Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry
Abstract
:1. Introduction
- (a)
- Construction of 1,2,3-triazole systems in biologically relevant compounds by means of classical and “green chemistry” conditions involving ultrasound chemistry and mechanochemistry.
- (b)
- Construction of 1,2,4-triazole systems in biologically relevant compounds by means of classical and “green chemistry” conditions involving ultrasound chemistry and mechanochemistry.
- (c)
- The mechanochemical cyclo-reversion of 1,2,3-triazole compounds and the scientific discussion on the topic that it could be extremely stimulating as mechanochemistry seems to provide a method by which reactive azide or alkyne intermediates could be selectively unmasked.
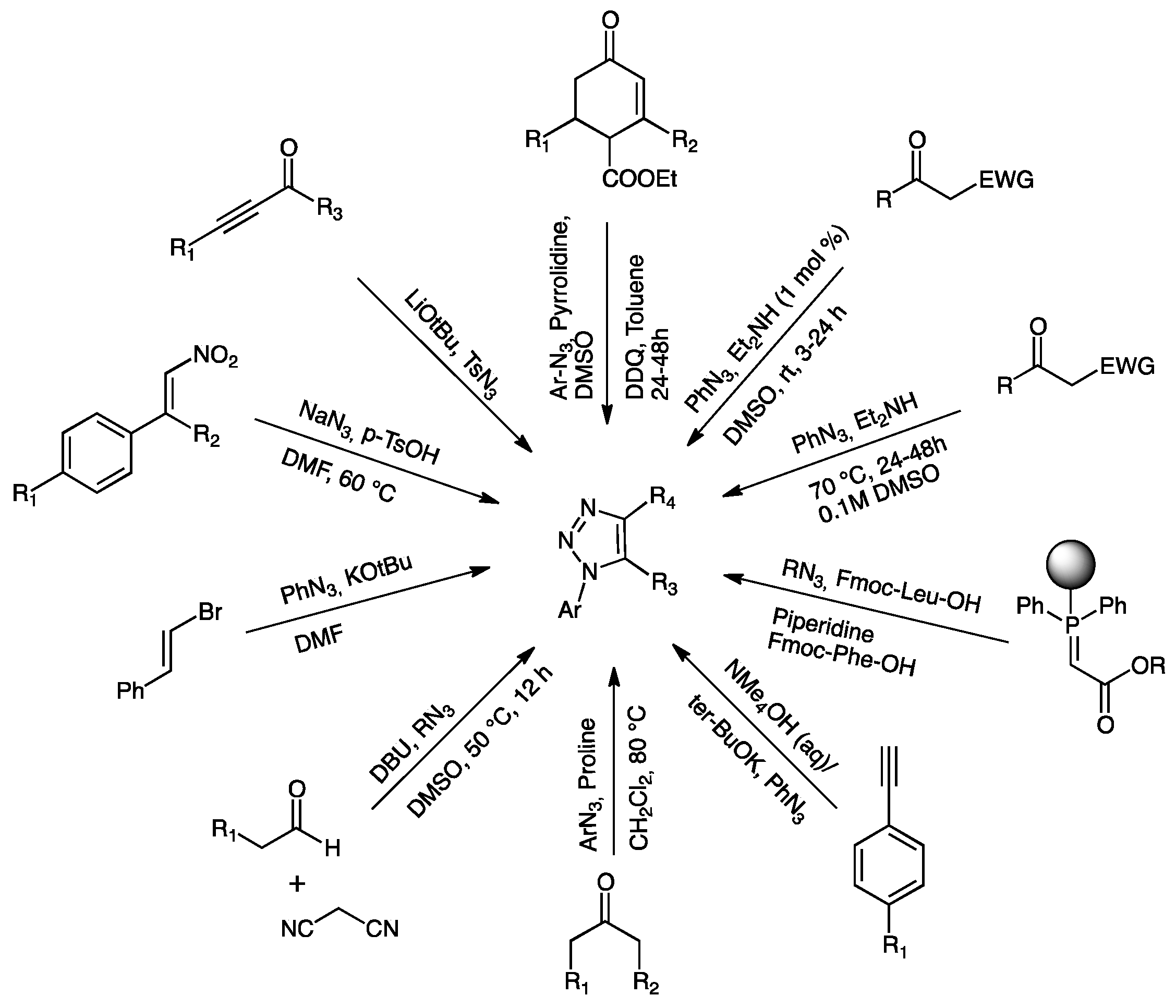
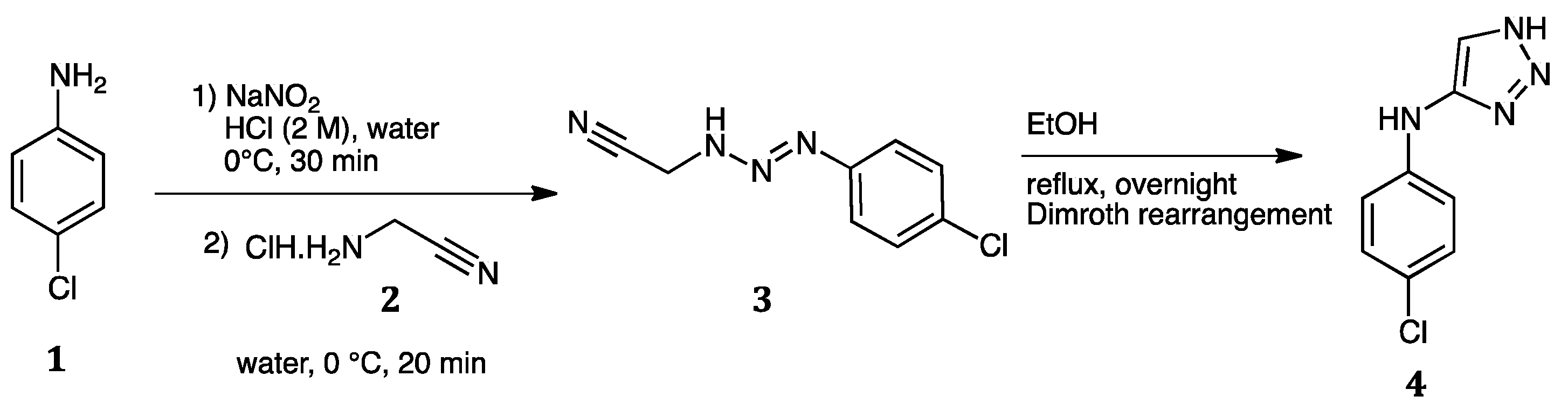
2. 1,2,3-Triazole Systems
2.1. Ultrasound Assisted Syntheses of 1,2,3-Triazoles
2.2. Mechanochemical Syntheses of 1,2,3-Triazoles
3. 1,2,4-Triazole Systems
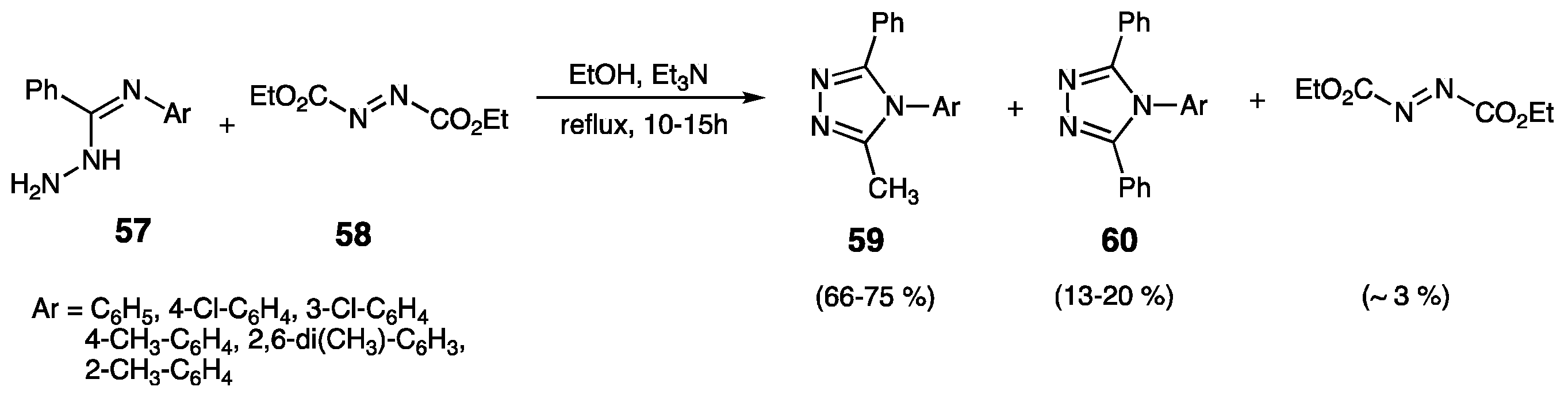
3.1. From Amidrazones
3.2. From Hydrazides
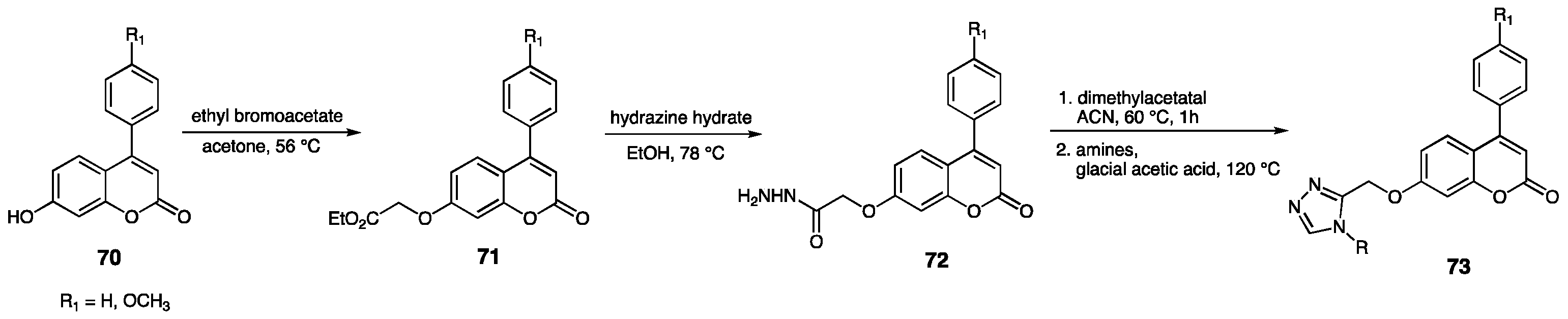
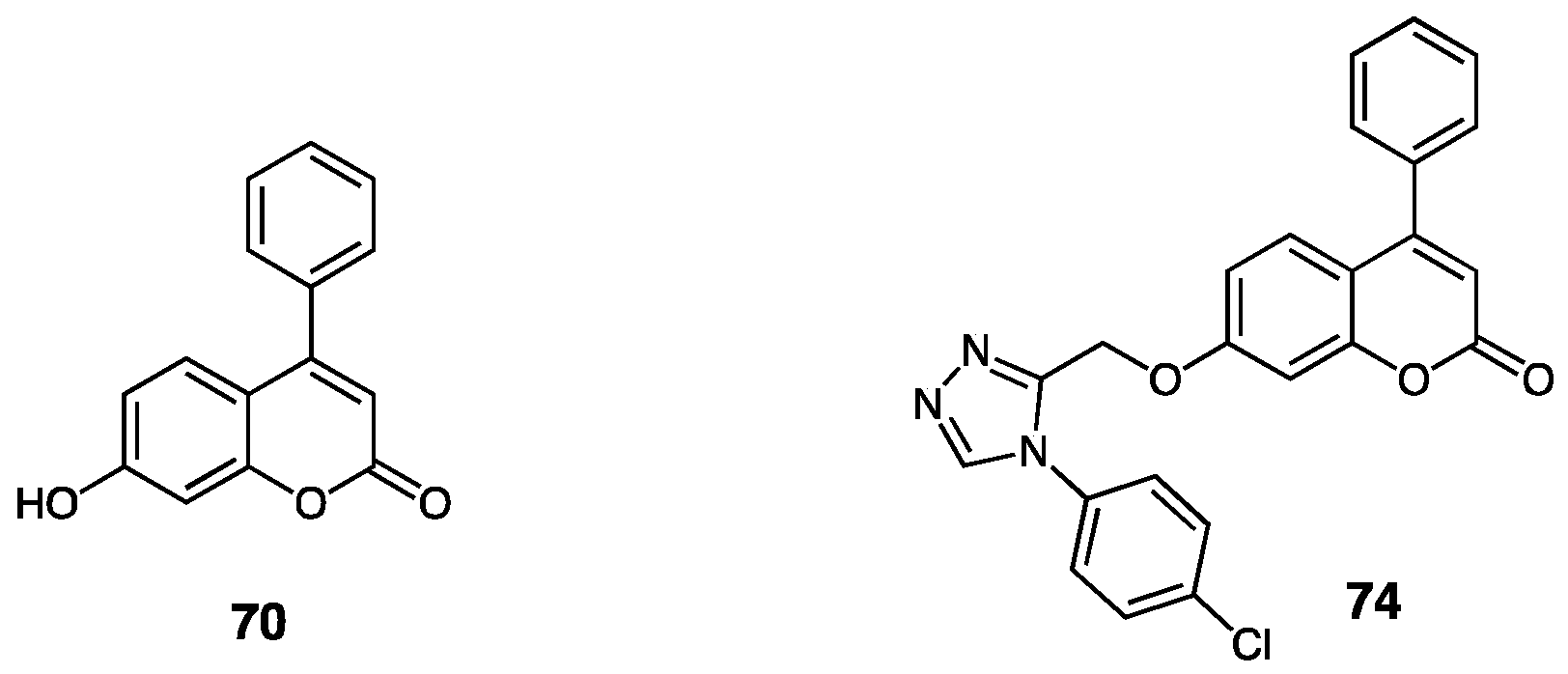
3.3. Annulated 1,2,4-Triazole Systems
4. Mechanochemical Cycloreversion of 1,2,3-Triazoles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Anastas, P.T.; Warner, J.C. Green Chemistry, Theory and Practice; Oxford University Press: Oxford, UK, 2000; ISBN 0198502346/9780198502340.
- Poliakoff, M.; Licence, P. Sustainable Technology: Green Chemistry. Available online: https://www.nature.com/articles/450810a (accessed on 16 September 2018).
- Varma, R.S. Chemical Activation by Mechanochemical Mixing, Microwave and Ultrasonic Irradiation. Green Chem. 2008, 10, 1129–1130. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2002; ISBN 978-3-527-60054-0. [Google Scholar]
- Bonrath, W.; Schmidt, R.A.P. Ultrasound in Synthetic Organic Chemistry. Adv. Org. Synth. 2005, 1, 81–117. [Google Scholar] [CrossRef]
- Li, J.T.; Wang, S.X.; Li, G.F.C.; Li, T.S. Some Applications of Ultrasound Irradiation in Organic Synthesis. Available online: http://www.eurekaselect.com/79542/article (accessed on 16 September 2018).
- Didenko, Y.T.; Suslick, K.S. The Energy Efficiency of Formation of Photons, Radicals and Ions during Single-Bubble Cavitation. Nature 2002, 418, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Flannigan, D.J. Inside a Collapsing Bubble: Sonoluminescence and the Conditions during Cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical Synthesis of Nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, R.B.N.; Varma, R.S. Alternative Energy Input: Mechanochemical, Microwave and Ultrasound-Assisted Organic Synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Gaudino, E.; Tagliapietra, S.; Mantegna, S.; Cravotto, G. Mechanochemical and Sonochemical Heterocyclizations. Chem. Heterocycl. Compd. 2016, 52, 856–865. [Google Scholar] [CrossRef]
- Stauch, T.; Dreuw, A. Advances in Quantum Mechanochemistry: Electronic Structure Methods and Force Analysis. Chem. Rev. 2016, 116, 14137–14180. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Technology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0865426848. [Google Scholar]
- Urakaev, F.K.; Boldyrev, V.V. Mechanism and kinetics of mechanochemical processes in comminuting devices: 1. Theory. Powder Technol. 2000, 107, 93–107. [Google Scholar] [CrossRef]
- Urakaev, F.K.; Boldyrev, V.V. Mechanism and kinetics of mechanochemical processes in comminuting devices: 2. Applications of the theory. Experiment. Powder Technol. 2000, 107, 197–206. [Google Scholar] [CrossRef]
- Boldyrev, V.V.; Tkáčová, K. Mechanochemistry of solids: Past, Present and Prospects. J. Mater. Synth. Process. 2000, 8, 121–132. [Google Scholar] [CrossRef]
- Cintas, P.; Tagliapietra, S.; Caporaso, M.; Tabasso, S.; Cravotto, G. Enabling Technologies Built on a Sonochemical Platform: Challenges and Opportunities. Ultrason. Sonochem. 2015, 25, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [Green Version]
- Dolotko, O.; Wiench, J.W.; Dennis, K.W.; Pecharsky, V.K.; Balema, V.P. Mechanically induced reactions in organic solids: Liquid eutectics or solid-state processes? New J. Chem. 2010, 34, 25–28. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, Y.; Xu, Y.; Weng, W. Mechanochemistry of Topological Complex Polymer Systems. Top. Curr. Chem. 2015, 369, 135–207. [Google Scholar] [CrossRef]
- Cintas, P.; Cravotto, G.; Barge, A.; Martina, K. Interplay between Mechanochemistry and Sonochemistry. In Polymer Mechanochemistry; Boulatov, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 239–284. ISBN 978-3-319-22825-9. [Google Scholar]
- Michalchuk, A.A.L.; Tumanov, I.A.; Boldyreva, E.V. Complexities of mechanochemistry: Elucidation of processes occurring in mechanical activators via implementation of a simple organic system. CrystEngComm 2013, 15, 6403–6412. [Google Scholar] [CrossRef]
- Wang, J. Characterization and Applications of Force-Induced Reactions. Ph.D. Thesis, Duke University, Durham, NC, USA, 2015. [Google Scholar]
- Haruta, N.; Sato, T.; Tanaka, K.; Baron, M. Reaction mechanism in the mechanochemical synthesis of dibenzophenazine: Application of vibronic coupling density analysis. Tetrahedron Lett. 2013, 54, 5920–5923. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Baron, M.; Chamayou, A.; Baltas, M.; Guidetti, B.; Haruta, N.; Tanaka, K.; Sato, T. Lowering the Activation Energy under Mechanochemical Conditions: The Case of 2,3-diphenylquinoxaline. ChemistrySelect 2016, 1, 984–988. [Google Scholar] [CrossRef]
- Ribas-Arino, J.; Shiga, M.; Marx, D. Understanding Covalent Mechanochemistry. Angew. Chem. Int. Ed. 2009, 48, 4190–4193. [Google Scholar] [CrossRef]
- Haruta, N.; Oliveira, P.F.M.; Sato, T.; Tanaka, K.; Baron, M. Force-Induced Dissolution of Imaginary Mode in Mechanochemical Reaction: Dibenzophenazine Synthesis. J. Phys. Chem. C 2019, 123, 21581–21587. [Google Scholar] [CrossRef] [Green Version]
- Smalø, H.S.; Rybkin, V.V.; Klopper, W.; Helgaker, T.; Uggerud, E. Mechanochemistry: The Effect of Dynamics. J. Phys. Chem. A 2014, 118, 7683–7694. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Bonnamour, J.; Métro, T.X.; Martinez, J.; Lamaty, F. Environmentally Benign Peptide Synthesis Using Liquid-Assisted Ball-Milling: Application to the Synthesis of Leu-Enkephalin. Green Chem. 2013, 15, 1116–1120. [Google Scholar] [CrossRef]
- Tumanov, I.A.; Achkasov, A.F.; Boldyreva, E.V.; Boldyrev, V.V. Following the products of mechanochemical synthesis step by step. CrystEngComm 2011, 13, 2213–2216. [Google Scholar] [CrossRef]
- Užarević, K.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Puškarić, A.; Friščić, T.; Halasz, I. Exploring the Effect of Temperature on a Mechanochemical Reaction by in Situ Synchrotron Powder X-ray Diffraction. Cryst. Growth Des. 2016, 16, 2342–2347. [Google Scholar] [CrossRef]
- Kubota, K.; Takahashi, R.; Ito, H. Mechanochemistry Allows Carrying out Sensitive Organometallic Reactions in Air: Glove-Box-and-Schlenk-Line-Free Synthesis of Oxidative Addition Complexes from Aryl Halides and Palladium(0). Chem. Sci. 2019, 10, 5837–5842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingner, F.J.L.; Giustra, Z.X.; Novosedlik, S.; Orthaber, A.; Gates, P.J.; Dyrager, C.; Pilarski, L.T. Mechanochemical Synthesis of (Hetero)Aryl Au(I) Complexes. Green Chem. 2020, 22, 5648–5655. [Google Scholar] [CrossRef]
- Takacs, L. The Historical Development of Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Ribas-Arino, J.; Marx, D. Covalent Mechanochemistry: Theoretical Concepts and Computational Tools with Applications to Molecular Nanomechanics. Chem. Rev. 2012, 112, 5412–5487. [Google Scholar] [CrossRef]
- Toda, F.; Tanaka, K.; Iwata, S. Oxidative Coupling Reactions of Phenols with Iron(III) Chloride in the Solid State. J. Org. Chem. 1989, 54, 3007–3009. [Google Scholar] [CrossRef]
- Kaupp, G. Solvent-Less Organic Synthesis. In Kirk-Othmer Encyclopedia of Chemical Technology; Othmer, K., Ed.; Wiley Online Library: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Baron, M. Towards a Greener Pharmacy by More Eco Design. Waste Biomass Valorization 2012, 3, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical Synthesis of Small Organic Molecules. Beilstein, J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef]
- Carlier, L.; Baron, M.; Chamayou, A.; Couarraze, G. Use of co-grinding as a solvent-free solid state method to synthesize dibenzophenazines. Tetrahedron Lett. 2011, 52, 4686–4689. [Google Scholar] [CrossRef] [Green Version]
- Carlier, L.; Baron, M.; Chamayou, A.; Couarraze, G. Greener pharmacy using solvent-free synthesis: Investigation of the mechanism in the case of dibenzophenazine. Powder Technol. 2013, 240, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Konnert, L.; Gauliard, A.; Lamaty, F.; Martinez, J.; Colacino, E. Solventless Synthesis of N-Protected Amino Acids in a Ball Mill. ACS Sustain. Chem. Eng. 2013, 1, 1186–1191. [Google Scholar] [CrossRef]
- Mashkouri, S.; Naimi-Jamal, M.R. Mechanochemical solvent-free and catalyst-free one-pot synthesis of pyrano[2,3-d]pyrimidine-2,4(1H,3H)-diones with quantitative yields. Molecules 2009, 14, 474–479. [Google Scholar] [CrossRef]
- Métro, T.X.; Bonnamour, J.; Reidon, T.; Sarpoulet, J.; Martinez, J.; Lamaty, F. Mechanosynthesis of amides in the total absence of organic solvent from reaction to product recovery. Chem. Commun. 2012, 48, 11781–11783. [Google Scholar] [CrossRef]
- Ranu, B.; Stolle, A. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Royal Society of Chemistry: Cambridge, UK, 2015; ISBN 978-1-84973-945-0. [Google Scholar]
- Rodríguez, B.; Rantanen, T.; Bolm, C. Solvent-free asymmetric organocatalysis in a ball mill. Angew. Chem. Int. Ed. 2006, 118, 7078–7080. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Štrukil, V.; Bartolec, B.; Portada, T.; Đilović, I.; Halasz, I.; Margetić, D. One-pot mechanosynthesis of aromatic amides and dipeptides from carboxylic acids and amines. Chem. Commun. 2012, 48, 12100–12102. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Z.; Jin, C.; Xu, L.; Wu, Q.; Su, W. Mechanically activated synthesis of 1,3,5-triaryl-2-pyrazolines by high speed ball milling. Green Chem. 2009, 11, 163–165. [Google Scholar] [CrossRef]
- Qiu, Y.; Puni, K.T.; Duplan, C.C.; Lindsay, A.C.; Sperry, J. Tetrahydrocarbazoles by mechanochemical Fisher indolization. Tetrahedron Lett. 2021, 72, 153068. [Google Scholar] [CrossRef]
- Nibin Joy, M.; Bodke, Y.D.; Telkar, S.; Bakulev, V.A. Synthesis of Coumarins Linked With 1,2,3-Triazoles under Microwave Irradiation and Evaluation of their Antimicrobial and Antioxidant Activity. J. Mex. Chem. Soc. 2020, 64, 53–73. [Google Scholar] [CrossRef] [Green Version]
- Nibin Joy, M.; Beliaev, N.; Beryozkina, T.V.; Bakulev, V.A. Design and the synthesis of 1-heteroaryl-1,2,3-triazoles connected to coumarins via ether linker. J. Heterocycl. Chem. 2020, 57, 3173–3185. [Google Scholar] [CrossRef]
- Rishikesan, R.; Ranjith, P.K.; Nibin Joy, M.; Ayyiliath, M.S.; Koti Reddy, E.; Rajeesh, P.; Karickal, R.H.; Vaishnav, B.; Kereyagalahally, H.N.; Muralidharan, A. Synthesis of some novel piperidine fused 5-thioxo-1H-1,2,4-triazoles as potential antimicrobial and antitubercular agents. J. Chem. Sci. 2021, 133, 3. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal Attributes of 1,2,3-Triazoles: Current Developments. Bioorganic Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.A.C.; Swan, M.K.; Latchem, M.J.; Boyall, D.; Pollard, J.R.; Hughes, S.W.; Westcott, J. New 4-Amino-1,2,3-Triazole Inhibitors of Indoleamine 2,3-Dioxygenase Form a Long-Lived Complex with the Enzyme and Display Exquisite Cellular Potency. ChemBioChem 2018, 19, 552–561. [Google Scholar] [CrossRef] [PubMed]
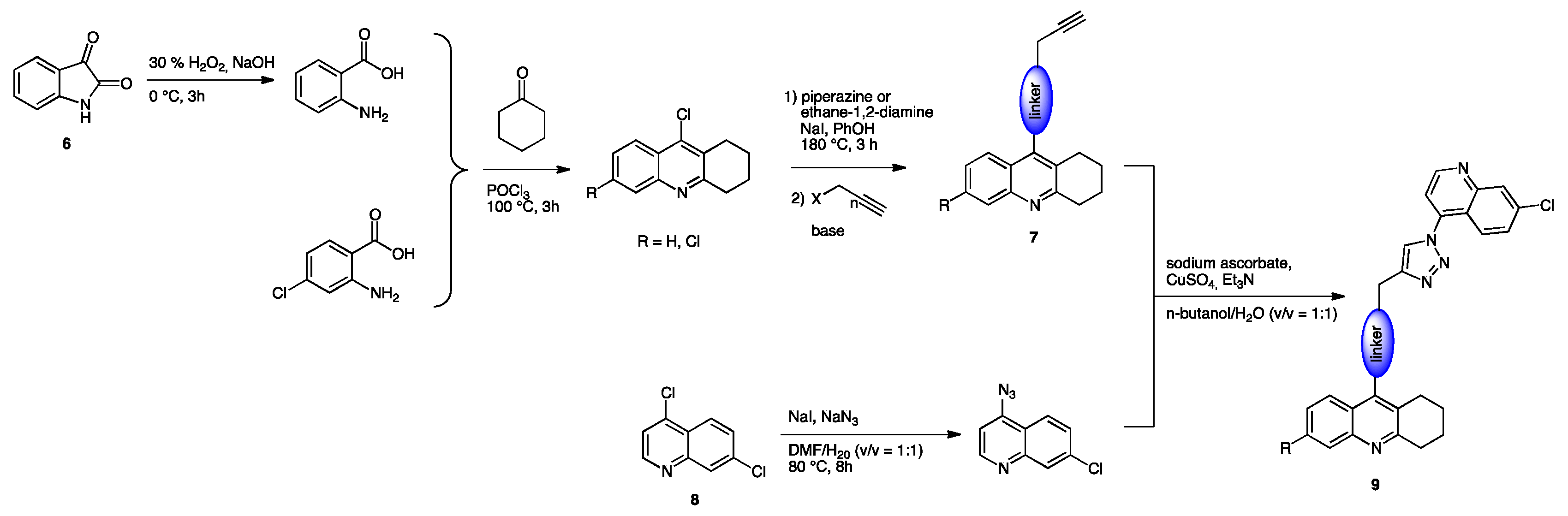
- Wu, G.; Gao, Y.; Kang, D.; Huang, B.; Huo, Z.; Liu, H.; Poongavanam, V.; Zhan, P.; Liu, X. Design, Synthesis and Biological Evaluation of Tacrine-1,2,3-Triazole Derivatives as Potent Cholinesterase Inhibitors. MedChemComm 2018, 9, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
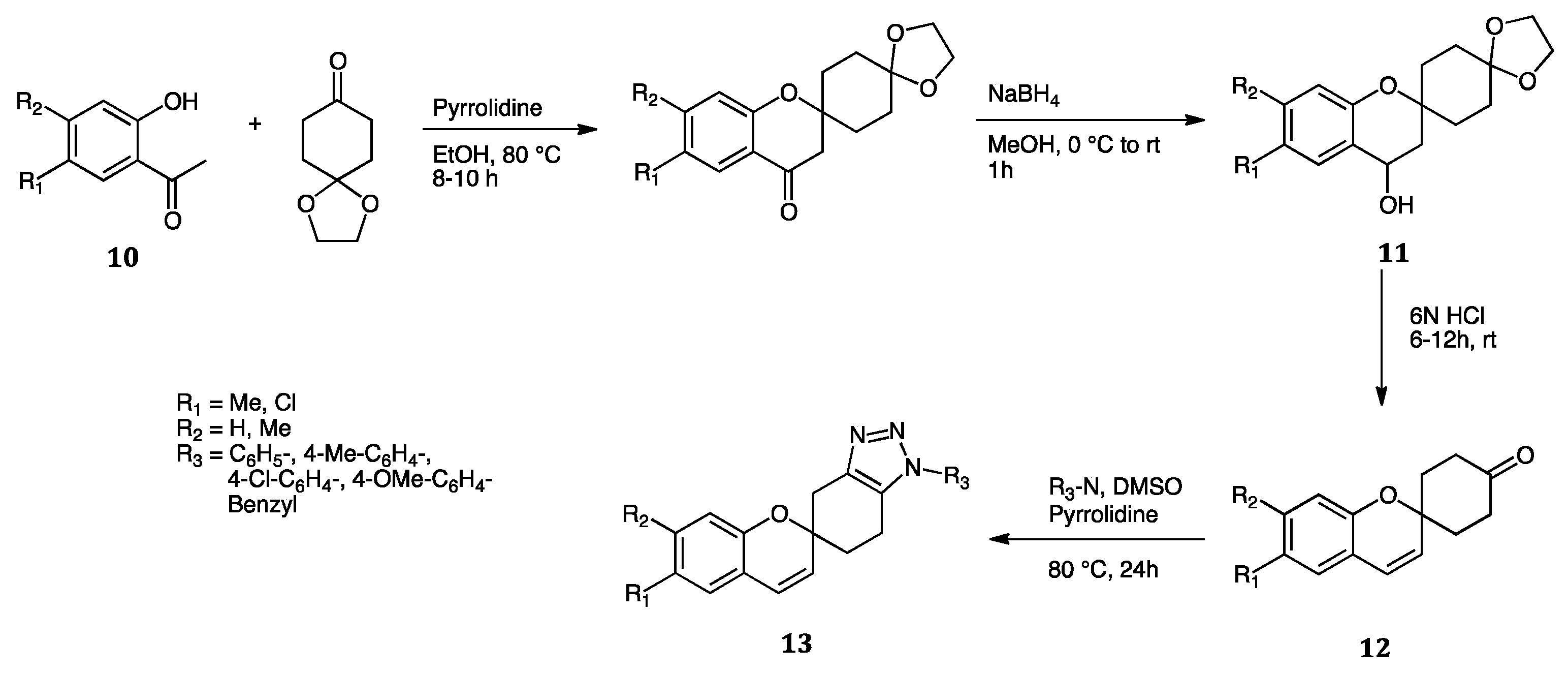
- Ashok, D.; Chiranjeevi, P.; Kumar, A.V.; Sarasija, M.; Krishna, V.S.; Sriram, D.; Balasubramanian, S. 1,2,3-Triazole-Fused Spirochromenes as Potential Anti-Tubercular Agents: Synthesis and Biological Evaluation. RSC Adv. 2018, 8, 16997–17007. [Google Scholar] [CrossRef] [Green Version]
- López-Rojas, P.; Janeczko, M.; Kubiński, K.; Amesty, Á.; Masłyk, M.; Estévez-Braun, A.; López-Rojas, P.; Janeczko, M.; Kubiński, K.; Amesty, Á.; et al. Synthesis and Antimicrobial Activity of 4-Substituted 1,2,3-Triazole-Coumarin Derivatives. Molecules 2018, 23, 199. [Google Scholar] [CrossRef] [Green Version]
- Mady, M.F.; Awad, G.E.A.; Jørgensen, K.B. Ultrasound-Assisted Synthesis of Novel 1,2,3-Triazoles Coupled Diaryl Sulfone Moieties by the CuAAC Reaction, and Biological Evaluation of Them as Antioxidant and Antimicrobial Agents. Eur. J. Med. Chem. 2014, 84, 433–443. [Google Scholar] [CrossRef]
- Nallapati, S.B.; Sreenivas, B.Y.; Bankala, R.; Parsa, K.V.L.; Sripelly, S.; Mukkanti, K.; Pal, M. 1,2,3-Triazoles Derived from Olanzapine: Their Synthesis via an Ultrasound Assisted CuAAC Method and Evaluation as Inhibitors of PDE4B. RSC Adv. 2015, 5, 94623–94628. [Google Scholar] [CrossRef]
- Rezki, N. A Green Ultrasound Synthesis, Characterization and Antibacterial Evaluation of 1,4-Disubstituted 1,2,3-Triazoles Tethering Bioactive Benzothiazole Nucleus. Molecules 2016, 21, 505. [Google Scholar] [CrossRef] [Green Version]
- Rezki, N.; Aouad, M.R. Green Ultrasound-Assisted Three-Component Click Synthesis of Novel 1H-1,2,3-Triazole Carrying Benzothiazoles and Fluorinated-1,2,4-Triazole Conjugates and Their Antimicrobial Evaluation. Acta Pharm. Zagreb Croat. 2017, 67, 309–324. [Google Scholar] [CrossRef] [Green Version]
- Vadivelu, M.; Sugirdha, S.; Dheenkumar, P.; Arun, Y.; Karthikeyan, K.; Praveen, C. Solvent-Free Implementation of Two Dissimilar Reactions Using Recyclable CuO Nanoparticles under Ball-Milling Conditions: Synthesis of New Oxindole-Triazole Pharmacophores. Green Chem. 2017, 19, 3601–3610. [Google Scholar] [CrossRef]
- Mack, J.; Shumba, M. Rate Enhancement of the Morita–Baylis–Hillman Reaction through Mechanochemistry. Green Chem. 2007, 9, 328–330. [Google Scholar] [CrossRef]
- Rubio, N.; Mei, K.C.; Klippstein, R.; Costa, P.M.; Hodgins, N.; Wang, J.T.W.; Festy, F.; Abbate, V.; Hider, R.C.; Chan, K.L.A.; et al. Solvent-Free Click-Mechanochemistry for the Preparation of Cancer Cell Targeting Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 18920–18923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, S.; Xue, F.; Lou, G.; Zhang, H.; Ma, S.; Duan, W.; Wang, W. Catalytic Enantioselective Henry Reactions of Isatins: Application in the Concise Synthesis of (S)-(−)-Spirobrassinin. Chem.—Eur. J. 2011, 17, 7791–7795. [Google Scholar] [CrossRef] [PubMed]
- DeLorbe, J.E.; Horne, D.; Jove, R.; Mennen, S.M.; Nam, S.; Zhang, F.L.; Overman, L.E. General Approach for Preparing Epidithiodioxopiperazines from Trioxopiperazine Precursors: Enantioselective Total Syntheses of (+)- and (−)-Gliocladine C, (+)-Leptosin D, (+)-T988C, (+)-Bionectin A, and (+)-Gliocladin A. J. Am. Chem. Soc. 2013, 135, 4117–4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, S.; Heiran, R.; Herrera, R.P.; Marqués-López, E. Isatin as a Strategic Motif for Asymmetric Catalysis. ChemCatChem 2013, 5, 2131–2148. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.; Agrawal, R.K.; Pandey, R. Synthesis and Systemic Toxicity Assessment of Quinine-Triazole Scaffold with Antiprotozoal Potency. Bioorganic Chem. 2019, 88, 102939. [Google Scholar] [CrossRef]
- Blasco, B.; Leroy, D.; Fidock, D.A. Antimalarial Drug Resistance: Linking Plasmodium Falciparum Parasite Biology to the Clinic. Nat. Med. 2017, 23, 917–928. [Google Scholar] [CrossRef]
- Adarsh, S.; Deepak, K.; Ram, K.A. Antileishmanial Drug Discovery: Synthetic Methods, Chemical Characteristics, and Biological Potential of Quinazolines and Its Derivatives. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2017, 16, 3–32. [Google Scholar]
- Scriven, E.F.V.; Turnbull, K. Azides: Their Preparation and Synthetic Uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar] [CrossRef]
- Sampath, S.; Vadivelu, M.; Ravindran, R.; Perumal, P.T.; Velkannan, V.; Karthikeyan, K. Synthesis of 1,2,3-Triazole Tethered 3-Hydroxy-2-Oxindoles: Promising Corrosion Inhibitors for Steel in Acidic Medium and Their Anti-Microbial Evaluation. ChemistrySelect 2020, 5, 2130–2134. [Google Scholar] [CrossRef]
- Singh, G.S.; Desta, Z.Y. Isatins as Privileged Molecules in Design and Synthesis of Spiro-Fused Cyclic Frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef]
- Hong, L.; Wang, R. Recent Advances in Asymmetric Organocatalytic Construction of 3,3′-Spirocyclic Oxindoles. Adv. Synth. Catal. 2013, 355, 1023–1052. [Google Scholar] [CrossRef]
- Trost, B.M.; Brennan, M.K. Asymmetric Syntheses of Oxindole and Indole Spirocyclic Alkaloid Natural Products. Synthesis 2009, 2009, 3003–3025. [Google Scholar] [CrossRef]
- Williams, R.M.; Cox, R.J. Paraherquamides, Brevianamides, and Asperparalines: Laboratory Synthesis and Biosynthesis. An Interim Report. Acc. Chem. Res. 2003, 36, 127–139. [Google Scholar] [CrossRef]
- Satyamaheshwar, P. 3-Substituted-3-Hydroxy-2-Oxindole, an Emerging New Scaffold for Drug Discovery with Potential Anti-Cancer and Other Biological Activities. Curr. Bioact. Compd. 2009, 5, 20–38. [Google Scholar] [CrossRef]
- Rottmann, M.; McNamara, C.; Yeung, B.K.S.; Lee, M.C.S.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D.M.; Dharia, N.V.; Tan, J.; et al. Spiroindolones, a Potent Compound Class for the Treatment of Malaria. Science 2010, 329, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Khetmalis, Y.M.; Shivani, M.; Murugesan, S.; Chandra Sekhar, K.V.G. Oxindole and Its Derivatives: A Review on Recent Progress in Biological Activities. Biomed. Pharmacother. 2021, 141, 111842. [Google Scholar] [CrossRef] [PubMed]
- Clubley, B.G. Chemical Inhibitors for Corrosion; Control, Royal Society Of Chemistry: Cambridge, UK, 1990; ISBN 978-0851868363. [Google Scholar]
- Sastri, V.S. Corrosion Inhibitors: Principles and Applications; Wiley: New York, NY, USA, 1998; ISBN 9780471976080. [Google Scholar]
- Zhou, C.H.; Wang, Y. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Tennefy, A.B. Pesticide Research Trends, 14th ed.; Nova Science Publishers: New York, NY, USA, 2008; ISBN 978-1604562002. [Google Scholar]
- Nakka, M.; Tadikonda, R.; Rayavarapu, S.; Sarakula, P.; Vidavalur, S. A Simple and Efficient Synthesis of 3,4,5-Trisubstituted/N-Fused 1,2,4-Triazoles via Ceric Ammonium Nitrate Catalyzed Oxidative Cyclization of Amidrazones with Aldehydes Using Polyethylene Glycol as a Recyclable Reaction Medium. Synthesis 2015, 47, 517–525. [Google Scholar] [CrossRef]
- Szőcs, B.; Bokor, É.; Szabó, K.E.; Kiss-Szikszai, A.; Tóth, M.; Somsák, L. Synthesis of 5-Aryl-3-C-Glycosyl- and Unsymmetrical 3,5-Diaryl-1,2,4-Triazoles from Alkylidene-Amidrazones. RSC Adv. 2015, 5, 43620–43629. [Google Scholar] [CrossRef] [Green Version]
- Aly, A.A.; Hassan, A.A.; Mohamed, N.K.; Ramadadfon, M.; Brown, A.B.; Abd El-Aal, A.S.; Bräse, S.; Nieger, M. Regioselective Formation of 1,2,4-Triazoles by the Reaction of Amidrazones in the Presence of Diethyl Azodicarboxylate and Catalyzed by Triethylamine. Mol. Divers. 2019, 23, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Kashaw, S.K.; Mishra, V.K.; Mishra, M.; Rajoriya, V.; Kashaw, V. Design and Synthesis of New Bioactive 1,2,4-Triazoles, Potential Antitubercular and Antimicrobial Agents. Indian J. Pharm. Sci. 2018, 80, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Sonawane, A.D.; Rode, N.D.; Nawale, L.; Joshi, R.R.; Joshi, R.A.; Likhite, A.P.; Sarkar, D. Synthesis and Biological Evaluation of 1,2,4-Triazole-3-Thione and 1,3,4-Oxadiazole-2-Thione as Antimycobacterial Agents. Chem. Biol. Drug Des. 2017, 90, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Shen, Q.K.; Li, J.J.; Tian, Y.S.; Quan, Z. Synthesis and Biological Evaluation of Novel 7-Hydroxy-4-Phenylchromen-2-One–Linked to Triazole Moieties as Potent Cytotoxic Agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- De, P.; Koumba Yoya, G.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffé, M.; Baltas, M. Design, Synthesis, and Biological Evaluation of New Cinnamic Derivatives as Antituberculosis Agents. J. Med. Chem. 2011, 54, 1449–1461. [Google Scholar] [CrossRef]
- Veau, D.; Krykun, S.; Mori, G.; Orena, B.S.; Pasca, M.R.; Frongia, C.; Lobjois, V.; Chassaing, S.; Lherbet, C.; Baltas, M. Triazolophthalazines: Easily Accessible Compounds with Potent Antitubercular Activity. ChemMedChem 2016, 11, 1078–1089. [Google Scholar] [CrossRef]
- Gonnet, L.; Guidetti, B.; André-Barrès, C.; Calvet, R.; Maturano, M.; Menendez, C.; Lherbet, C.; Baron, M.; Chamayou, A.; Baltas, M. Study of the Two Steps and One-Pot Two-Step Mechanochemical Synthesis of Annulated 1,2,4-Triazoles. ACS Sustain. Chem. Eng. 2020, 8, 3114–3125. [Google Scholar] [CrossRef]
- Gonnet, L. Mécanosynthèses Organiques: Étude Cinétique de la Réaction de Diels-Alder et Synthèses de 1,2,4-Triazoles à Activités Biologiques. Ph.D. Thesis, Ecole des Mines d’Albi-Carmaux, Albi, France, 2019. (In French). [Google Scholar]
- Wang, Q.; Chittaboina, S.; Barnhill, H.N. Advances in 1,3-Dipolar Cycloaddition Reaction of Azides and Alkynes—A Prototype of “Click” Chemistry. Lett. Org. Chem. 2005, 2, 293–301. [Google Scholar] [CrossRef]
- Brantley, J.N.; Wiggins, K.M.; Bielawski, C.W. Unclicking the Click: Mechanically Facilitated 1,3-Dipolar Cycloreversions. Science 2011, 333, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- McNutt, M. Editorial Retraction. Science 2015, 347, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brantley, J.N. Mechanical Activations of Synthetic and Biological Systems. Ph.D. Thesis, University of Texas, Austin, TX, USA, 2014. [Google Scholar]
- Brantley, J.N.; Konda, S.S.M.; Makarov, D.E.; Bielawski, C.W. Regiochemical Effects on Molecular Stability: A Mechanochemical Evaluation of 1,4- and 1,5-Disubstituted Triazoles. J. Am. Chem. Soc. 2012, 134, 9882–9885. [Google Scholar] [CrossRef] [PubMed]
- Stauch, T.; Dreuw, A. Force-Induced Retro-Click Reaction of Triazoles Competes with Adjacent Single-Bond Rupture. Chem. Sci. 2017, 8, 5567–5575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupička, M.; Dopieralski, P.; Marx, D. Unclicking the Click: Metal-Assisted Mechanochemical Cycloreversion of Triazoles Is Possible. Angew. Chem. Int. Ed. 2017, 56, 7745–7749. [Google Scholar] [CrossRef] [PubMed]
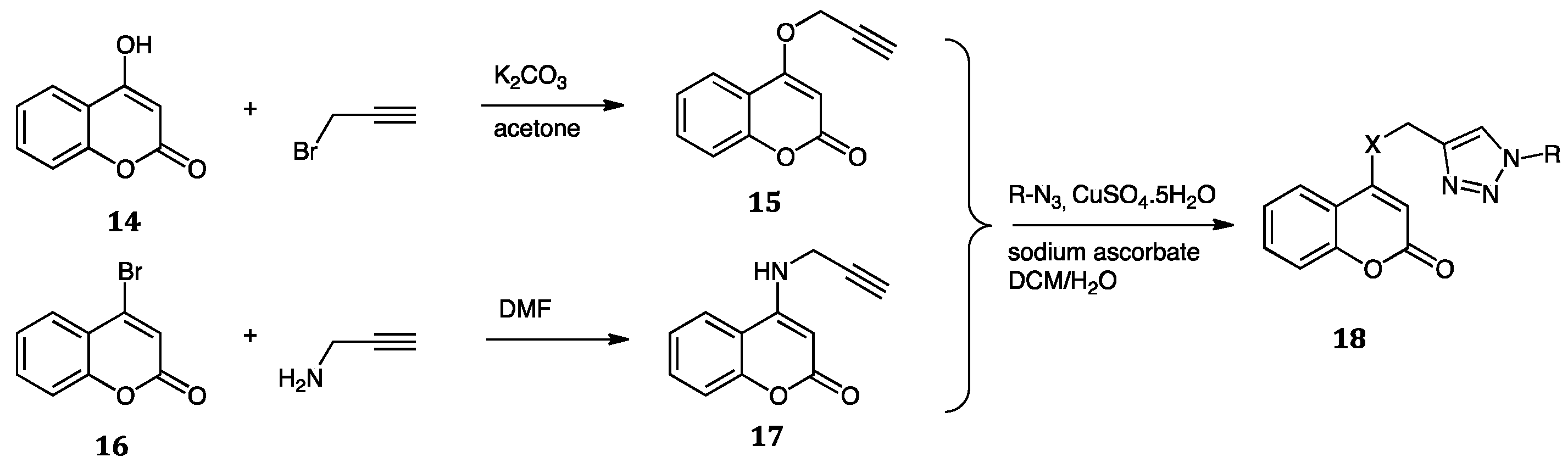
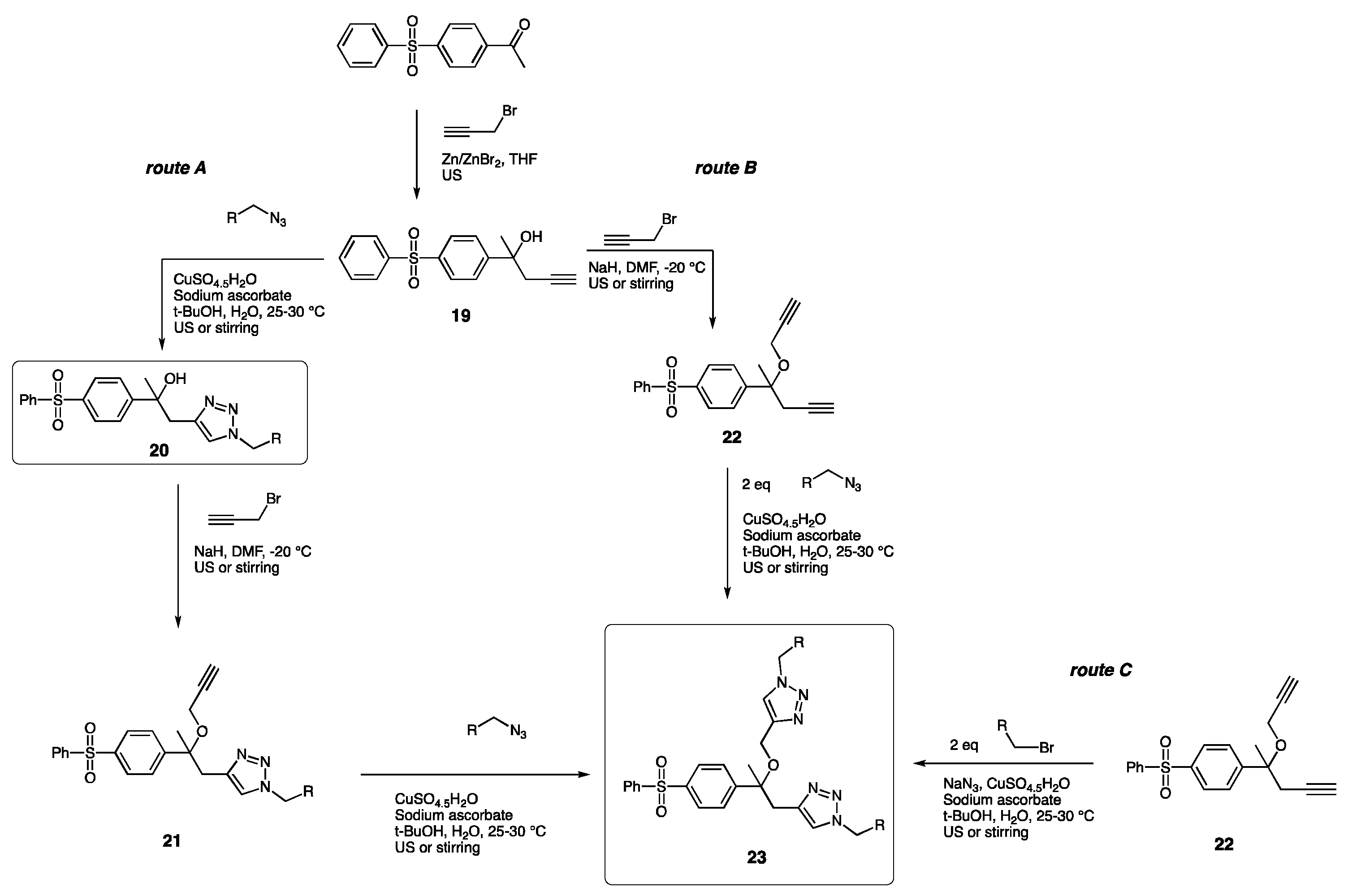
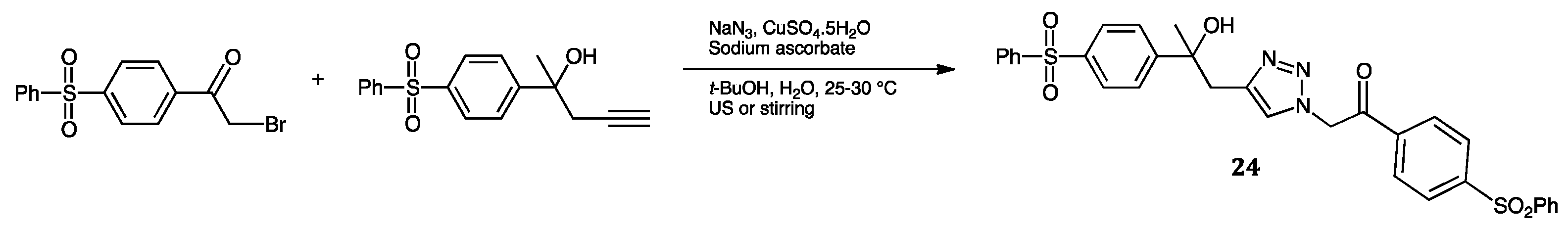
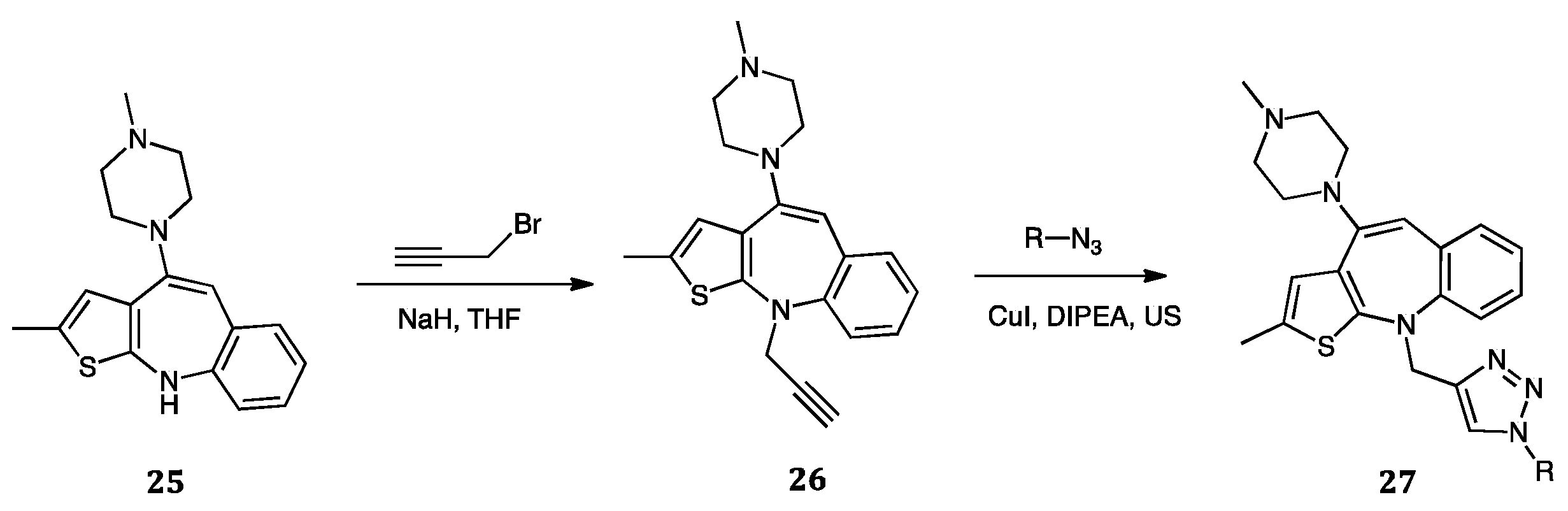

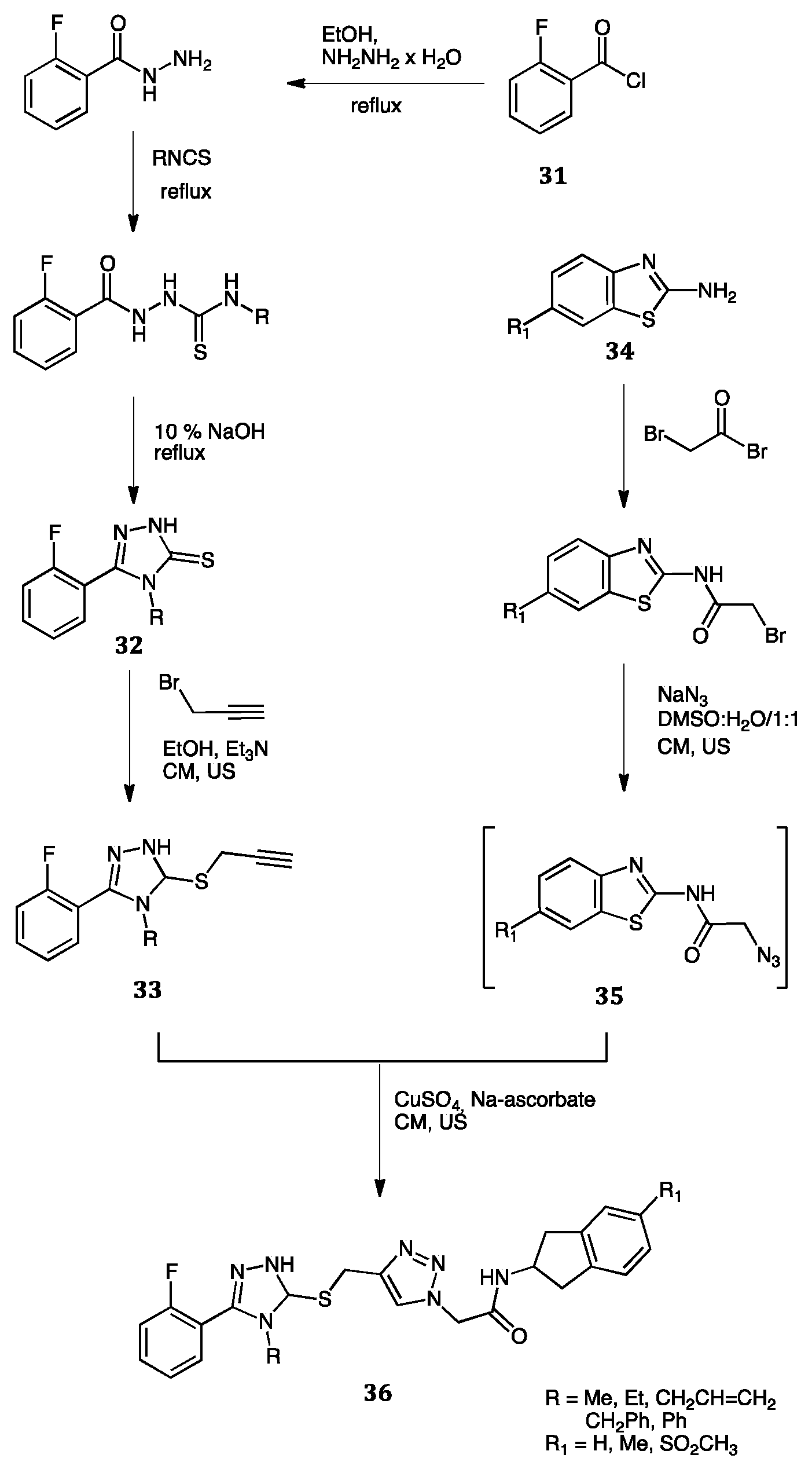

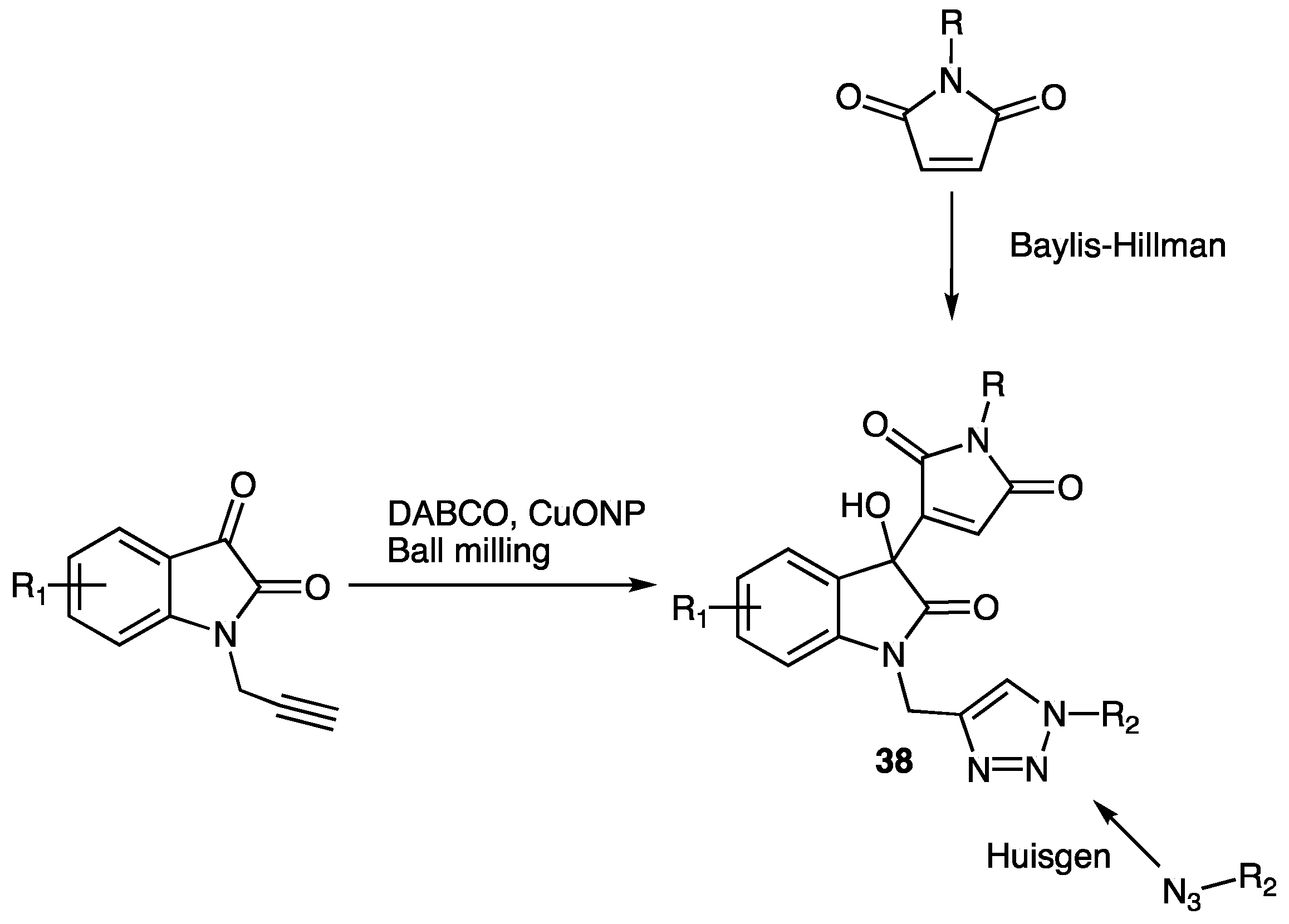
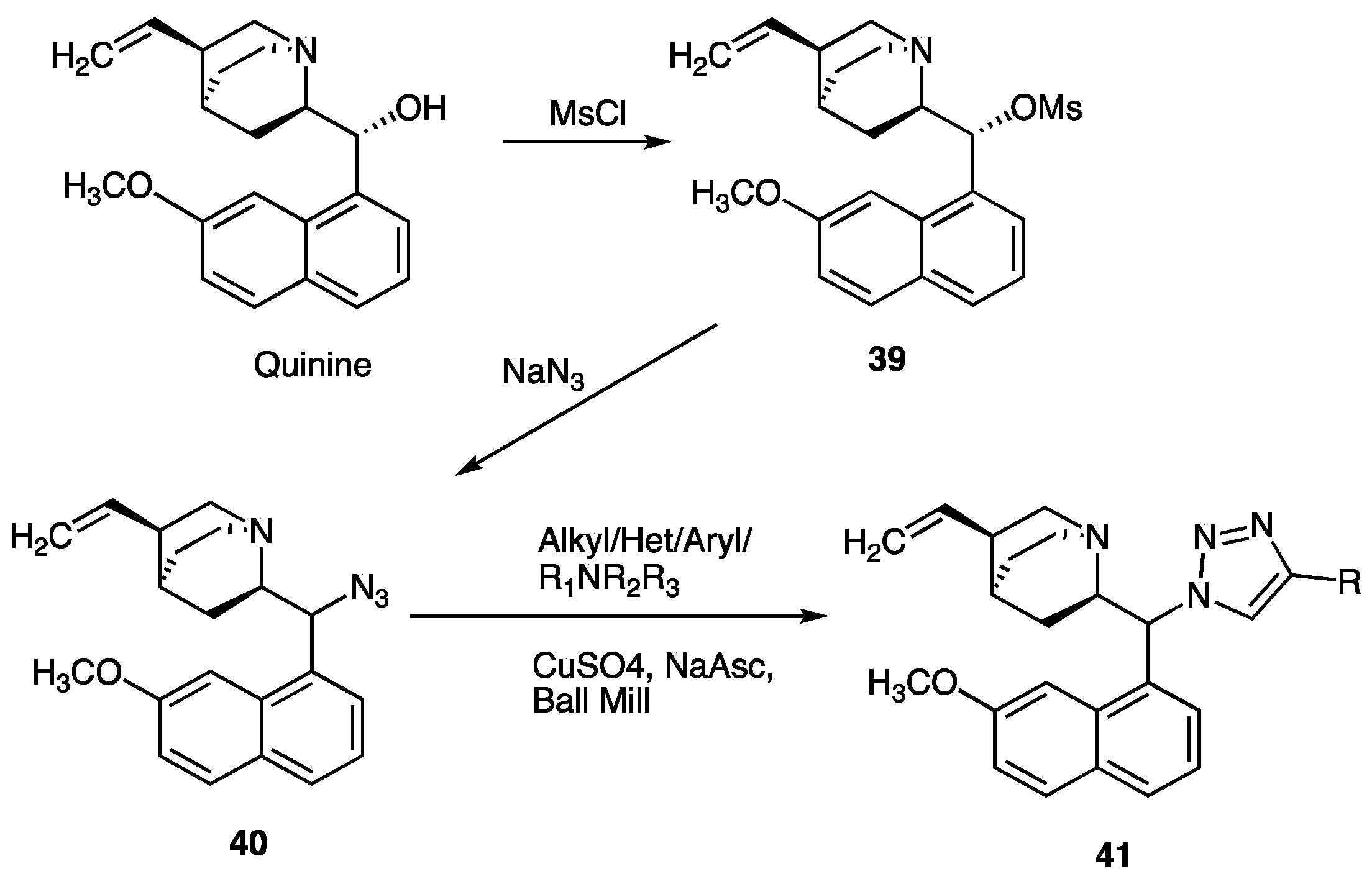
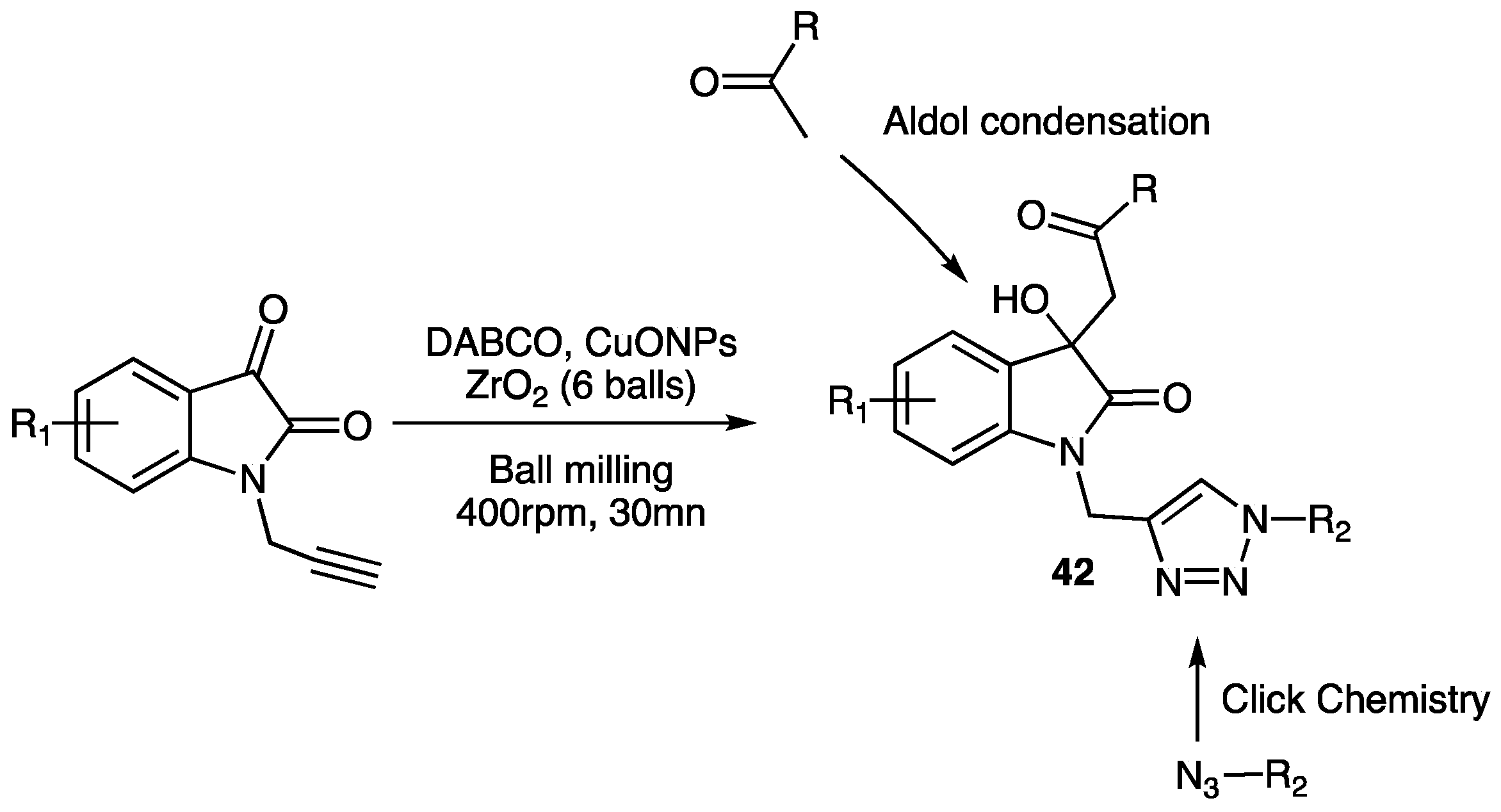
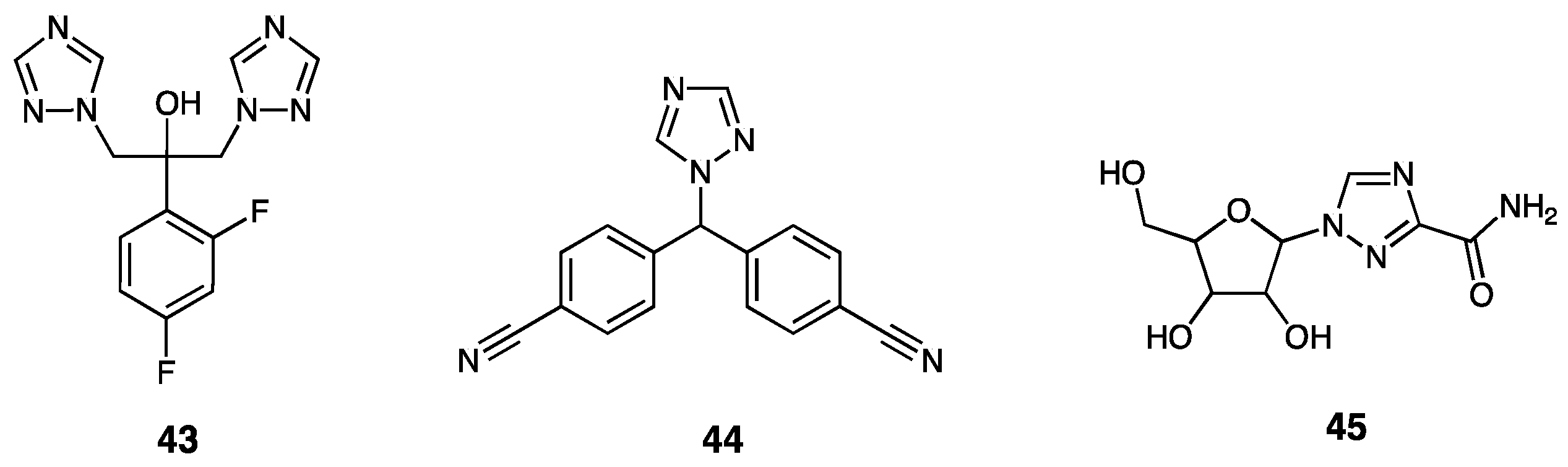

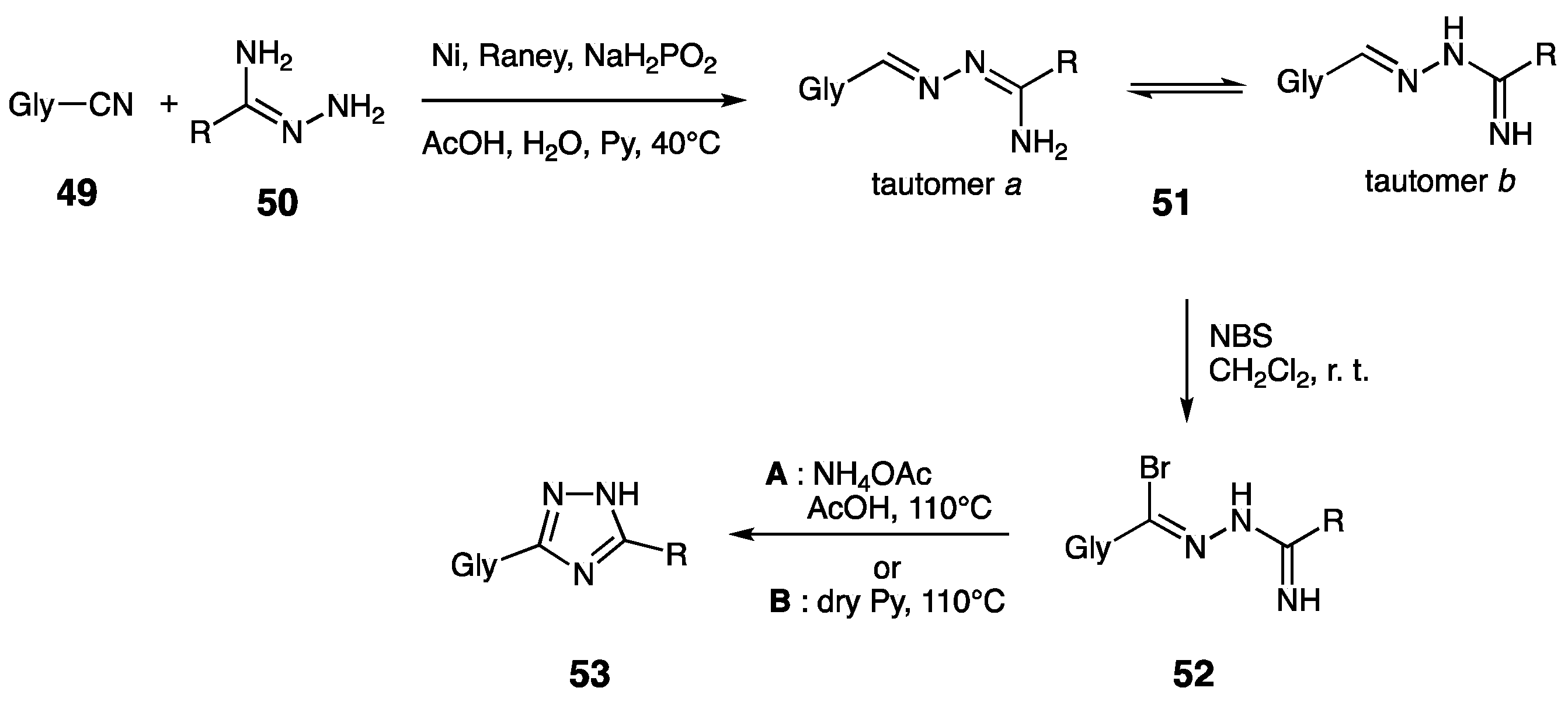
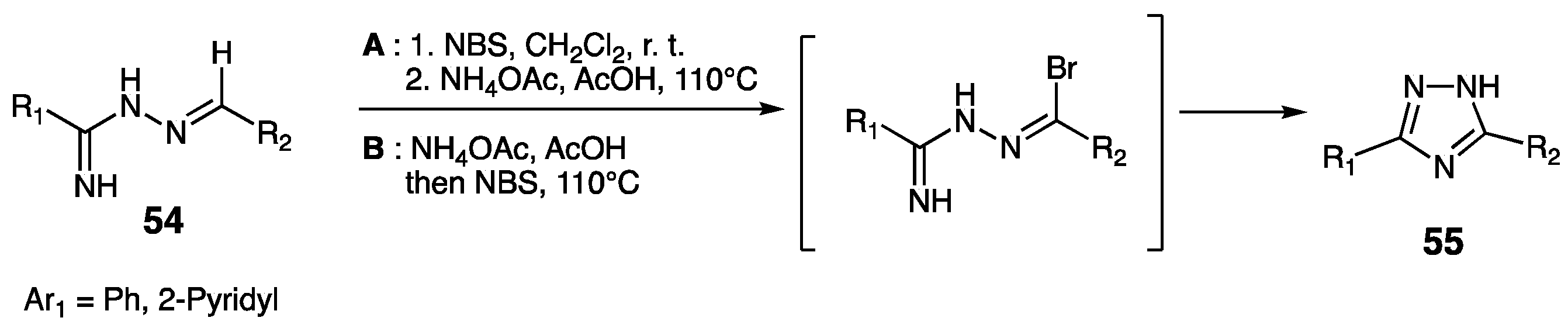
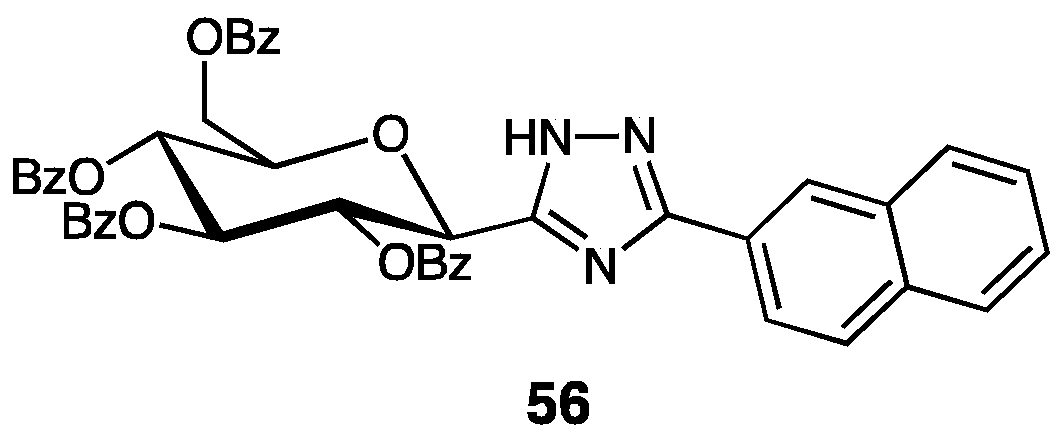


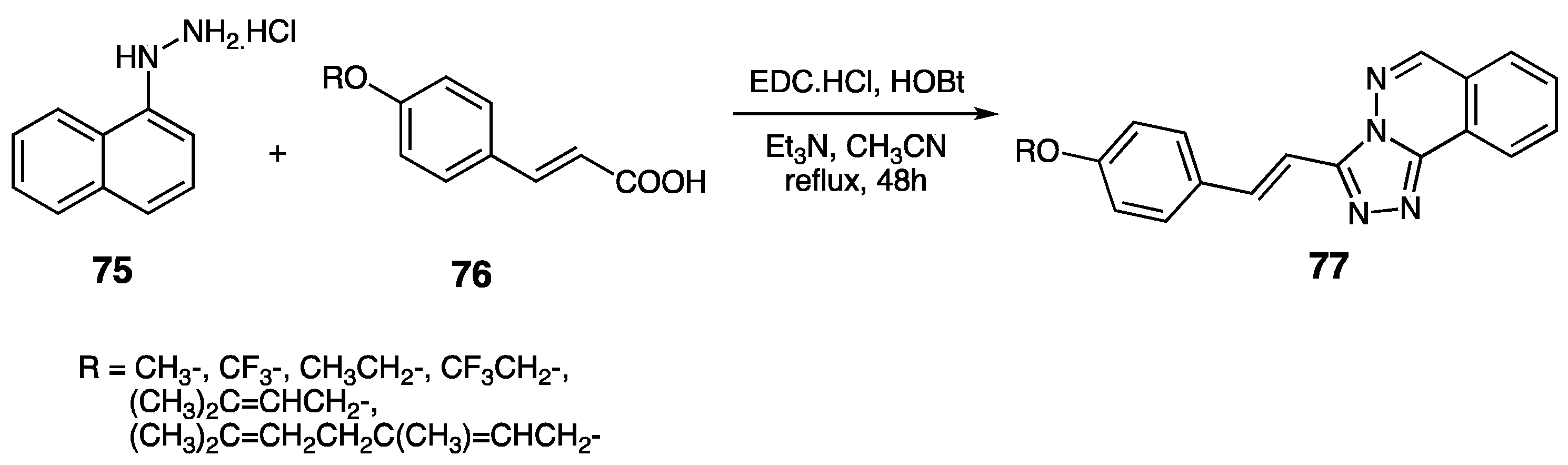
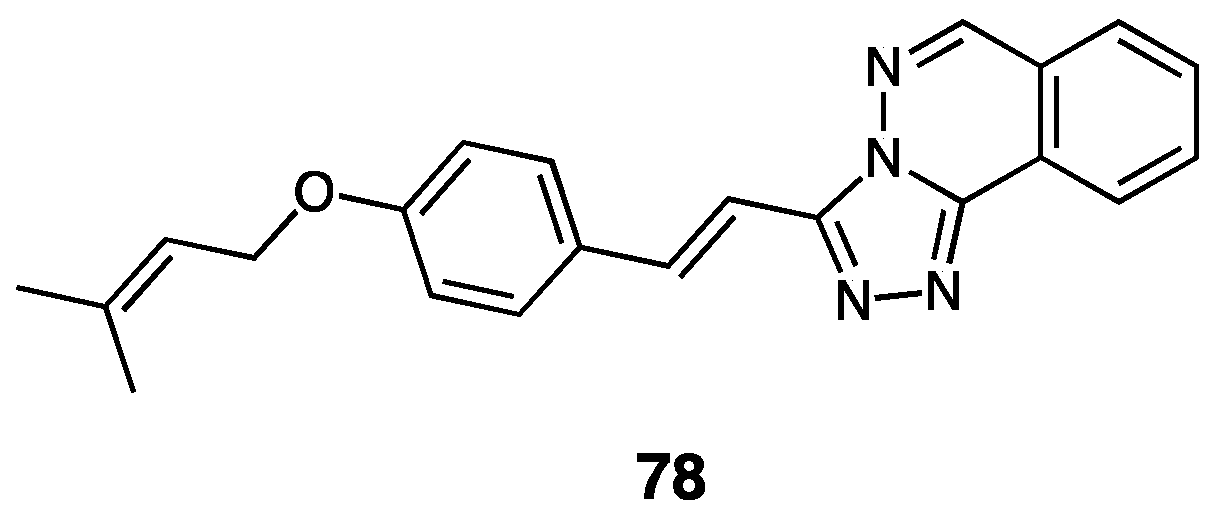
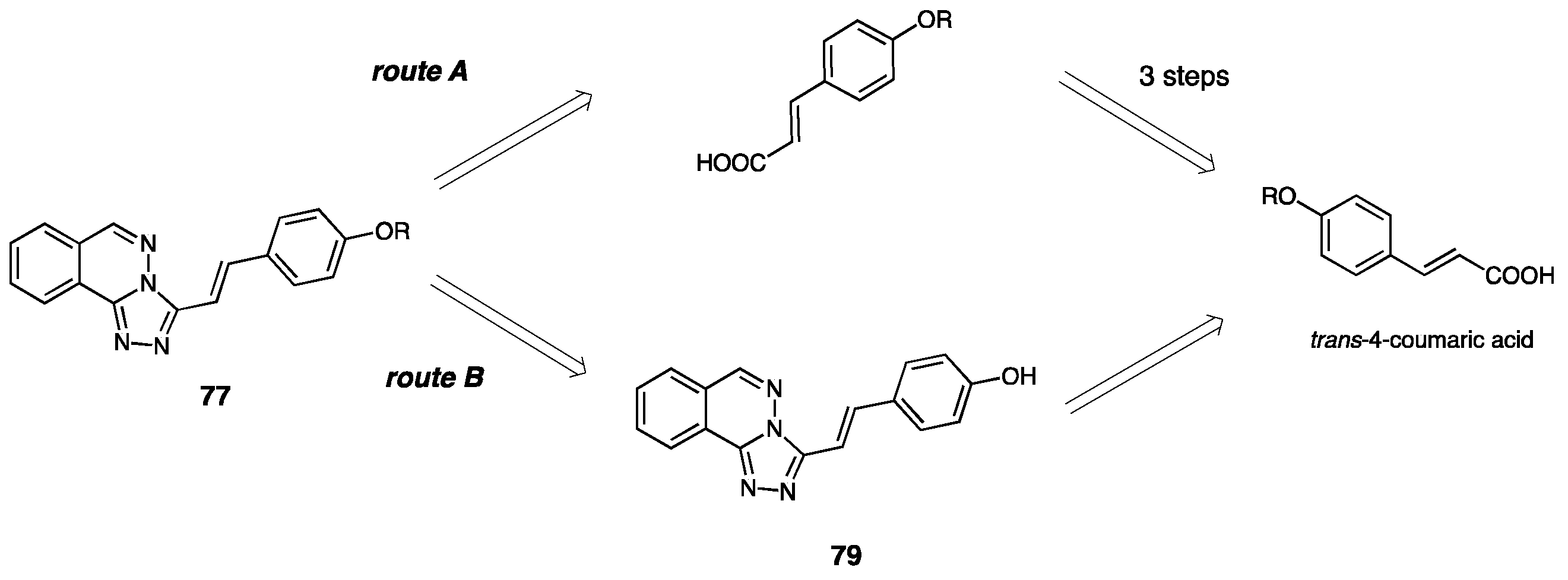
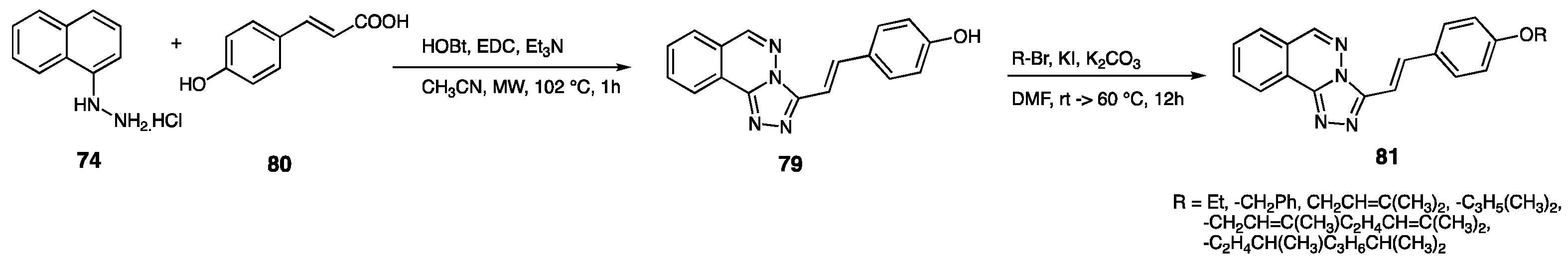
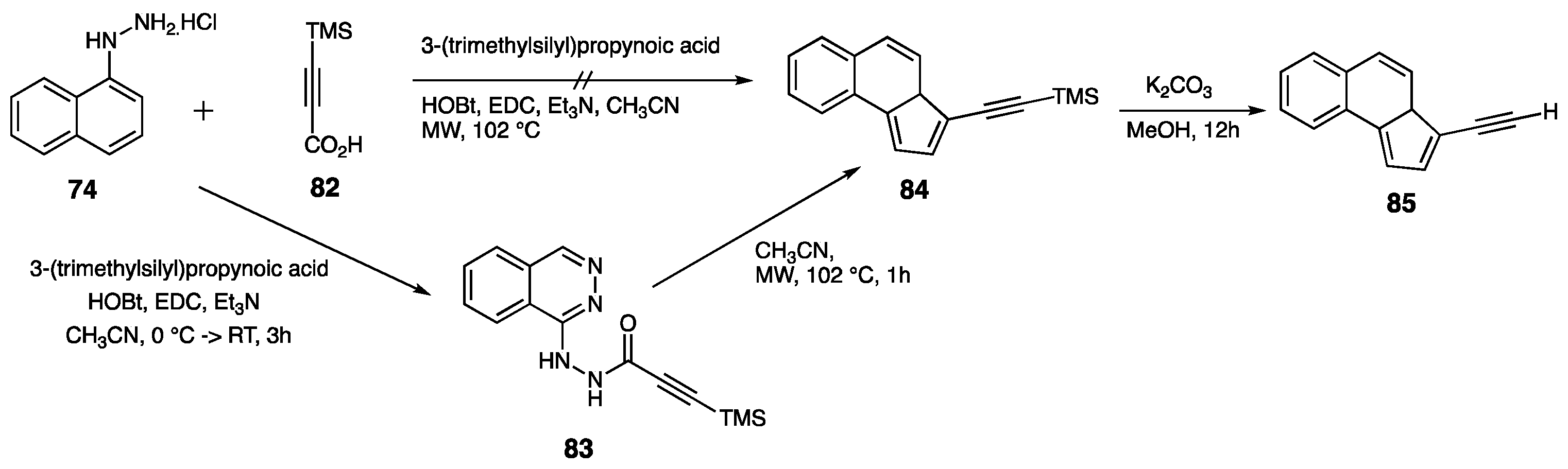
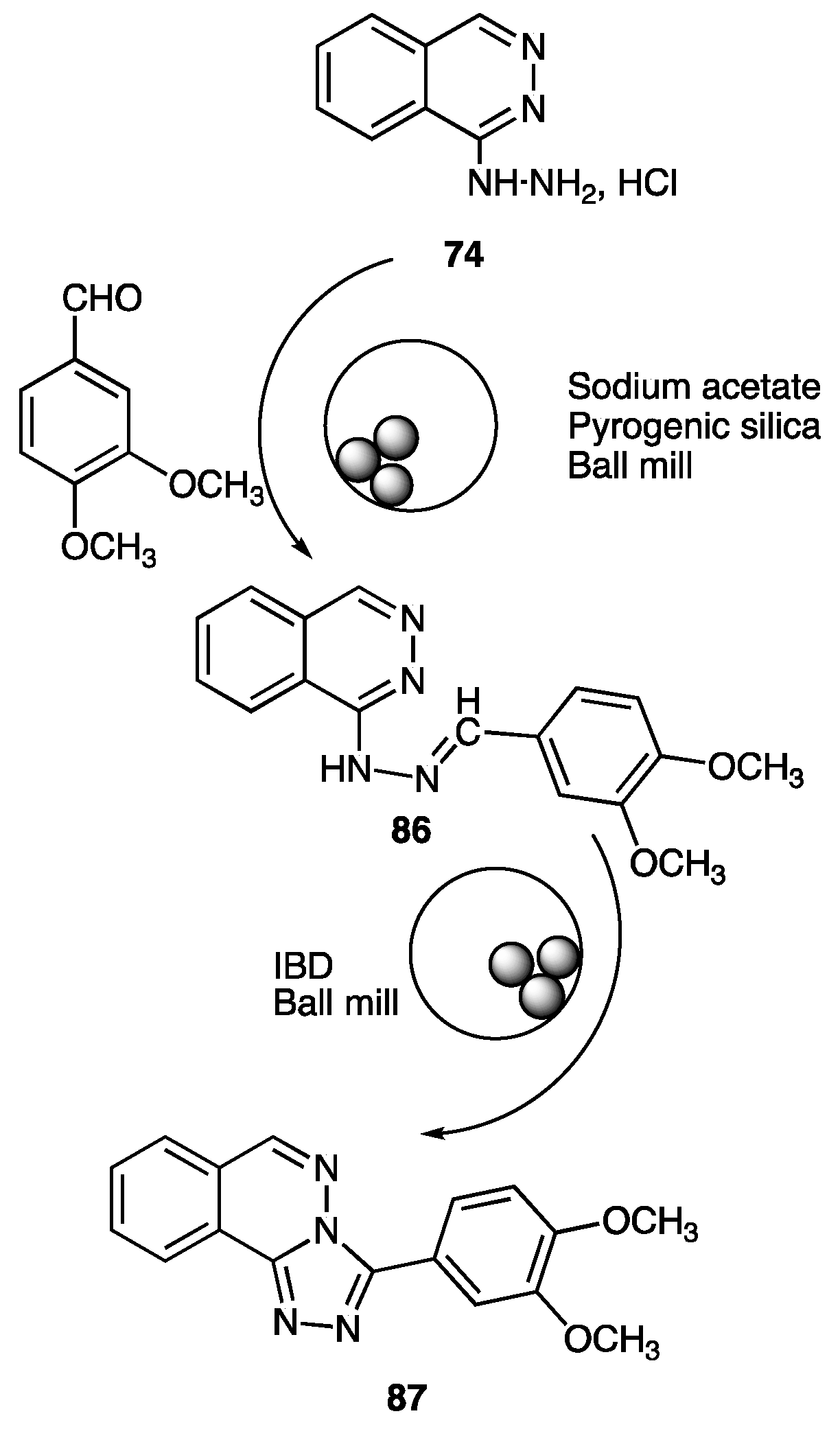
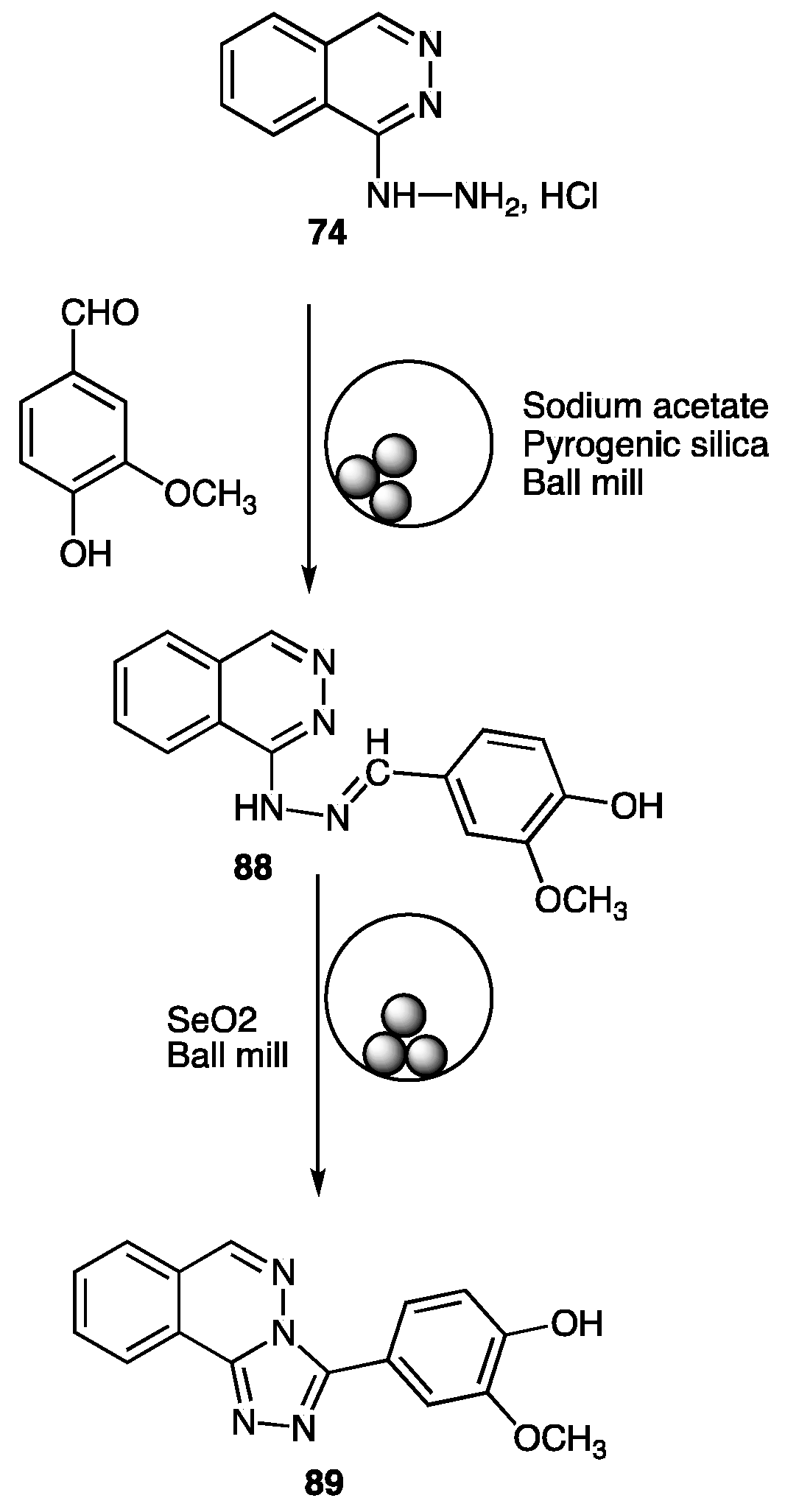


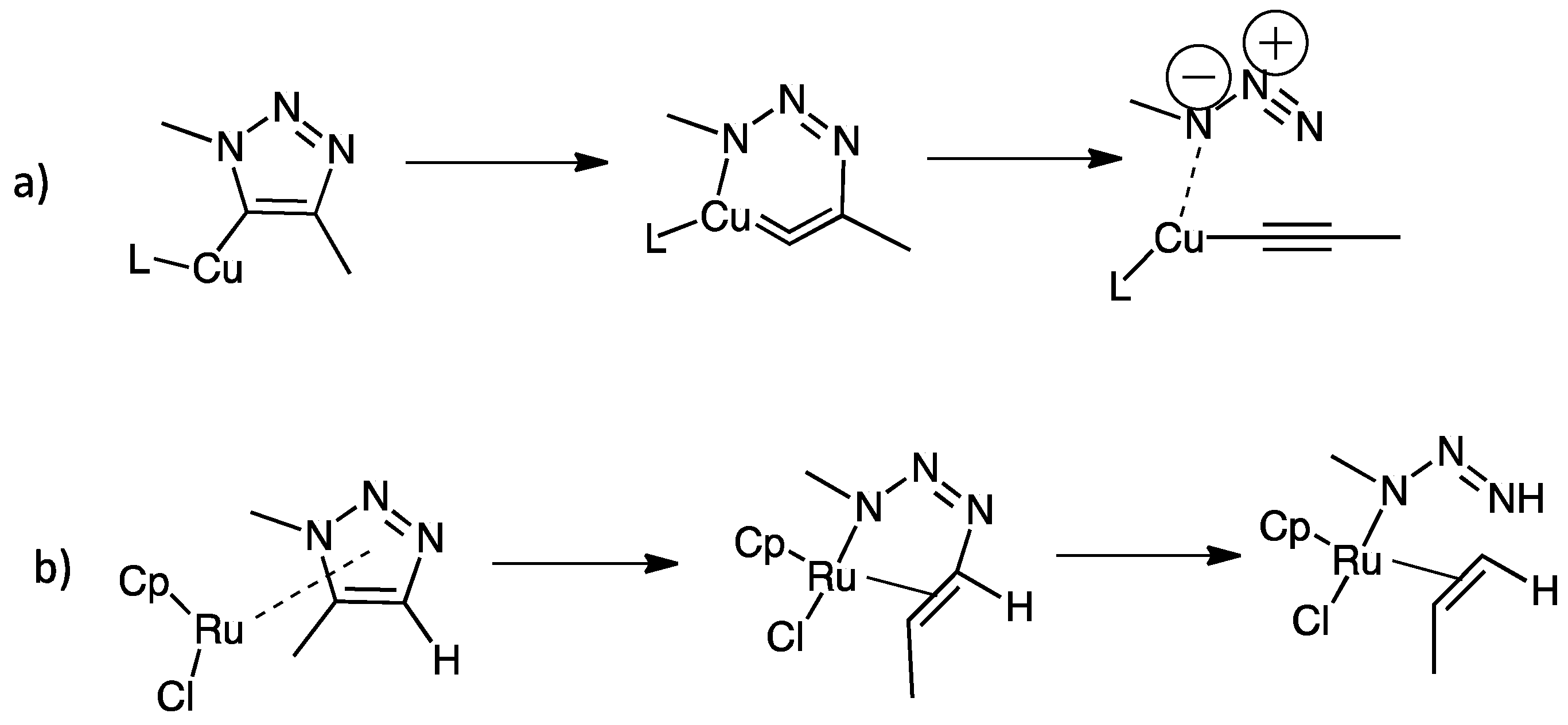
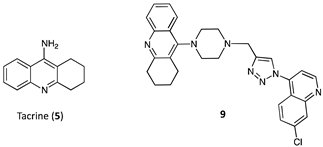
| Compounds | Inhibition (%) at 100 µM | IC50 (µM) | ||
|---|---|---|---|---|
| AChE | BChE | AChE | BChE | |
| 9 (R = H, linker = piperazine) | 78.69 | 91.80 | 4.89 | 3.61 |
| Tacrine 5 | 86.22 | 99.63 | 0.316 | 0.066 |
| Compounds 13 | MIC (µg/mL) | MIC (µM) | Cytotoxicity in % Inhibition at 50 µg/mL | ||
|---|---|---|---|---|---|
| R1 | R2 | R3 | |||
| Me | H | C6H5- | 1.56 | 4.74 | 30.23 |
| Me | H | 4-OMe-C6H5- | 1.56 | 4.34 | 33.14 |
| Cl | H | Benzyl | 1.56 | 4.11 | 29.36 |
| Cl | Me | C6H5- | 3.125 | 8.60 | 24.90 |
| Cl | Me | 4-Cl-C6H5- | 3.125 | 7.87 | 24.76 |
| Compounds 18 | Enterococcus faecalis | |
|---|---|---|
| X | R | |
| O | C6H5- | 50 |
| O | 2-OMe-C6H4- | 12.5 |
| O | 4-F-C6H4- | 50 |
| NH | 3-NO2-C6H4- | 50 |
| NH | C11H23 | 50 |
| Compounds | R | A. niger MIC (µg/mL) |
|---|---|---|
| 20 | C6H5- | 25 |
| 23 | C6H5- | 25 |
| 23 | (4-SO2Ph)C6H4PhCO- | 25 |
| 23 | (4-F)C6H4PhCO- | 25 |
| 23 | (4-Br)C6H4PhCO- | 25 |
| Compounds 27 | Average % Inhibition against PDE4B |
|---|---|
| R | |
| -CH3Ph | 72.82 |
| -CH2C6H3(Cl-o)(CF3-p) | 75.37 |
| -C6H4F-p | 74.83 |
| Compounds 30 | Gram-Positive Organisms | Gram-Negative Organisms | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | R1 | Sp | Bs | Sa | Pa | Ec | Kp | Af | Ca |
| -SO2Me | -CH(OH)(Ph) | 8 | 8 | 4 | 4 | 8 | 8 | 8 | 8 |
| -SO2Me | -C2H4OH | 4 | 4 | 8 | 4 | 4 | 8 | 4 | 4 |
| -SO2Me | -C3H6OH | 4 | 4 | 8 | 4 | 4 | 8 | 4 | 4 |
| Compounds 38 | Yield (%) | |||
|---|---|---|---|---|
| R | R1 | R2 | ||
| 38a | Me | H | benzyl | 96 |
| 38b | Me | H | p-methylbenzyl | 98 |
| 38c | Me | H | p-methoxybenzyl | 99 |
| 38d | Me | H | hexyl | 80 |
| 38e | Me | H | phenyl | 70 |
| 38f | Me | H | ethoxycarbonylmethyl | 90 |
| 38g | Me | H | p-nitrobenzyl | 93 |
| 38h | Me | 5-Cl | benzyl | 99 |
| 38i | Me | 5-Cl | p-methylbenzyl | 90 |
| 38j | Me | 5-Cl | p-nitrobenzyl | 90 |
| 38k | Me | 5-Cl | p-methoxybenzyl | 98 |
| 38l | Me | 5-Cl | phenyl | 71 |
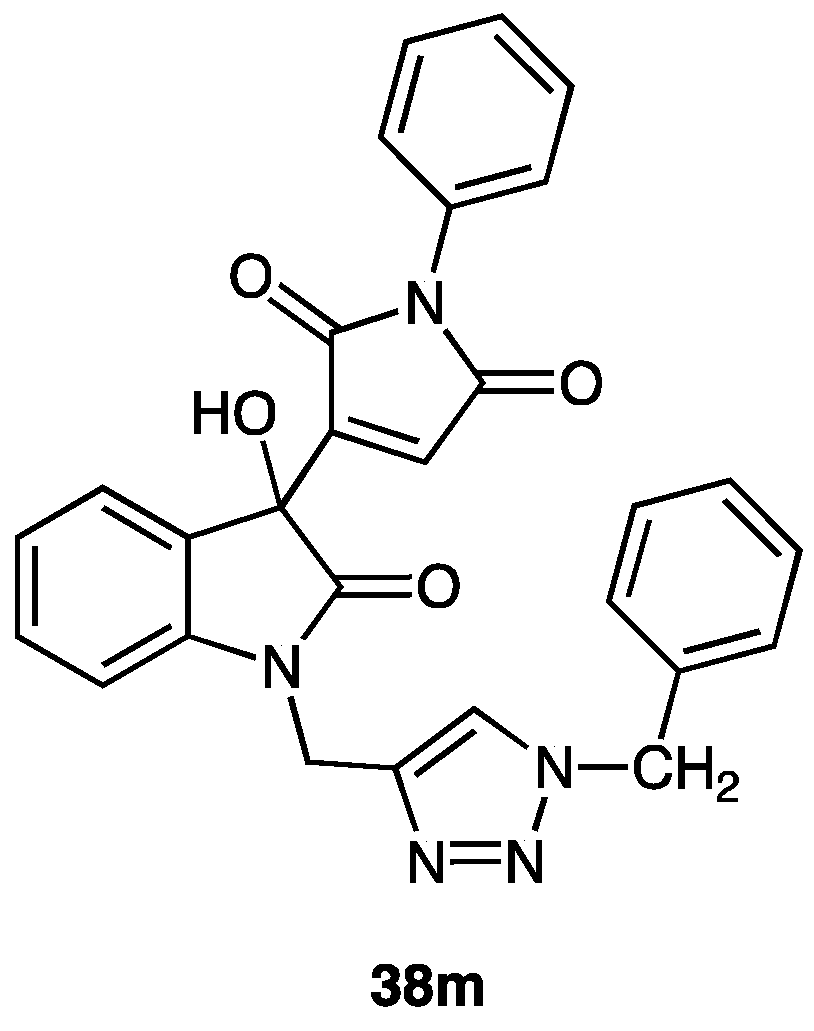
| 38m | phenyl | H | benzyl | 90 |
| 38n | benzyl | H | benzyl | 93 |
| 38o | Me | 5-Br | benzyl | 96 |
| 38p | Me | 5-Me | benzyl | 88 |
| Compounds 41 | R | Yield (%) | Antimalarial Assay (IC50, (µM)) | Antileishmanial Assay(IC50, (µM)) | Toxicity Profile NOAEL (mg/kg) |
|---|---|---|---|---|---|
| a | 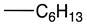 | 91 | 0.25 | 8.26 | >1000 |
| b | 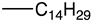 | 89 | 0.25 | 4.4 | >1000 |
| c | 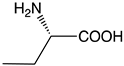 | 56 | 0.25 | 1.78 | >1000 |
| d | 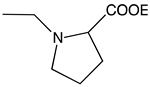 | 82 | 0.37 | 3.95 | >1000 |
| e | 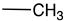 | 78 | 0.76 | 4.06 |
| Compounds 42 | R | R1 | R2 | Yield (%) | Biological Effect |
|---|---|---|---|---|---|
| a |  | H | 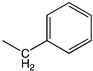 | 90 | Anti-fungal Anti-bacterial |
| b |  | 5-Me | 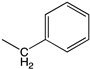 | 92 | Main activity (f.i.): Corrosion inhibition |
| Compounds 64 | Percent Reduction in RLU | |
|---|---|---|
| R | M. tuberculosis H37Rv | Clinical Isolates, S, H R and E resistant M. tuberculosis |
| 4-F-C6H4- | 80.50 | 50.72 |
| 4-CH3-C6H4- | 82.01 | 52.79 |
| CH=CH-C6H5 | 83.49 | 52.47 |
| Compounds 64 | Minimum Inhibitory Concentration (MIC, µg/mL) | |||
|---|---|---|---|---|
| R | Gram-Positive Bacteria | Fungi | ||
| S. aureus | B. subtilis | A. niger | C. albicans | |
| 4-F-C6H4- | 12.5 | 10.2 | 125 | 106.3 |
| -CH=CH-C6H5 | 75 | 81.3 | 11.7 | 10.9 |
| Compound | Anti-Tubercular Activity against MTB (μg/mL) | Cytotoxic Activity of Triazole Thiones against Human Cancer Cell Lines (µg/mL) | Aqueous Solubility (µM) | |||
|---|---|---|---|---|---|---|
| Dormant Stage | Active Stage | THP-1 | A549 | PANC-1 | ||
| MIC | MIC | GI90 | GI90 | GI90 | ||
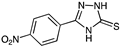 | 0.64 | 9.05 | >100 | >100 | >100 | >1280 |
 | 0.46 | >30 | >100 | >100 | >100 | >3200 |
| Compound | Time (h) | Yield (%) | E-factor | PMI |
|---|---|---|---|---|
| 86 | 1/0.5 | 100/100 | 14/4 | 15/5 |
| 87 | 4/0.75 | 84/97 | 74/12 | 75/13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonnet, L.; Baron, M.; Baltas, M. Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry. Molecules 2021, 26, 5667. https://doi.org/10.3390/molecules26185667
Gonnet L, Baron M, Baltas M. Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry. Molecules. 2021; 26(18):5667. https://doi.org/10.3390/molecules26185667
Chicago/Turabian StyleGonnet, Lori, Michel Baron, and Michel Baltas. 2021. "Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry" Molecules 26, no. 18: 5667. https://doi.org/10.3390/molecules26185667
APA StyleGonnet, L., Baron, M., & Baltas, M. (2021). Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry. Molecules, 26(18), 5667. https://doi.org/10.3390/molecules26185667






