Synthesis of Proposed Structure of Aaptoline A, a Marine Sponge-Derived 7,8-Dihydroxyquinoline, and Its Neuroprotective Properties in C. elegans
Abstract
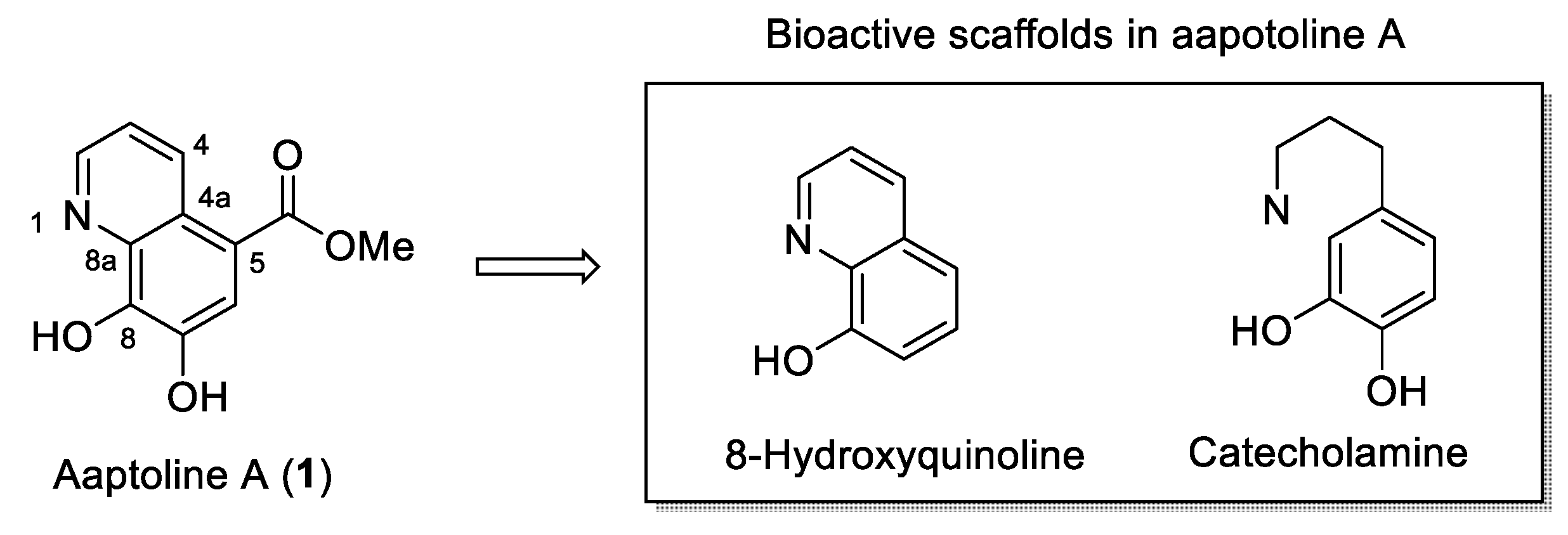
:1. Introduction
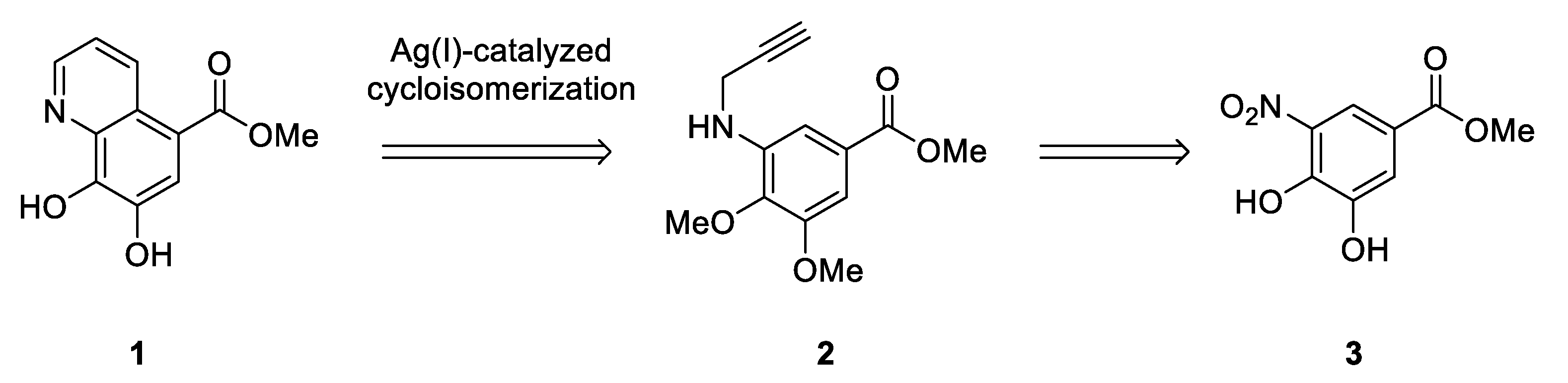
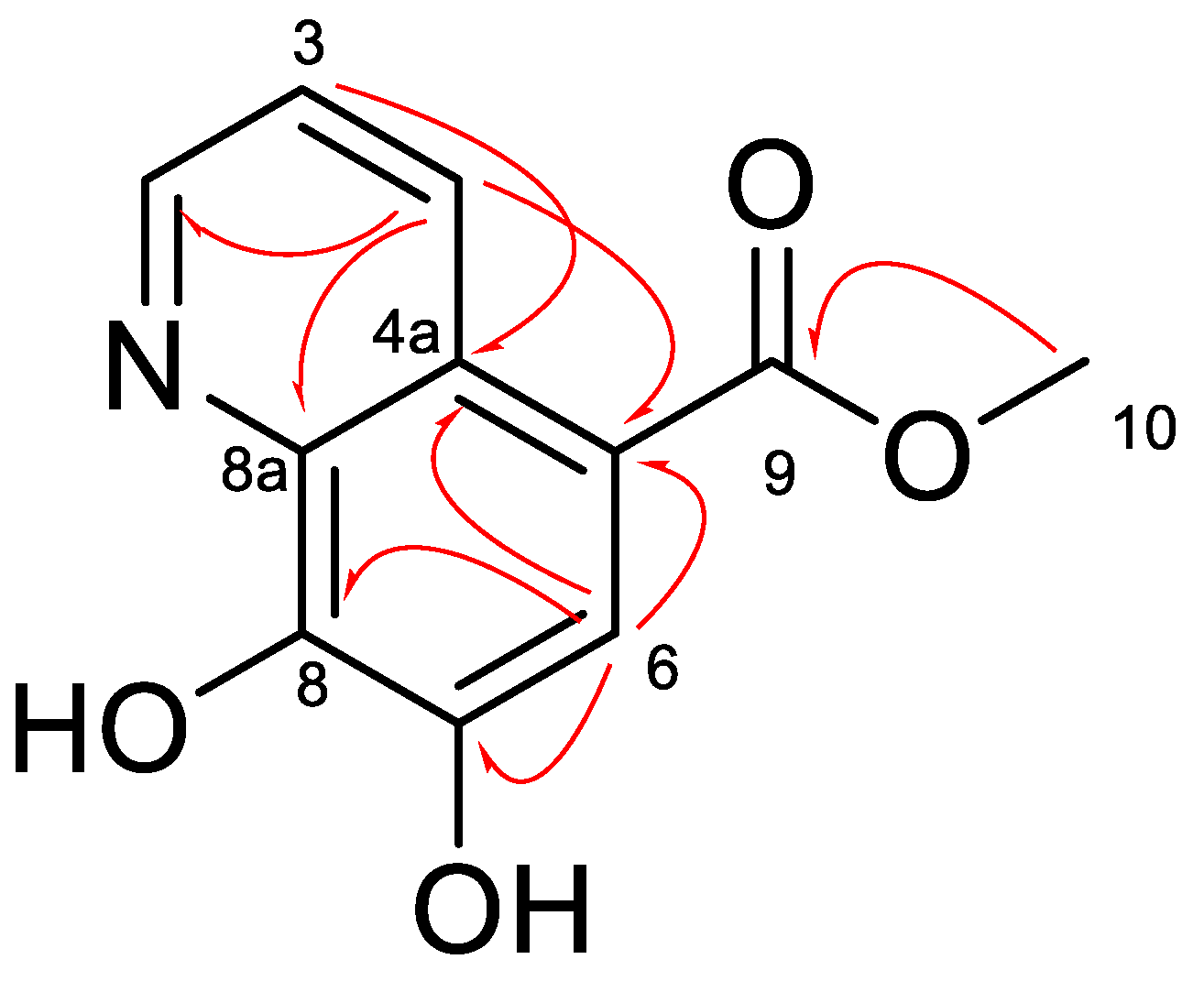
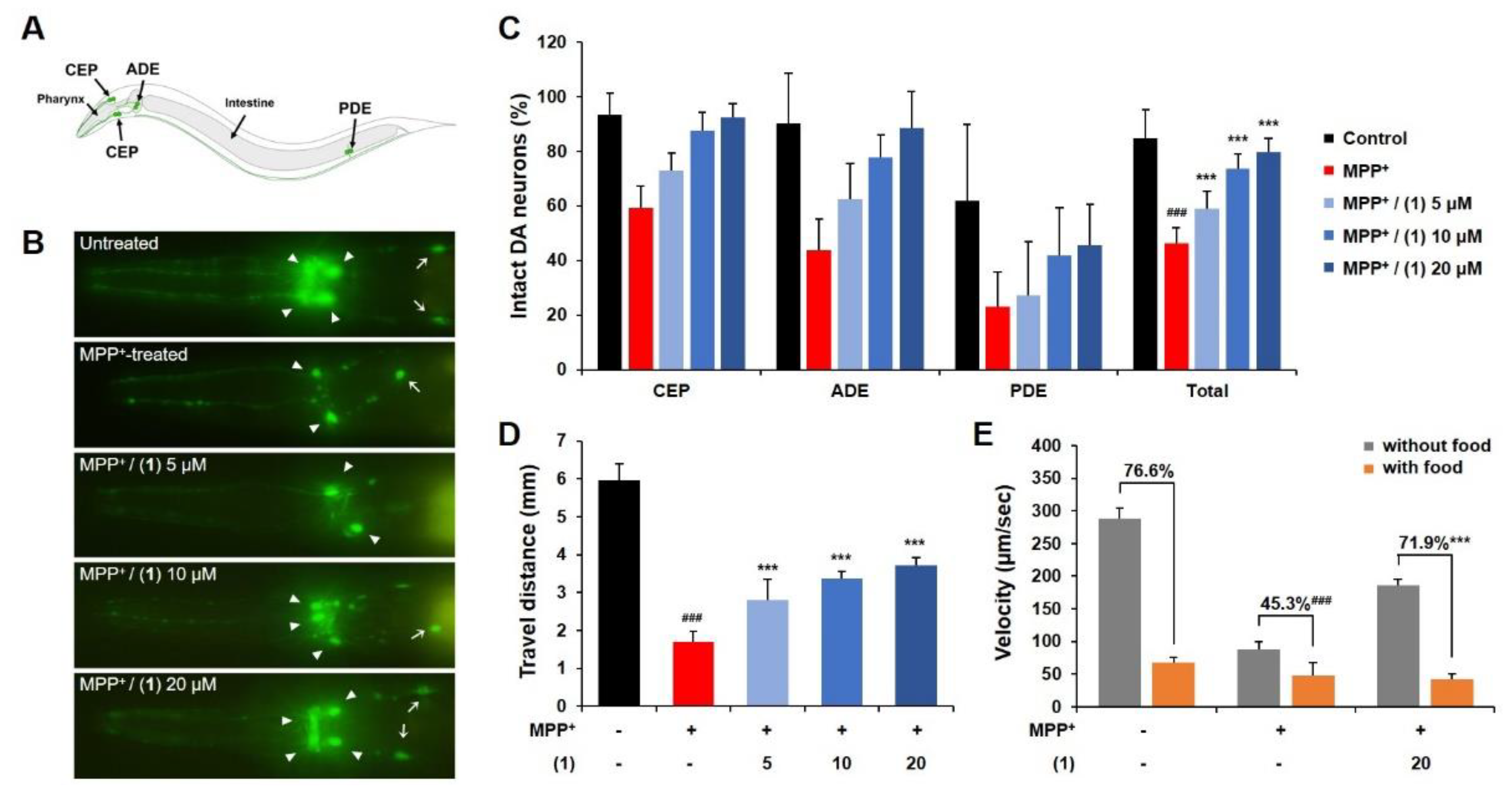
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. General Experimental
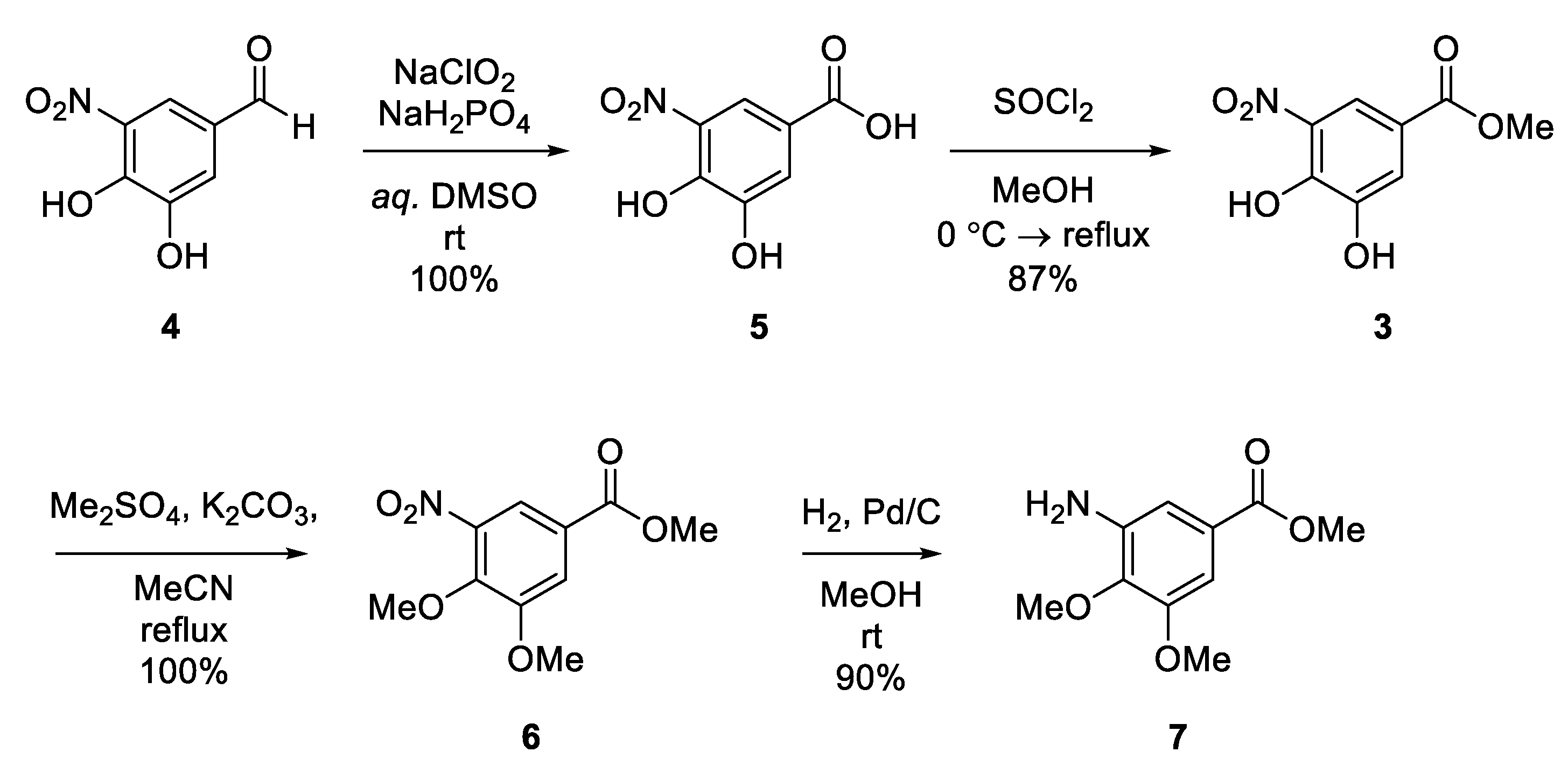
3.1.2. 3,4-Dihydroxy-5-nitrobenzoic Acid (5)
3.1.3. Methyl 3,4-Dihydroxy-5-nitrobenzoate (3)
3.1.4. Methyl 3,4-Dimethoxy-5-nitrobenzoate (6)
3.1.5. Methyl 3-Amino-4,5-dimethoxybenzoate (7)
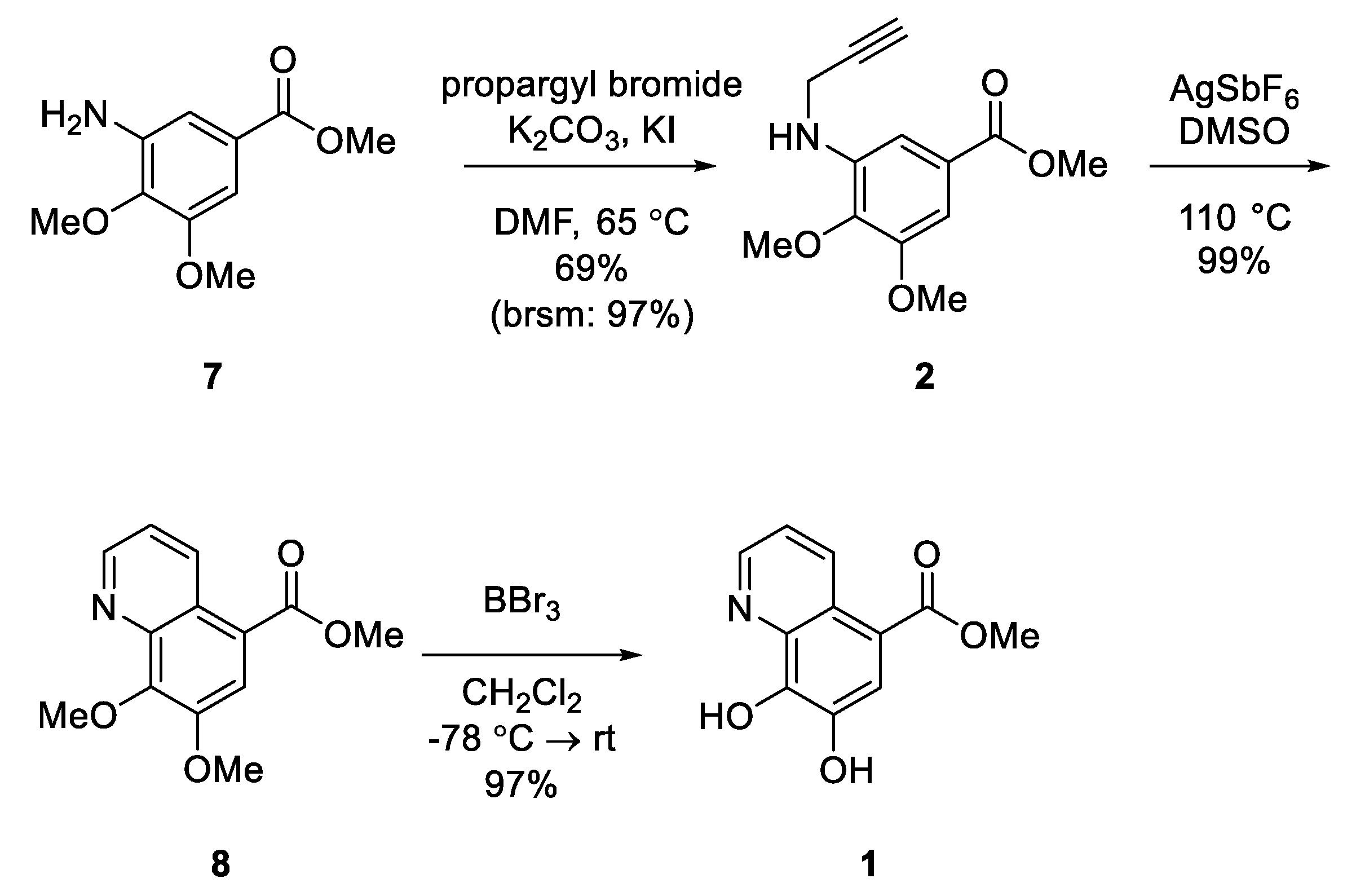
3.1.6. Methyl 3,4-Dimethoxy-5-(prop-2-yn-1-ylamino)benzoate (2)
3.1.7. Methyl 7,8-Dimethoxyquinoline-5-carboxylate (8)
3.1.8. Aaptoline A (1)
3.2. Biology
3.2.1. C. elegans Incubation
3.2.2. Fluorescence Microscopy
3.2.3. Behavioral Assay
3.2.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. MedChemComm 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent Advances in the Synthesis and Biological Activity of 8-Hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.R.; Steglich, W. Effective syntheses of quinoline-7,8-diol, 5-amino-l-DOPA, and 3-(7,8-dihydroxyquinolin-5-yl)-l-alanine. Tetrahedron 2003, 59, 9231–9237. [Google Scholar] [CrossRef]
- Heinrich, M.R.; Steglich, W.; Banwell, M.G.; Kashman, Y. Total synthesis of the marine alkaloid halitulin. Tetrahedron 2003, 59, 9239–9247. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov. Disord. 1998, 13, 24–34. [Google Scholar] [PubMed]
- Braungart, E.; Gerlach, M.; Riederer, P.; Baumeister, R.; Hoener, M. Caenorhabditis elegans MPP+ Model of Parkinson’s Disease for High-Throughput Drug Screenings. Neurodegener. Dis. 2004, 1, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Seifert, M.; Baumeister, R. Caenorhabditis elegans as a Model System for Parkinson’s Disease. Neurodegener. Dis. 2007, 4, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kudo, Y.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; De Voogd, N.J. Aaptoline A, a New Quinoline Alkaloid from the Marine Sponge Aaptos suberitoides. Heterocycles 2014, 88, 591. [Google Scholar] [CrossRef]
- Tang, W.; Yu, H.; Lu, J.; Lin, H.; Sun, F.; Wang, S.; Yang, F. Aaptolines A and B, Two New Quinoline Alkaloids from the Marine Sponge Aaptos aaptos. Chem. Biodivers. 2020, 17, e2000074. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Luxami, V.; Paul, K. Insights of 8-hydroxyquinolines: A novel target in medicinal chemistry. Bioorganic Chem. 2021, 108, 104633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, B.; Wang, X.; Lei, S.; Shi, Z.; Zhao, J.; Liu, Q.; Peng, R. The mono(catecholamine) derivatives as iron chelators: Synthesis, solution thermodynamic stability and antioxidant properties research. R. Soc. Open Sci. 2018, 5, 171492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.T.; Ahn, S.; A Yoon, J. Total Synthesis of the Natural Pyridocoumarins Goniothaline A and B. Synthesis 2018, 51, 552–556. [Google Scholar] [CrossRef] [Green Version]
- A Yoon, J.; Lim, C.; Han, Y.T. Preliminary Study on Novel Expedient Synthesis of 5-Azaisocoumarins by Transition Metal-Catalyzed Cycloisomerization. Front. Chem. 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- A Yoon, J.; Han, Y.T. Efficient Synthesis of Pyrido[3,2-c]coumarins via Silver Nitrate Catalyzed Cycloisomerization and Application to the First Synthesis of Polyneomarline C. Synthesis 2019, 51, 4611–4618. [Google Scholar] [CrossRef]
- Corbett, J.W.; Elliott, R.L.; Freeman-Cook, K.D.; Griffith, D.A.; Phillion, D.P. Pyrazolospiroketone acetyl-CoA carboxylase inhibitors. WO Patent Application No. 2009144554A1, 3 December 2009. [Google Scholar]
- Bai, H.; Bailey, S.; Bhumralkar, D.R.; Bi, F.C.; Guo, F.; He, M.; Humphries, P.S.; Ling, A.L.; Lou, J.; Nukui, S.; et al. Fused phenylamindo heterocyclic compounds. U.S. Patent Application No. 20080280875A1, 13 November 2008. [Google Scholar]
- Kiss, L.E.; Ferreira, H.; Torrão, L.; Bonifácio, M.J.; Palma, N.; Soares-Da-Silva, P.; Learmonth, D.A. Discovery of a Long-Acting, Peripherally Selective Inhibitor of Catechol-O-methyltransferase. J. Med. Chem. 2010, 53, 3396–3411. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.C.; Nandi, R.K.; Ganai, S.; Taher, A. Regioselective synthesis of annulated quinoline and pyridine derivatives by silver-catalyzed 6-endo-dig cycloisomerization. Synlett 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H. C. elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Han, Y.T.; Cha, D.S. Neuroprotective effect of damaurone D in a C. elegans model of Parkinson’s disease. Neurosci. Lett. 2021, 747, 135623. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Yang, W.; Cha, D.S.; Han, Y.T. Synthesis of Proposed Structure of Aaptoline A, a Marine Sponge-Derived 7,8-Dihydroxyquinoline, and Its Neuroprotective Properties in C. elegans. Molecules 2021, 26, 5964. https://doi.org/10.3390/molecules26195964
Kim S, Yang W, Cha DS, Han YT. Synthesis of Proposed Structure of Aaptoline A, a Marine Sponge-Derived 7,8-Dihydroxyquinoline, and Its Neuroprotective Properties in C. elegans. Molecules. 2021; 26(19):5964. https://doi.org/10.3390/molecules26195964
Chicago/Turabian StyleKim, Soobin, Wooin Yang, Dong Seok Cha, and Young Taek Han. 2021. "Synthesis of Proposed Structure of Aaptoline A, a Marine Sponge-Derived 7,8-Dihydroxyquinoline, and Its Neuroprotective Properties in C. elegans" Molecules 26, no. 19: 5964. https://doi.org/10.3390/molecules26195964
APA StyleKim, S., Yang, W., Cha, D. S., & Han, Y. T. (2021). Synthesis of Proposed Structure of Aaptoline A, a Marine Sponge-Derived 7,8-Dihydroxyquinoline, and Its Neuroprotective Properties in C. elegans. Molecules, 26(19), 5964. https://doi.org/10.3390/molecules26195964




