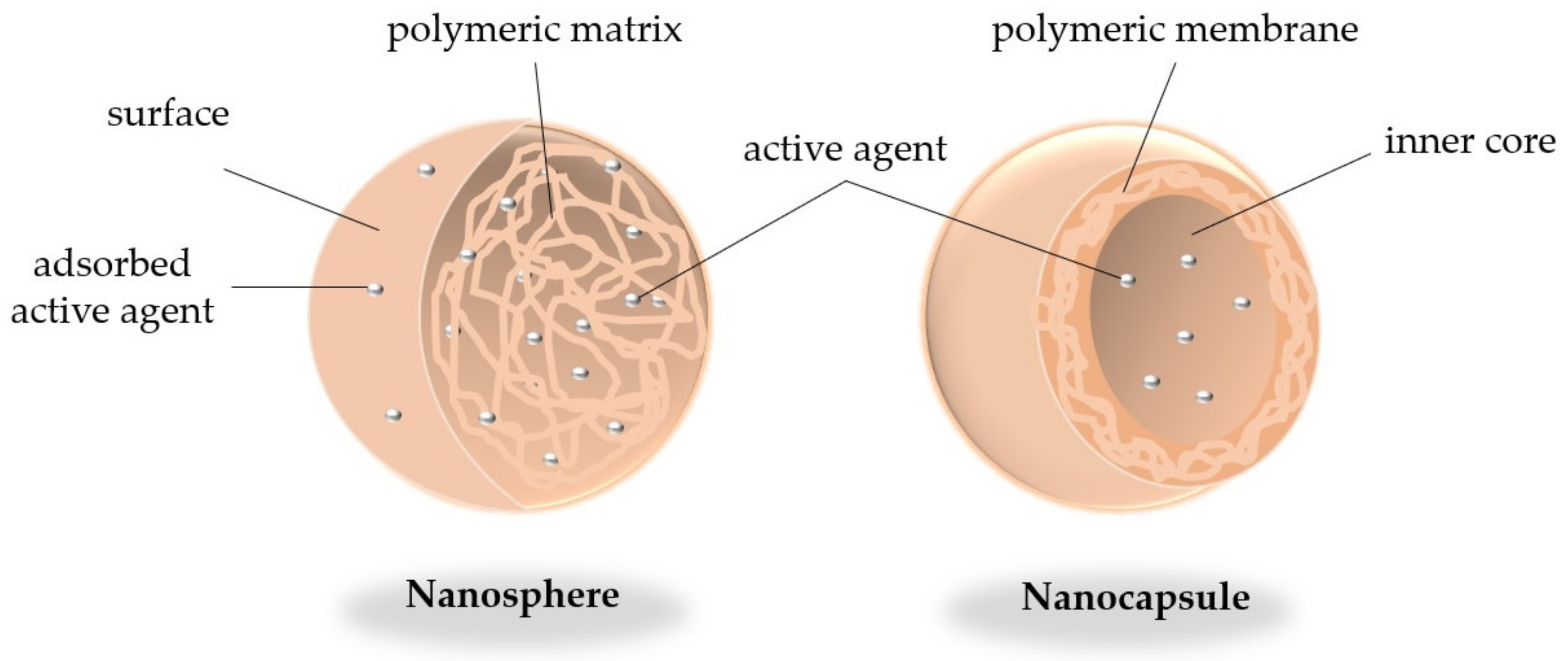
2. Brief Overview of Nano-Encapsulated Polyphenols: Nature and Physiochemical Polymer Benefits
2.1. Proteins
2.2. Polysaccharides
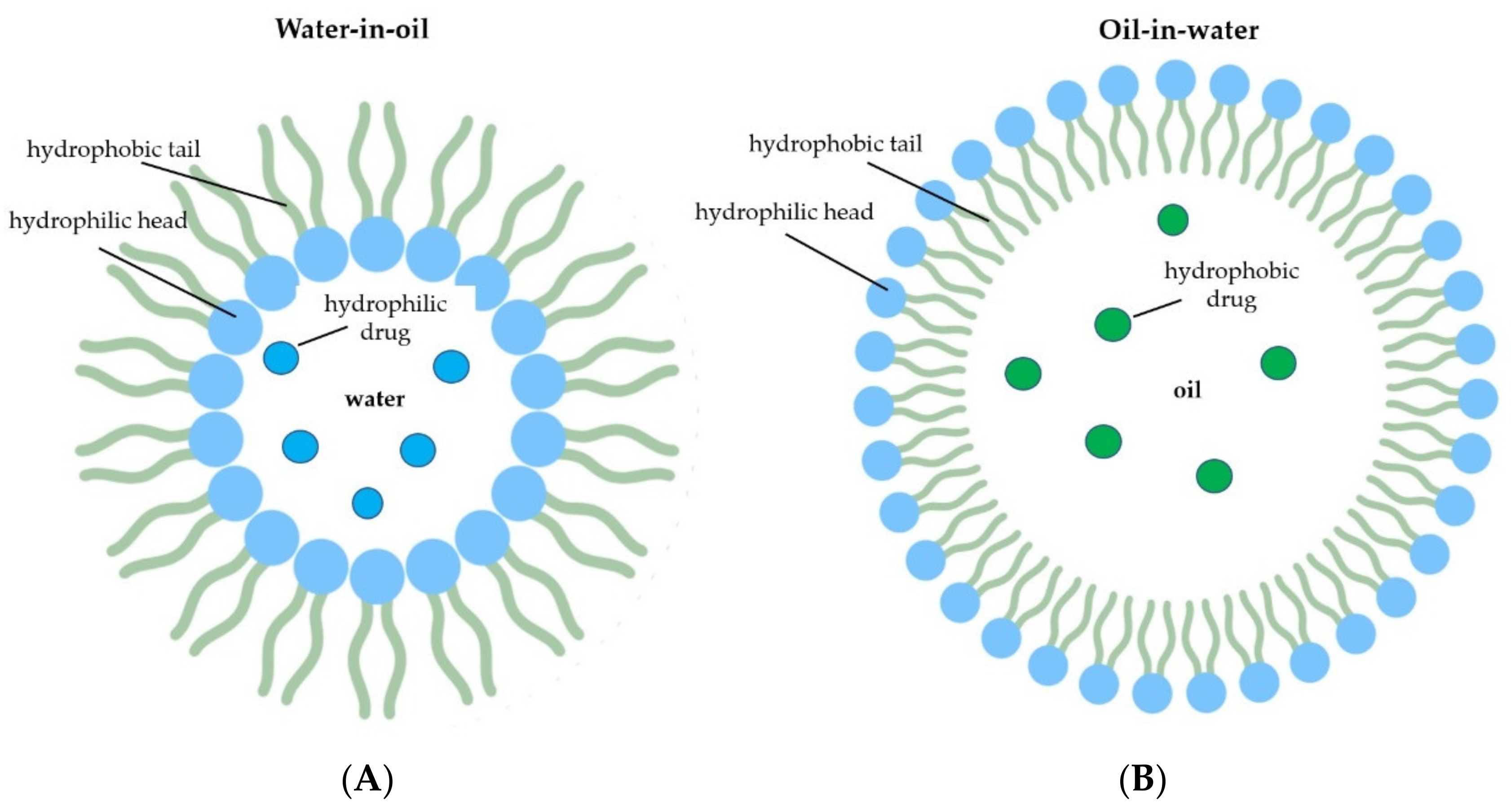
2.3. Nanoemulsions
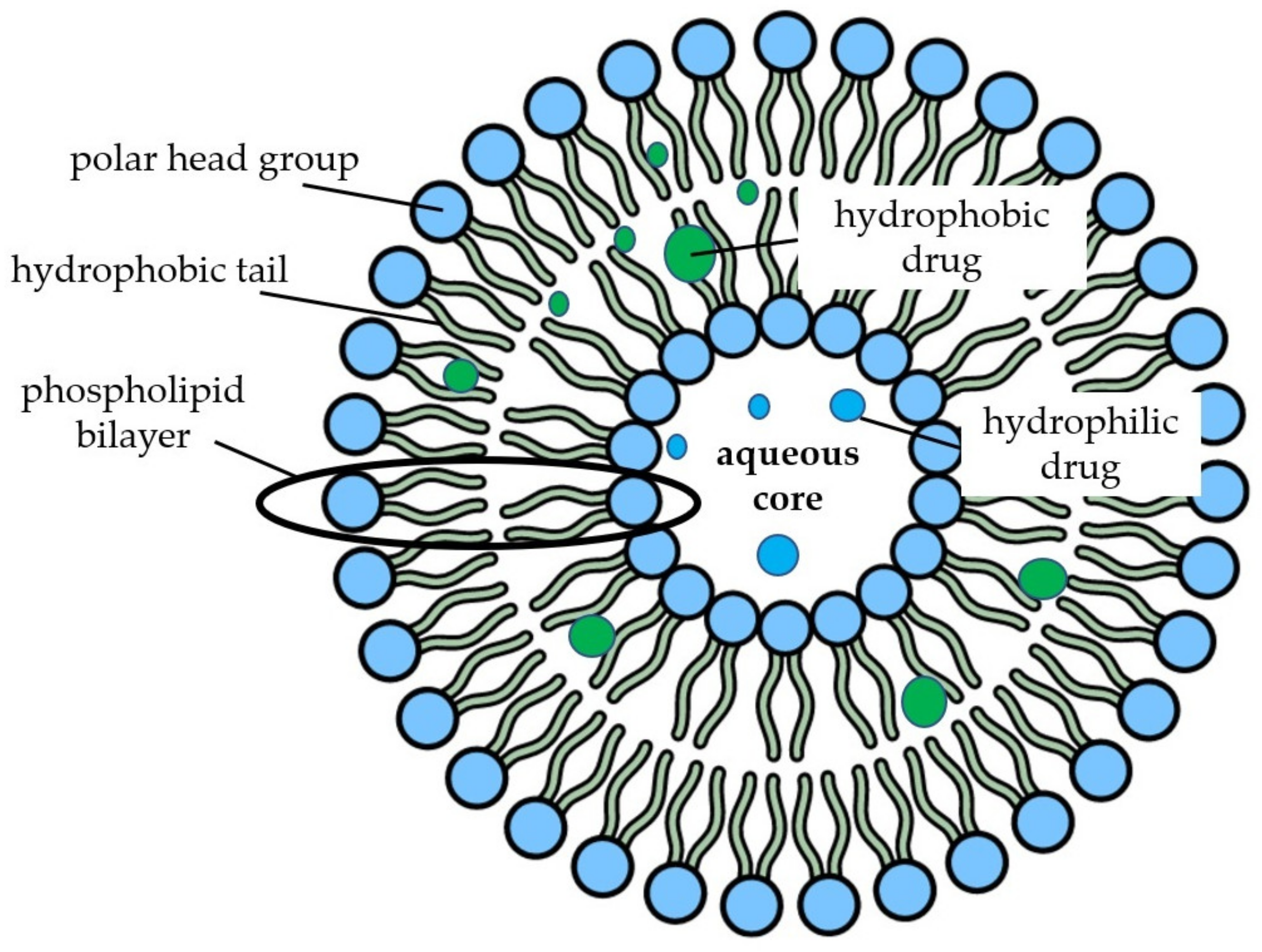
2.4. Nanoliposomes and Niosomes
3. Nano-Encapsulated Polyphenols Bioaccessibility and Bioavailability
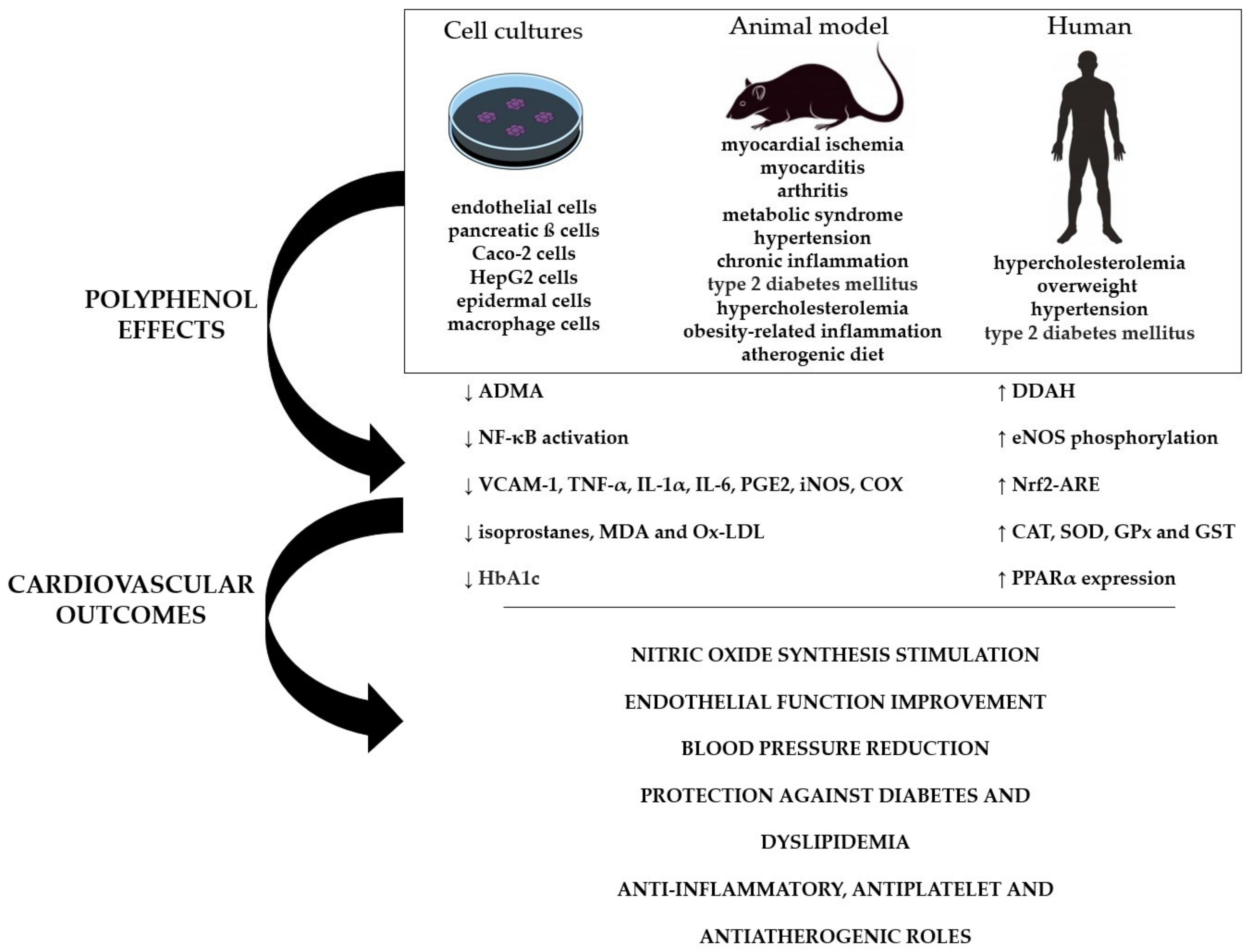
4. Critical Analysis of the Effectiveness of Nano-Encapsulated Polyphenols on Cardiovascular Performance: Pre-Clinical and Clinical Trials
5. Nanoparticles: Safety and Cytotoxicity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food. Sci. Food Saf. 2020, 20, 1036–1074. [Google Scholar] [CrossRef] [PubMed]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of phenolic compounds from color and flavor problems to health benefits. J. Agric. Food. Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.V.T.; Baião, D.S.; Silva, F.O.; Alves, G.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. Betanin, a natural food additive: Stability, bioavailability, antioxidant and preservative ability assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [Green Version]
- Baião, D.D.S.; de Freitas, C.S.; Gomes, L.P.; da Silva, D.; Correa, A.C.N.T.F.; Pereira, P.R.; Aguila, E.M.D.; Paschoalin, V.M.F. Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases. Nutrients 2017, 9, 1044. [Google Scholar] [CrossRef]
- Marx, W.; Kelly, J.; Marshall, S.; Nakos, S.; Campbell, K.; Itsiopoulos, C. The effect of polyphenol-rich interventions on cardiovascular risk factors in haemodialysis: A systematic review and meta-analysis. Nutrients 2017, 9, 1345. [Google Scholar] [CrossRef] [Green Version]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomized double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekalina, N.I. Resveratrol has a positive effect on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiad Lek. 2017, 70, 286–291. [Google Scholar] [PubMed]
- Liu, Y.J.; Zhan, J.; Liu, X.L.; Wang, Y.; Ji, J.; He, Q.Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qin, S.; Huang, L.; Tang, Y.; Ren, H.; Hu, H. Efficacy and safety of Rhizoma curcumea longae with respect to improving the glucose metabolism of patients at risk for cardiovascular disease: A meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet. 2019, 32, 591–606. [Google Scholar] [CrossRef]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and vascular function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef] [Green Version]
- Appeldoorn, M.M.; Venema, D.P.; Peters, T.H.; Koenen, M.E.; Arts, I.C.; Vincken, J.P.; Gruppen, H.; Keijer, J.; Hollman, P.C. Some phenolic compounds increase the nitric oxide level in endothelial cells in vitro. J. Agric. Food. Chem. 2009, 57, 7693–7699. [Google Scholar] [CrossRef]
- Li, P.G.; Sun, L.; Han, X.; Ling, S.; Gan, W.T.; Xu, J.W. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt-independent and PKA-dependent mechanism. Pharmacology 2012, 89, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Hu, C.P.; Chen, M.F.; Deng, P.Y.; Li, Y.J. Epigallocatechin gallate preserves endothelial function by reducing the endogenous nitric oxide synthase inhibitor level. Can. J. Physiol. Pharmacol. 2006, 84, 163–171. [Google Scholar] [CrossRef]
- Frombaum, M.; Therond, P.; Djelidi, R.; Beaudeux, J.L.; Bonnefont-Rousselot, D.; Borderie, D. Piceatannol is more effective than resveratrol in restoring endothelial cell dimethylarginine dimethylaminohydrolase expression and activity after high-glucose oxidative stress. Free. Radic. Res. 2011, 45, 293–302. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent advances of natural polyphenols activators for keap1-nrf2 signaling pathway. Chem. Biodivers. 2019, 16, 1900400. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food. Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Scazzocchio, B.; Vari, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPAR gamma activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: A review. Int. J. Mol. Sci. 2019, 21, 140. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Rubió, L.; Macià, A.; Motilva, M.J. Impact of various factors on pharmacokinetics of bioactive polyphenols: An overview. Curr. Drug. Metab. 2014, 15, 62–76. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on nature: The role of nanomedicine in the development of clinical natural drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A successful tool to increase solubility, stability and optimise bioefficacy of natural constituents. Curr. Med. Chem. 2018, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Suganya, V.; Anuradha, V. Microencapsulation and nano-encapsulation: A review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nano-capsules for Drug Delivery: An Updated Review of the Last Decade. Recent Pat Drug Deliv. Formul. 2018, 12, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends. Food. Sci. Tech. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nano-encapsulation techniques for food bioactive components: A review. Food Bioprocess. Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Mitragotri, S.; Rai, B. Permeation of nanoparticles across the intestinal lipid membrane: Dependence on shape and surface chemistry studied through molecular simulations. Nanoscale 2020, 12, 6318–6333. [Google Scholar] [CrossRef] [PubMed]
- Bazana, M.T.; Codevilla, C.F.; da Silva, C.D.B.; Menezes, C.R. Nano-encapsulação de licopeno em alimentos. Ciência Nat. 2015, 37, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Kreuter, J. Nanoparticle-based dmg delivery systems. J. Control. Release 1991, 16, 169–176. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-brain delivery methods using nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, N.; Jaitley, V.; Florence, A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug. Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef]
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug. Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef]
- Morris, A.L.; Mohiuddin, S.S. Biochemistry, Nutrients. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554545 (accessed on 5 May 2021).
- Xing, F.; Cheng, G.; Yi, K.; Ma, L. Nano-encapsulation of capsaicin by complex coacervation of gelatin, acacia, and tannins. J. Appl. Polym. Sci. 2005, 96, 2225–2229. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, F.; Li, X.; Zhang, X.; Abbas, S. Formation of heat-resistant nano-capsules of jasmine essential oil via gelatin/gum arabic based complex coacervation. Food Hydrocoll. 2013, 35, 305–314. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T. Encapsulation of active ingredients in complex polysaccharide-protein coacervates. Adv. Colloid. And. Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Llavata-Cabrero, B.; Martínez-Sanz, M.; Fabra, M.J.; López-Rubio, A. Self-assembled gelatin-ι-carrageenan encapsulation structures for intestinal-targeted release applications. J. Colloid. Interface Sci. 2018, 517, 113–123. [Google Scholar] [CrossRef]
- Wong, B.T.; Day, L.; Augustin, M.A. Deamidated wheat protein-dextran Maillard conjugates: Effect of size and location of polysaccharide conjugated on steric stabilization of emulsions at acidic pH. Food Hydrocol. 2011, 25, 1424–1432. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, S.H.; Tsai, G.J.; Tang, D.W.; Mi, F.L.; Peng, Y.P. Novel technology for the preparation of self-assembled catechin/gelatin nanoparticles and their characterization. J. Agric. Food. Chem. 2010, 58, 6728–6734. [Google Scholar] [CrossRef]
- Queiroz-Reyes, C.; Ronquillo-de Jesús, E.; Duran-Caballero, N.; Aguilar-Méndez, M. Development and characterization of gelatin nanoparticles loaded with a cocoa-derived polyphenolic extract. Fruits 2014, 69, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, S.; Prasad, N.R.; Ganamani, A.; Balamurugan, E. Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed. Prev. Nutr. 2013, 3, 64–73. [Google Scholar] [CrossRef]
- Song, X.; Gan, K.; Qin, S.; Chen, L.; Liu, X.; Chen, T.; Liu, H. Preparation and characterization of general-purpose gelatin-based co-loading flavonoids nano-core structure. Sci. Rep. 2019, 9, 6365. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Chuah, Y.J.; Xu, C.; Wang, D.A. Albumin conjugates and assemblies as versatile bio-functional additives and carriers for biomedical applications. J. Mater. Chem. B. 2019, 7, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, P.; Tufoni, M.; Bonavita, M.E. Clinical use of albumin. Blood Transfus. 2013, 11, 18–25. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Huang, M. Structure and enzymatic activities of human serum albumin. Curr. Pharm. Des. 2015, 21, 1831–1836. [Google Scholar] [CrossRef]
- Kim, B.; Seo, B.; Park, S.; Lee, C.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.G.; Youn, Y.S. Albumin nanoparticles with synergistic antitumor efficacy against metastatic lung cancers. Colloids Surf. B Biointerfaces 2017, 158, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Dadparvar, M.; Wagner, S.; Wien, S.; Worek, F.H.; Von Briesen, H.; Kreuter, J. Freeze-drying of HI-6-loaded recombinant human serum albumin nanoparticles for improved storage stability. Eur. J. Pharm. Biopharm. 2014, 88, 510–517. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Manjili, H.K.; Danafar, H.; Davaran, S. Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications. Int. J. Biol. Macromol. 2018, 108, 909–915. [Google Scholar] [CrossRef]
- Pedrozo, R.C.; Antonio, E.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing the flavonoid rutin produced by nano spray drying. Braz. J. Pharm. Sci. 2020, 56, 2020. [Google Scholar] [CrossRef] [Green Version]
- Antônio, E.; Khalil, N.M.; Mainardes, R.M. Bovine Serum albumin nanoparticles containing quercetin: Characterization and antioxidant activity. J. Nanosci. Nanotechnol. 2016, 16, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Bakshi, H.A.; Hakkim, F.L.; Haggag, Y.A.; Al-Batanyeh, K.M.; Al Zoubi, M.S.; Al-Trad, B.; Nasef, M.M.; Satija, S.; Mehta, M.; et al. Albumin nano-encapsulation of piceatannol enhances its anticancer potential in colon cancer via downregulation of nuclear p65 and HIF-1α. Cancers 2020, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Yassa, N.W.; Khalil, S.; Saleh, S.R.; Ghareeb, D.A.; El Demellawy, M.A.; El-Sayed, M.M. Ipriflavone and Ipriflavone loaded albumin nanoparticles reverse lipopolysaccharide induced neuroinflammation in rats. PLoS ONE 2020, 15, e0237929. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy. Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Dalgleish, D.G. On the structural models of bovine casein micelles-review and possible improvements. Soft Matter. 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Holt, C. Casein and casein micelle structures, functions and diversity in 20 species. Int. Dairy J. 2016, 60, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Fokkink, R.; Ni, Y.; Kleijn, M. Bovine beta-casein micelles as delivery systems for hydrophobic flavonoids. Food Hydrocoll. 2019, 96, 653–662. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nano-capsules. J. Agric. Food. Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef] [Green Version]
- Heep, G.; Almeida, A.; Marcano, R.; Vieira, D.; Mainardes, R.M.; Khalil, N.M.; Sarmento, B. Zein-casein-lysine multicomposite nanoparticles are effective in modulate the intestinal permeability of ferulic acid. Int. J. Biol. Macromol. 2019, 138, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, L.; Yu, C.; Jin, P.; Qin, D.; Xu, Y.; Yin, J.; Liu, Z.; Du, Q. Enhanced antiarthritic efficacy by nanoparticles of (-)-epigallocatechin gallate-glucosamine-casein. J. Agric. Food. Chem. 2019, 67, 6476–6486. [Google Scholar] [CrossRef]
- Rivera, M.C.; Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Hollow chitosan/alginate nano-capsules for bioactive compound delivery. Int. J. Biol. Macromol. 2015, 79, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ge, S.; Yang, J.; Xu, Y.; Zhao, M.; Xiong, L.; Sun, Q. Adsorption mechanism of polyphenols onto starch nanoparticles and enhanced antioxidant activity under adverse conditions. J. Funct. Foods. 2016, 26, 632–644. [Google Scholar] [CrossRef]
- Shamekhi, F.; Tamjid, E.; Khajeh, K. Development of chitosan coated calcium-alginate nano-capsules for oral delivery of liraglutide to diabetic patients. Int. J. Biol. Macromol. 2018, 120, 460–467. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Lertsutthiwong, P.; Rojsitthisak, P. Chitosan-alginate nano-capsules for encapsulation of turmeric oil. Pharmazie 2011, 66, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Schoubben, A.; Blasi, P.; Giovagnoli, S.; Rossi, C.; Ricci, M. Development of a scalable procedure for fine calcium alginate particle preparation. Chem. Eng. Sci. 2010, 160, 363–369. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nano-capsules. Mater. Sci. Eng. C 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Balaji, R.; Raghunathan, S.; Revathy, R. Levofloxacin: Formulation and in-vitro evaluation of alginate and chitosan nanospheres. Egypt. Pharm. 2015, 14, 30–35. [Google Scholar] [CrossRef]
- Bhunchu, S.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Curcumin diethyl disuccinate encapsulated in chitosan/alginate nanoparticles for improvement of its in vitro citotoxicity against MDA-MB-231 human breast cancer cells. Pharmazie 2016, 71, 691–700. [Google Scholar] [CrossRef]
- Li, P.; Dai, Y.N.; Zhang, J.P.; Wang, A.Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and evaluation of alginate–chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Saralkar, P.; Dash, A.K. Alginate nanoparticles containing curcumin and resveratrol: Preparation, characterization, and in vitro evaluation against du145 prostate cancer cell line. AAPS Pharm. Sci. Tech. 2017, 18, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Nalini, T.; Basha, S.K.; Sadiq, A.M.M.; Kumari, V.S.; Kaviyarasu, K. Development and characterization of alginate / chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug. Deliv. Sci. Technol. 2019, 52, 65–72. [Google Scholar] [CrossRef]
- Fernandes, A.; Sousa, A.; Azevedo, J.; Mateus, N.; de Freitas, V. Effect of cyclodextrins on the thermodynamic and kinetic properties of cyanidin-3-O-glucoside. Food Res. Int. 2013, 51, 748–755. [Google Scholar] [CrossRef]
- Kittisuban, P.; Lee, B.H.; Suphantharika, M.; Hamaker, B.R. Slow glucose release property of enzyme-synthesized highly branched maltodextrins differs among starch sources. Carbohydr. Polym. 2014, 107, 182–191. [Google Scholar] [CrossRef]
- Gidley, M.J. Factors affecting the crystalline type (A C) of native starches and model compounds: A rationalization of observed effects in terms of polymorphic structures. Carbohydr. Res. 1987, 161, 301–304. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Wani, I.A.; Jabeen, M.; Geelani, H.; Masoodi, F.A.; Saba, I.; Muzaffar, S. Effect of gamma irradiation on physicochemical properties of Indian Horse Chestnut (Aesculus indica Colebr.) starch. Food Hydrocoll. 2014, 35, 253–263. [Google Scholar] [CrossRef]
- Chin, F.C.; Yazid, S.N.A.M.; Pang, S.C. Preparation and characterization of starch nanoparticles for controlled release of curcumin. Int. J. Polym. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Gani, A. Ultrasonicated resveratrol loaded starch nano-capsules: Characterization, bioactivity and release behavior under in-vitro digestion. Carbohydr. Polym. 2021, 251, 117111. [Google Scholar] [CrossRef] [PubMed]
- Contri, R.V.; Soares, R.M.D.; Pohlmann, A.R.; Guterres, S.S. Structural analysis of chitosan hydrogels containing polymeric nano-capsules. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Gatti, T.H.H.; Eloy, J.O.; Ferreira, L.M.B.; da Silva, I.C.; Pavan, F.R.; Gremião, M.P.D.; Chorilli, M. Insulin-loaded polymeric mucoadhesive nanoparticles: Development, characterization and cytotoxicity evaluation. Braz. J. Pharm. 2016, 54, e17314. [Google Scholar] [CrossRef] [Green Version]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.M.; Lu, E.S.; Zhi, L.Z.; Chen, M.J.; Shi, D.; Yang, J.; Tang, Z. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of mir-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rodriguez, G.R.R. Parameters influencing the size of chitosan-tpp nano- and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef] [Green Version]
- Stoica, R.; Somoghi, R.; Ion, R.M. Preparation of chitosan-tripolyphosphate nanoparticles for the encapsulation of polyphenols extracted from rose hips. Dig. J. Nanomater. Biostructures. 2013, 8, 955–963. [Google Scholar]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian. J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.; Souza, H.; Campiña, J.; Andrade, C.; Silva, A.; Gonçalves, M.; Paschoalin, V. Edible chitosan films and their nanosized counterparts exhibit antimicrobial activity and enhanced mechanical and barrier properties. Molecules 2019, 24, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends. Food. Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Roy, P.; Parveen, S.; Ghosh, P.; Ghatak, K.; Dasgupta, S. Flavonoid loaded nanoparticles as an effective measure to combat oxidative stress in Ribonuclease A. Biochimie 2019, 162, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 981–991. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.; Yue, X.; Fu, R.; Liang, J.; Gao, X. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food. Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef]
- Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Nunes, M.R.; Benvenutti, E.V.; Luz, S.R.; D’Avila, S.F.; Rutz, J.K. Microencapsulation of gallic acid in chitosan, b-cyclodextrin and xanthan. Ind. Crops. Prod. 2013, 46, 138–146. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Percival, S.S.; Cheng, Q. Preparation, characterization, and induction of cell apoptosis of cocoa procyanidins-gelatin-chitosan nanoparticle. Eur. J. Pharm. Biopharm. 2012, 82, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticle for delivery of quercetin. Colloids. Surf. B. 2010, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Pan, C.; Sun, Y.; Hou, Z.; Ye, H.; Zeng, X. Optimization of fabrication parameters to produce chitosan-tripolyphosphate nanoparticles for delivery of tea catechins. J. Agric. Food. Chem. 2008, 56, 7451–7458. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 42–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, N.; Ranjan, S.; Gandhi, M. Nanoemulsions in food: Market demand. Environ. Chem. Lett. 2019, 17, 1003–1009. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food. Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Sarkar, D. Encapsulation of bioactive compounds using nanoemulsions. Environ. Chem. Lett. 2018, 16, 59–70. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of Lipid-Based Encapsulation on the Bioaccessibility and Bioavailability of Phenolic Compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Hou, R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2018, 242, 527–532. [Google Scholar] [CrossRef]
- Frozza, R.L.; Bernardi, A.; Paese, K.; Hoppe, J.B.; Silva, T.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Characterization of trans-resveratrol-loaded lipid-core nano-capsules and tissue distribution studies in rats. J. Biomed. Nanotechnol. 2010, 6, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsì, F. Evaluation of the stability and antioxidant activity of nano-encapsulated resveratrol during in vitro digestion. J. Agric. Food. Chem. 2011, 59, 12352–12360. [Google Scholar] [CrossRef]
- Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials 2020, 10, 2464. [Google Scholar] [CrossRef]
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci. 2019, 84, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Taylor, T.M.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Liposomal nano-capsules in food science and agriculture. Crit. Rev. Food. Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef]
- Matos, C.; Moutinho, C.; Lobão, P. Liposomes as a model for the biological membrane: Studies on daunorubicin bilayer interaction. J. Membr. Biol. 2012, 245, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.K. Current demands for food-approved liposome nanoparticles in food and safety sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef] [Green Version]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 19, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.C.N.T.F.; Pereira, P.R.; Paschoalin, V.M.F. Preparation and characterization of nanoliposomes for the entrapment of bioactive hydrophilic globular proteins. J. Vis. Exp. 2019, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Fidan-Yardimci, M.; Akay, S.; Sharifi, F.; Sevimli-Gur, C.; Ongen, G.; Yesil-Celiktas, O. A novel niosome formulation for encapsulation of anthocyanins and modelling intestinal transport. Food. Chem. 2019, 293, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [Green Version]
- Khan, D.H.; Bashir, S.; Figueiredo, P.; Santos, H.A.; Khan, M.I.; Peltonen, L. Process optimization of ecological probe sonication technique for production of rifampicin loaded niosomes. J. Drug. Deliv. Sci. Technol. 2019, 50, 27–33. [Google Scholar] [CrossRef]
- Giordani, B.; Basnet, P.; Mishchenko, E.; Luppi, B.; Škalko-Basnet, N. Utilizing Liposomal quercetin and gallic acid in localized treatment of vaginal candida infections. Pharmaceutics 2019, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.R. Bioactive entrapment and targeting using nanocarrier technologies: An introduction. In Nanocarrier Technologies: Frontiers of Nanotherapy; Mozafari, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–16. [Google Scholar]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Zhou, W.; Liu, W.; Liu, C.; McClements, D.J. Encapsulation of lipophilic polyphenols into nanoliposomes using pH-driven method: Advantages and disadvantages. J. Agric. Food Chem. 2019, 67, 7506–7511. [Google Scholar] [CrossRef]
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC. Adv. 2017, 7, 25978–25986. [Google Scholar] [CrossRef] [Green Version]
- Shin, G.H.; Chung, S.K.; Kim, J.T.; Joung, H.J.; Park, H.J. Preparation of chitosan-coated nanoliposomes for improving the mucoadhesive property of curcumin using the ethanol injection method. J. Agric. Food. Chem. 2013, 61, 11119–11126. [Google Scholar] [CrossRef]
- Filipović-Grcić, J.; Skalko-Basnet, N.; Jalsenjak, I. Mucoadhesive chitosan-coated liposomes: Characteristics and stability. J. Microencapsul. 2001, 18, 3–12. [Google Scholar] [CrossRef]
- Andersen, T.; Bleher, S.; Eide, F.G.; Tho, I.; Mattsson, S.; Škalko-Basnet, N. Chitosan in mucoadhesive drug delivery: Focus on local vaginal therapy. Mar. Drugs. 2015, 13, 222–236. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, E.B.; Almeda, R.A.; Vidallon, M.L.P.; Reyes, C.T. Enhanced bioactivity and efficient delivery of quercetin through nanoliposomal encapsulation using rice bran phospholipids. J. Sci. Food. Agric. 2019, 99, 1980–1989. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: A review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Simon, C.D.; Sagdic, O.; Capanoglu, E. Effect of dietary fiber (inulin) addition on phenolics and in vitro bioaccessibility of tomato sauce. Int. Food Res. J. 2018, 106, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Tew, B.Y.; Xu, X.; Wang, H.J.; Murphy, P.A.; Hendrich, S. A diet high in wheat fiber decreases the bioavailability of soybean isoflavones in a single meal fed to women. J. Nutr. 1996, 126, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, M.; Iwami, T.; Hirayama, K.; Itoh, K. High fiber diet supplemented with rice bran hemicellulose may reduce daidzein absorption in mice. Food Sci. Technol. 2009, 15, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Nizamova, A.; Ziyatdinova, G.; Budnikov, G. Electrogenerated bromine as a coulometric reagent for the estimation of the bioavailability of polyphenols. J. Anal. Chem. 2011, 66, 301–309. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends. Food. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Peñalva, R.; Esparza, I.; Morales-Gracia, J.; González-Navarro, C.J.; Larrañeta, E.; Irache, J.M. Casein nanoparticles in combination with 2-hydroxypropyl-β-cyclodextrin improves the oral bioavailability of quercetin. Int. J. Pharm. 2019, 570, 118652. [Google Scholar] [CrossRef]
- Basavaraj, S.; Betageri, G.V. Improved oral delivery of resveratrol using proliposomal formulation: Investigation of various factors contributing to prolonged absorption of unmetabolized resveratrol. Expert. Opin. Drug. Deliv. 2014, 11, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, M.D.; Vesa, C.M.; et al. Pleotropic effects of polyphenols in cardiovascular system. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Epigenetic and disease targets by polyphenols. Curr. Pharm. Des. 2013, 19, 6156–6185. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the predimed. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatakrishnan, K.; Chiu, H.F.; Cheng, J.C.; Chang, Y.H.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Tsai, K.S.; Wang, C.K. Comparative studies on the hypolipidemic, antioxidant and hepatoprotective activities of catechin-enriched green and oolong tea in a double-blind clinical trial. Food Funct. 2018, 9, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Gholami, A.; Hooshmand, E.; Tavallaie, S.; Nobakht, M.G.B.F. The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Drug Metab. Pers. Ther. 2019, 34. [Google Scholar] [CrossRef]
- Barrera-Reyes, P.K.; Hernández-Ramírez, N.; Cortés, J.; Poquet, L.; Redeuil, K.; Rangel-Escareño, C.; Kussmann, M.; Silva-Zolezzi, I.; Tejero, M.E. Gene expression changes by high-polyphenols cocoa powder intake: A randomized crossover clinical study. Eur. J. Nutr. 2019, 58, 1887–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vors, C.; Couillard, C.; Paradis, M.E.; Gigleux, I.; Marin, J.; Vohl, M.C.; Couture, P.; Lamarche, B. Supplementation with resveratrol and curcumin does not affect the inflammatory response to a high-fat meal in older adults with abdominal obesity: A randomized, placebo-controlled crossover trial. J. Nutr. 2018, 148, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute cocoa flavanols intake has minimal effects on exercise-induced oxidative stress and nitric oxide production in healthy cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 28. [Google Scholar] [CrossRef] [Green Version]
- Boarescu, P.M.; Boarescu, I.; Bocșan, I.C.; Pop, R.M.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Râjnoveanu, R.M.; Bolboacă, S.D. Curcumin Nanoparticles protect against isoproterenol induced myocardial infarction by alleviating myocardial tissue oxidative stress, electrocardiogram, and biological changes. Molecules 2019, 24, 2802. [Google Scholar] [CrossRef] [Green Version]
- Boarescu, P.M.; Chirilă, I.; Bulboacă, A.E.; Bocșan, I.C.; Pop, R.M.; Gheban, D.; Bolboacă, S.D. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid. Med. Cell. Longev. 2019, 2019, 7847142. [Google Scholar] [CrossRef] [PubMed]
- Boarescu, P.M.; Boarescu, I.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Pop, R.M.; Râjnoveanu, R.M.; Bolboacă, S.D. Antioxidant and anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants 2019, 8, 504. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Ray, A.; Rana, S.; Banerjee, D.; Mitra, A.; Datta, R.; Naskar, S.; Sarkar, S. Improved bioavailability of targeted Curcumin delivery efficiently regressed cardiac hypertrophy by modulating apoptotic load within cardiac microenvironment. Toxicol. Appl. Pharmacol. 2016, 290, 54–65. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Larraneta, E.; González-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. J. Agric. Food. Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef] [Green Version]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef]
- Penalva, R.; González-Navarro, C.J.; Gamazo, C.; Esparza, I.; Irache, J.M. Zein nanoparticles for oral delivery of quercetin: Pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomedicine 2017, 13, 103–110. [Google Scholar] [CrossRef]
- Beconcini, D.; Felice, F.; Zambito, Y.; Fabiano, A.; Piras, A.M.; Macedo, M.H.; Sarmento, B.; Di Stefano, R. Anti-inflammatory effect of cherry extract loaded in polymeric nanoparticles: Relevance of particle internalization in endothelial cells. Pharmaceutics 2019, 11, 500. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef]
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020, 14, 247–250. [Google Scholar] [CrossRef]
- Daiber, A.; Hahad, O.; Andreadou, I.; Steven, S.; Daub, S.; Münzel, T. Redox-related biomarkers in human cardiovascular disease-classical footprints and beyond. Redox Biol. 2021, 42, 101875. [Google Scholar] [CrossRef] [PubMed]
- Bulboacă, A.E.; Porfire, A.; Bolboacă, S.D.; Nicula, C.A.; Feștilă, D.G.; Roman, A.; Râjnoveanu, R.M.; Râjnoveanu, A.; Dogaru, G.; Boarescu, P.M.; et al. Protective effects of liposomal curcumin on oxidative stress/antioxidant imbalance, metalloproteinases 2 and -9, histological changes and renal function in experimental nephrotoxicity induced by gentamicin. Antioxidants 2021, 10, 325. [Google Scholar] [CrossRef]
- Wu, G.R.; Cheserek, M.; Shi, Y.H.; Shen, L.Y.; Yu, J.; Le, G.W. Elevated plasma dityrosine in patients with hyperlipidemia compared to healthy individuals. Ann. Nutr. Metab. 2015, 66, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yücel, Ç.; Karatoprak, G.Ş.; Aktaş, Y. Nanoliposomal Resveratrol as a Novel Approach to Treatment of Diabetes Mellitus. J. Nanosci. Nanotechnol. 2018, 18, 3856–3864. [Google Scholar] [CrossRef]
- Rozanska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food. Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Colorado, D.; Fernandez, M.; Orozco, J.; Lopera, Y.; Muñoz, D.L.; Acín, S.; Balcazar, N. Metabolic Activity of Anthocyanin Extracts Loaded into Non-ionic Niosomes in Diet-Induced Obese Mice. Pharm. Res. 2020, 37. [Google Scholar] [CrossRef]
- Wilkinson, L.J.; White, R.J.; Chipman, J.K. Silver and nanoparticles of silver in wound dressings: A review of efficacy and safety. J. Wound. Care. 2011, 20, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Song, M.Y.; Song, K.S.; Ryu, H.R.; Yoon, J.U.; Jeon, K.S.; Jeong, J.; Han, B.S.; et al. Subchronic inhalation toxicity of gold nanoparticles. Part. Fibre. Toxicol. 2011, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E.; Roblegg, E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Saha, K.; Moyano, D.F.; Rotello, V.M. Protein coronas suppress the hemolytic activity of hydrophilic and hydrophobic nanoparticles. Mater Horiz. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Kharazian, B.; Hadipour, N.L.; Ejtehadi, M.R. Understanding the nanoparticle–protein corona complexes using computational and experimental methods. Int. J. Biochem. Cell. Biol. 2016, 75, 162–174. [Google Scholar] [CrossRef]
- Higashisaka, K.; Nagano, K.; Yoshioka, Y.; Tsutsumi, Y. Nano-safety research: Examining the associations among the biological effects of nanoparticles and their physicochemical properties and kinetics. Biol. Pharm. Bull. 2017, 40, 243–248. [Google Scholar] [CrossRef] [Green Version]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, 5327. [Google Scholar] [CrossRef] [Green Version]
- Siegrist, S.; Cörek, E.; Detampel, P.; Sandström, J.; Wick, P.; Huwyler, J. Preclinical hazard evaluation strategy for nanomedicines. Nanotoxicology 2019, 13, 73–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration (FDA). PART 182 Substances generally recognized as safe. In Code of Federal Regulations; Title 21, Volume 3, Cite: 21CFR182, Revised on 1 April 2021; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2021. [Google Scholar]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Cruz, C.N.; Chen, M.L.; Kapoor, M.; Lee, S.L.; Tyner, K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017, 12, 523–529. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Chamcheu, J.C.; Mukhtar, H. Impact of nanotechnology in cancer: Emphasis on nanochemoprevention. Int. J. Nanomed. 2012, 7, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Bebawy, M.; Loo, C.Y.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Fabrication of curcumin micellar nanoparticles with enhanced anti-cancer activity. J. Biomed. Nanotechnol. 2015, 11, 1093–1105. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Parliament and the Council of the European Union. Directive 2010/63/eu of the european parliament and of the council of 22 september 2010 on the protection of animals used for scientific purposes. OJEU 2010, 53, 33–79. [Google Scholar]
- Felice, F.; Zambito, Y.; Belardinelli, E.; D’Onofrio, C.; Fabiano, A.; Balbarini, A.; Di Stefano, R. Delivery of natural polyphenols by polymeric nanoparticles improves the resistance of endothelial progenitor cells to oxidative stress. Eur. J. Pharm. Sci. 2013, 50, 393–399. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Tang, X.J.; Du, L.; Li, F. In vitro and in vivo evaluation of functionalized chitosan-Pluronic micelles loaded with myricetin on glioblastoma cancer. Nanomedicine 2016, 12, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Saifi, M.A.; Khan, W.; Godugu, C. Cytotoxicity of nanomaterials: Using nanotoxicology to address the safety concerns of nanoparticles. Pharm. Nanotechnol. 2018, 6, 3–16. [Google Scholar] [CrossRef]
- Siddique, M.I.; Katas, H.; Jamil, A.; Mohd Amin, M.C.I.; Ng, S.F.; Zulfakar, M.H.; Nadeem, S.M. Potential treatment of atopic dermatitis: Tolerability and safety of cream containing nanoparticles loaded with hydrocortisone and hydroxytyrosol in human subjects. Drug Deliv. Transl. Res. 2017, 9, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez-Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [Green Version]
| Wall Polymer | Polyphenol | Cytotoxicity Assay | Cell Culture | Exposure Time | NP Concentration | Cell Viability | Reference |
|---|---|---|---|---|---|---|---|
| Thiolated quaternary ammonium-chitosan | Grape seed polyphenols | WST-1 | EPCs | 3 h | 0–4.9 μg·mL−1 | 100% | [202] |
| Soy protein nanoemulsion | Catechins | MTT | Caco-2 | 24 h | 75 and 500 μg·mL−1 | 95 and 85% | [203] |
| Starch NPs | Catechin, epicatechin, epigallocatechin, proanthocyanidin, individually encapsulated and assayed | MTT | MEF | 24 h | 0.015–0.250 μg·mL−1 | >80% | [75] |
| Transcutol-based nanoemulsion | Curcumin | MTT | nasal epithelium | 72 h | 0.012–1 mg·mL−1 | 100% | [127] |
| Chitosan-alginate NPs | Quercetin | MTT LDH | HepG2 | 24 h | 10 μg·mL−1 | 100% | [77] |
| Zein-casein-lysine NPs | Ferulic acid | MTT | Caco-2 | 24 h | 0.1–1000 μg·mL−1 | 94% | [72] |
| Zein-casein-lysine NPs | Ferulic acid | MTT | Caco-2 | 4 h | 100 and 300 μg·mL−1 | 100% | [72] |
| Liposomes NPs | Resveratrol | MTT | ß-pancreatic | 24 h | 25 μg·mL−1 | >90% | [181] |
| Quaternary ammonium chitosan NPs | Cherry extract | WST-1 | HUVEC | 2 h | 2 μg·mL−1 GAE | 77% | [174] |
| liposome-phosphatidylcholine from soybean | Quercetin and gallic acid alone and co-loaded | CC8-K | RAW 264.7 | 24 h | 1, 10 and 50 μg·mL−1 | 100% | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trindade, L.R.; da Silva, D.V.T.; Baião, D.d.S.; Paschoalin, V.M.F. Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases. Molecules 2021, 26, 4621. https://doi.org/10.3390/molecules26154621
Trindade LR, da Silva DVT, Baião DdS, Paschoalin VMF. Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases. Molecules. 2021; 26(15):4621. https://doi.org/10.3390/molecules26154621
Chicago/Turabian StyleTrindade, Lucileno Rodrigues, Davi Vieira Teixeira da Silva, Diego dos Santos Baião, and Vania Margaret Flosi Paschoalin. 2021. "Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases" Molecules 26, no. 15: 4621. https://doi.org/10.3390/molecules26154621
APA StyleTrindade, L. R., da Silva, D. V. T., Baião, D. d. S., & Paschoalin, V. M. F. (2021). Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases. Molecules, 26(15), 4621. https://doi.org/10.3390/molecules26154621








