Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives
Abstract
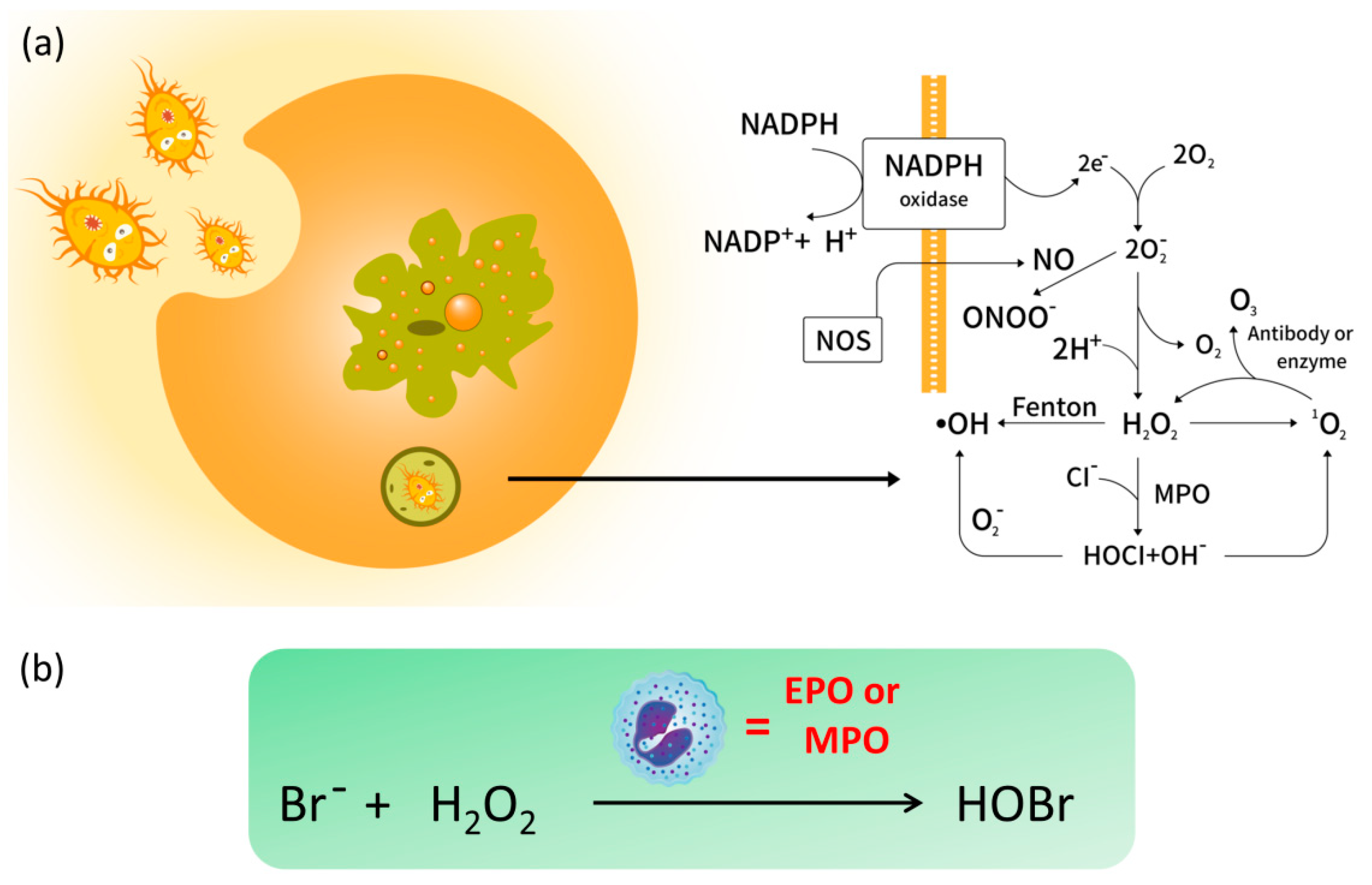
:1. Introduction
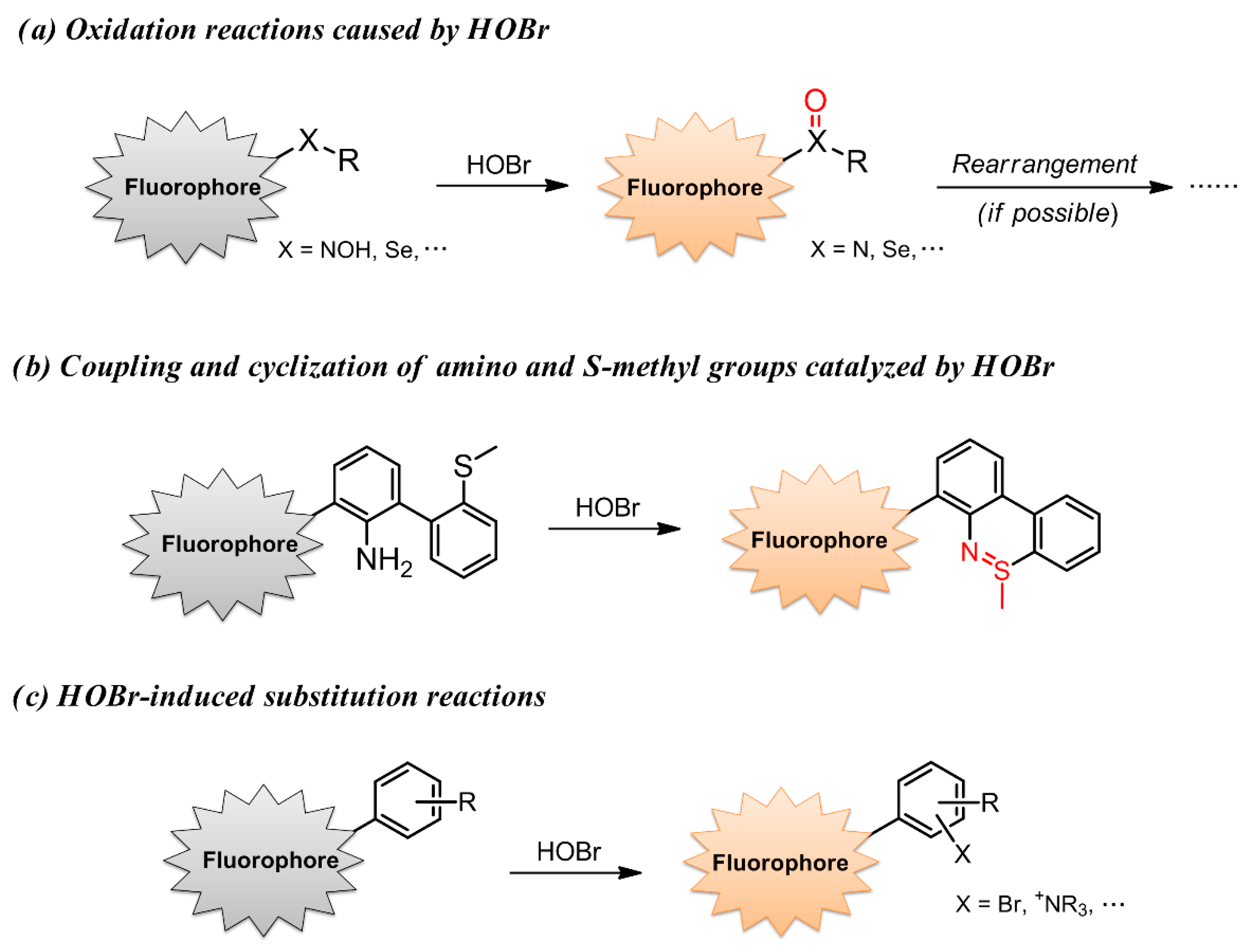
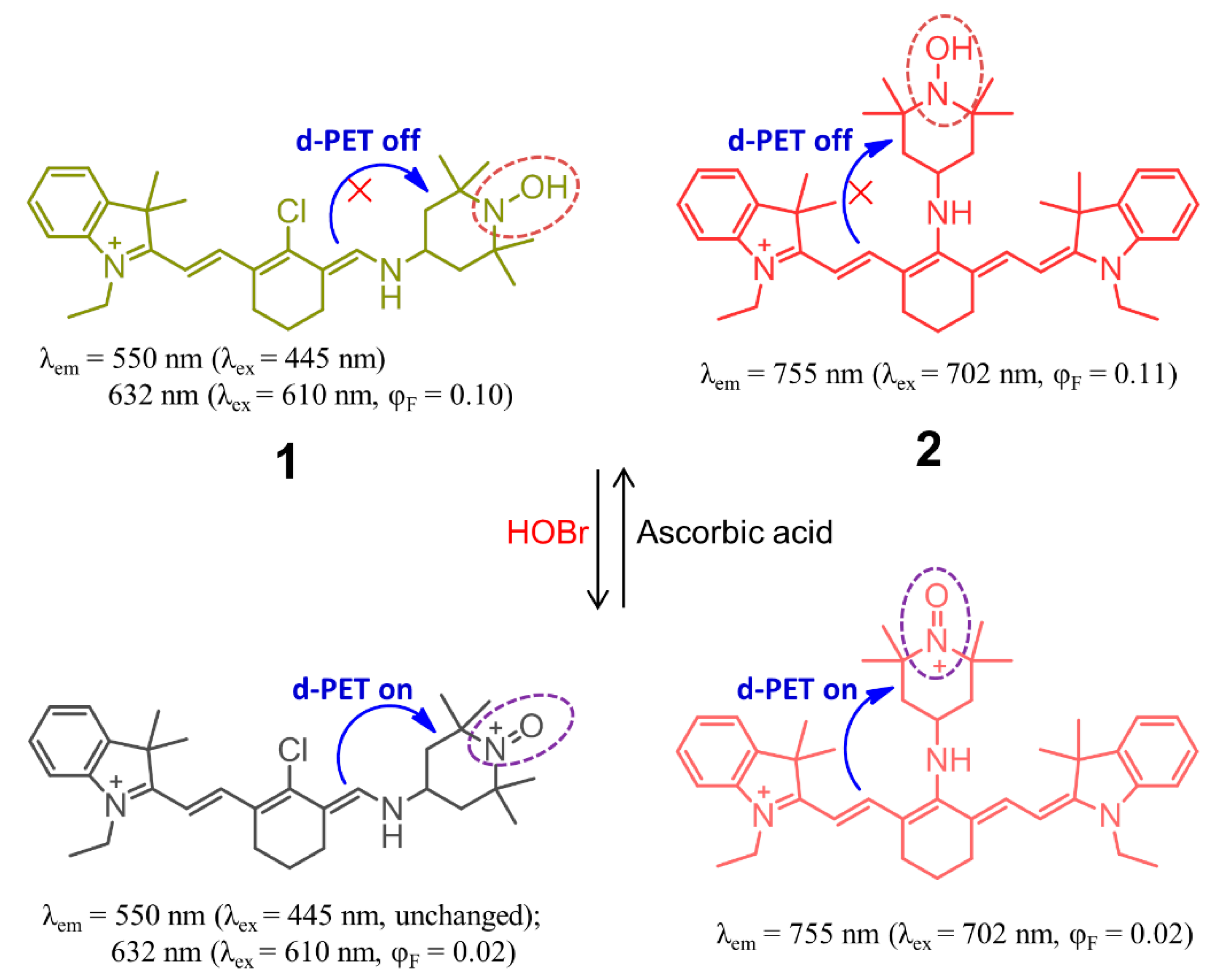
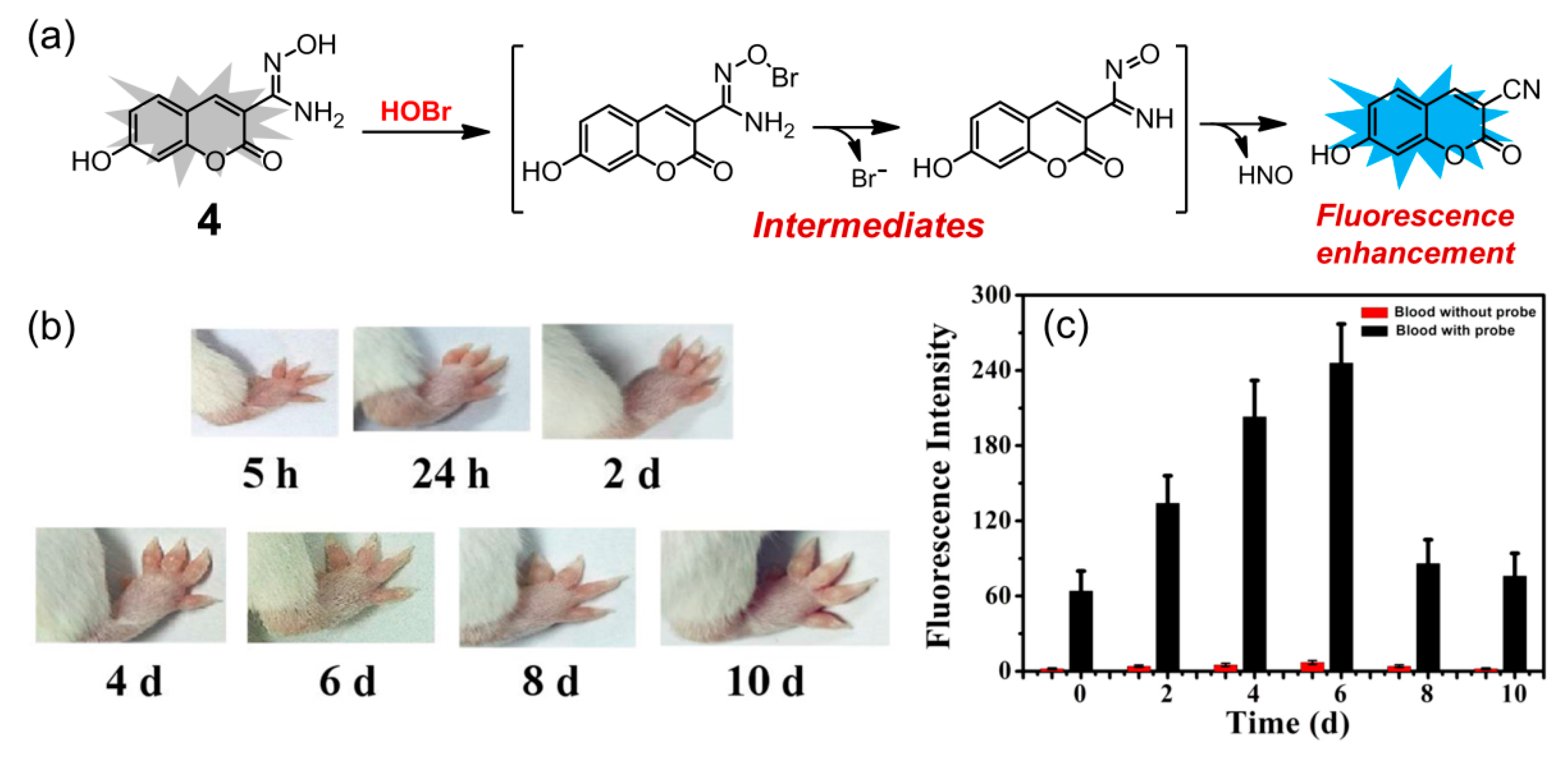
2. Probes Based on the Oxidation Reactions Caused by HOBr
3. Coupling and Cyclization of Amino and S-Methyl Groups Catalyzed by HOBr
4. Probes Based on Substitution Reactions Caused by HOBr
5. Summary and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular chemistry. Science 1993, 260, 1762–1763. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Miyata, Y.; Mukae, Y.; Harada, J.; Matsuda, T.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Sakai, H. Pathological and Pharmacological Roles of Mitochondrial Reactive Oxygen Species in Malignant Neoplasms: Therapies Involving Chemical Compounds, Natural Products, and Photosensitizers. Molecules 2020, 25, 5252. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.-C.; Sedgwick, A.C.; Zang, Y.; Chen, G.-R.; He, X.-P.; Li, J.; Yoon, J.; James, T.D. Sensors, Imaging Agents, and Theranostics to Help Understand and Treat Reactive Oxygen Species Related Diseases. Small Methods 2019, 3, 1900013. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, L.; Lin, Y.; Ding, C.; Lu, C. Luminescent probes for hypochlorous acid in vitro and in vivo. Analyst 2020, 145, 5068–5089. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, L.; Jangili, P.; Sharma, A.; Li, W.; Hou, J.-T.; Qin, C.; Yoon, J.; Kim, J.S. Design and applications of fluorescent detectors for peroxynitrite. Coordin. Chem. Rev. 2018, 374, 36–54. [Google Scholar] [CrossRef]
- Wu, H.; Song, Q.; Ran, G.; Lu, X.; Xu, B. Recent developments in the detection of singlet oxygen with molecular spectroscopic methods. TrAC Trend. Anal. Chem. 2011, 30, 133–141. [Google Scholar] [CrossRef]
- Lane, A.E.; Tan, J.T.M.; Hawkins, C.L.; Heather, A.K.; Davies, M.J. The myeloperoxidase-derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signalling via the mitogen-activated protein kinase (MAPK) pathway in macrophages. Biochem. J. 2010, 430, 161–169. [Google Scholar] [CrossRef] [Green Version]
- McCall, A.S.; Cummings, C.F.; Bhave, G.; Vanacore, R.; Page-McCaw, A.; Hudson, B.G. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 2014, 157, 1380–1392. [Google Scholar] [CrossRef] [Green Version]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.; James, S.A.; Kirby, J.K.; Rodgers, R.J.; Harris, H.H. Distribution and speciation of bromine in mammalian tissue and fluids by X-ray fluorescence imaging and X-ray absorption spectroscopy. Metallomics 2015, 7, 756–765. [Google Scholar] [CrossRef]
- Brown, K.L.; Darris, C.; Rose, K.L.; Sanchez, O.A.; Madu, H.; Avance, J.; Brooks, N.; Zhang, M.-Z.; Fogo, A.; Harris, R.; et al. Hypohalous Acids Contribute to Renal Extracellular Matrix Damage in Experimental Diabetes. Diabetes 2015, 64, 2242–2253. [Google Scholar] [CrossRef] [Green Version]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Aldridge, R.E.; Chan, T.; van Dalen, C.J.; Senthilmohan, R.; Winn, M.; Venge, P.; Town, G.I.; Kettle, A.J. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radical Biol. Med. 2002, 33, 847–856. [Google Scholar] [CrossRef]
- Ximenes, V.F.; Morgon, N.H.; de Souza, A.R. Hypobromous acid, a powerful endogenous electrophile: Experimental and theoretical studies. J. Inorg. Biochem. 2015, 146, 61–68. [Google Scholar] [CrossRef]
- Guo, C.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular fluorescent sensors: An historical overview and update. Coordin. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef]
- Hira, J.; Uddin, M.J.; Haugland, M.M.; Lentz, C.S. From Differential Stains to Next Generation Physiology: Chemical Probes to Visualize Bacterial Cell Structure and Physiology. Molecules 2020, 25, 4949. [Google Scholar] [CrossRef]
- Chilka, P.; Desai, N.; Datta, B. Small Molecule Fluorescent Probes for G- Quadruplex Visualization as Potential Cancer Theranostic Agents. Molecules 2019, 24, 752. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent Probes for the Visualization of Cell Viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gulzar, A.; Yang, P.; Bi, H.; Yang, D.; Gai, S.; He, F.; Lin, J.; Xing, B.; Jin, D. Recent advances in near-infrared emitting lanthanide-doped nanoconstructs: Mechanism, design and application for bioimaging. Coordin. Chem. Rev. 2019, 381, 104–134. [Google Scholar] [CrossRef]
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing. Coordin. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Li, L.; Dong, X.; Li, J.; Wei, J. A short review on NIR-II organic small molecule dyes. Dyes Pigment. 2020, 183, 108756. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. AIE-based cancer theranostics. Coordin. Chem. Rev. 2020, 402, 213076. [Google Scholar] [CrossRef]
- Feng, H.-T.; Lam, J.W.Y.; Tang, B.Z. Self-assembly of AIEgens. Coordin. Chem. Rev. 2020, 406, 213142. [Google Scholar] [CrossRef]
- Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens for Activity-Based Sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-Q.; Wei, T.-B.; Ma, X.-Q.; Yang, Q.-Y.; Zhang, Y.-F.; Sun, Y.-J.; Shi, B.-B.; Yao, H.; Zhang, Y.-M.; Lin, Q. 1,8-Naphthalimide-based fluorescent chemosensors: Recent advances and perspectives. J. Mater. Chem. C 2020, 8, 13501–13529. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Meher, N.; Iyer, P.K. Functional 1,8-Naphthalimide AIE/AIEEgens: Recent Advances and Prospects. ACS Appl. Mater. Interfaces 2018, 10, 12081–12111. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.; Martinho, J.M. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem. Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and controlled photosensitization with BODIPY dyes. Coordin. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coordin. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Z.; Feng, R.; Ou, Z.; Wang, S.; Yang, L.; Ma, L.-J. An intermolecular pyrene excimer-based ratiometric fluorescent probes for extremely acidic pH and its applications. Dyes Pigment. 2020, 174, 108102. [Google Scholar] [CrossRef]
- Krasheninina, O.A.; Novopashina, D.S.; Apartsin, E.K.; Venyaminova, A.G. Recent Advances in Nucleic Acid Targeting Probes and Supramolecular Constructs Based on Pyrene-Modified Oligonucleotides. Molecules 2017, 22, 2108. [Google Scholar] [CrossRef] [Green Version]
- Ostergaard, M.E.; Hrdlicka, P.J. Pyrene-functionalized oligonucleotides and locked nucleic acids (LNAs): Tools for fundamental research, diagnostics, and nanotechnology. Chem. Soc. Rev. 2011, 40, 5771–5788. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.T.; Yuan, Y.X.; Xiong, J.B.; Zheng, Y.S.; Tang, B.Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem. Soc. Rev. 2018, 47, 7452–7476. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, P.; Kaur, N.; Mittal, L.S.; Kumar, K. Perylene diimides: Will they flourish as reaction-based probes? Anal. Methods 2020, 12, 3560–3574. [Google Scholar] [CrossRef]
- Kwon, O.S.; Song, H.S.; Park, T.H.; Jang, J. Conducting Nanomaterial Sensor Using Natural Receptors. Chem. Rev. 2019, 119, 36–93. [Google Scholar] [CrossRef]
- Khorasani, M.Y.; Langari, H.; Sany, S.B.T.; Rezayi, M.; Sahebkar, A. The role of curcumin and its derivatives in sensory applications. Mater. Sci. Eng. C 2019, 103, 109792. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Dehaen, W. Small-molecule-based fluorescent probes for f-block metal ions: A new frontier in chemosensors. Coordin. Chem. Rev. 2021, 427, 213524. [Google Scholar] [CrossRef]
- Fang, Y.; Deng, Y.; Dehaen, W. Tailoring pillararene-based receptors for specific metal ion binding: From recognition to supramolecular assembly. Coordin. Chem. Rev. 2020, 415, 213313. [Google Scholar] [CrossRef]
- Huang, J.; Fang, Y.; Dehaen, W. Macrocyclic Arenes Functionalized with BODIPY: Rising Stars among Chemosensors and Smart Materials. Chemosensors 2020, 8, 51. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.W.; Chen, L.; Yuan, J.; Liu, Y.; Teng, L.; Huan, S.Y.; Yuan, L.; Zhang, X.B.; Tan, W. Learning from Artemisinin: Bioinspired Design of a Reaction-Based Fluorescent Probe for the Selective Sensing of Labile Heme in Complex Biosystems. J. Am. Chem. Soc. 2020, 142, 2129–2133. [Google Scholar] [CrossRef]
- Murfin, L.C.; Weber, M.; Park, S.J.; Kim, W.T.; Lopez-Alled, C.M.; McMullin, C.L.; Pradaux-Caggiano, F.; Lyall, C.L.; Kociok-Kohn, G.; Wenk, J.; et al. Azulene-Derived Fluorescent Probe for Bioimaging: Detection of Reactive Oxygen and Nitrogen Species by Two-Photon Microscopy. J. Am. Chem. Soc. 2019, 141, 19389–19396. [Google Scholar] [CrossRef] [Green Version]
- Nan, X.; Huyan, Y.; Li, H.; Sun, S.; Xu, Y. Reaction-based fluorescent probes for Hg2+, Cu2+ and Fe3+/Fe2+. Coordin. Chem. Rev. 2021, 426, 213580. [Google Scholar] [CrossRef]
- Hou, J.-T.; Zhang, M.; Liu, Y.; Ma, X.; Duan, R.; Cao, X.; Yuan, F.; Liao, Y.-X.; Wang, S.; Xiu Ren, W. Fluorescent detectors for hydroxyl radical and their applications in bioimaging: A review. Coordin. Chem. Rev. 2020, 421, 213457. [Google Scholar] [CrossRef]
- Ma, C.; Zhong, G.; Zhao, Y.; Zhang, P.; Fu, Y.; Shen, B. Recent development of synthetic probes for detection of hypochlorous acid/hypochlorite. Spectrochim. Acta A 2020, 240, 118545. [Google Scholar] [CrossRef]
- Zheng, D.-J.; Yang, Y.-S.; Zhu, H.-L. Recent progress in the development of small-molecule fluorescent probes for the detection of hydrogen peroxide. TrAC Trend. Anal. Chem. 2019, 118, 625–651. [Google Scholar] [CrossRef]
- Prolo, C.; Rios, N.; Piacenza, L.; Alvarez, M.N.; Radi, R. Fluorescence and chemiluminescence approaches for peroxynitrite detection. Free Radic. Biol. Med. 2018, 128, 59–68. [Google Scholar] [CrossRef]
- You, Y. Chemical tools for the generation and detection of singlet oxygen. Org. Biomol. Chem. 2018, 16, 4044–4060. [Google Scholar] [CrossRef]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Ng, K.K.-H.; Hu, J.J.; Ye, S.; Yang, D. Small-Molecule-Based Fluorescent Sensors for Selective Detection of Reactive Oxygen Species in Biological Systems. Annu. Rev. Biochem. 2019, 88, 605–633. [Google Scholar] [CrossRef]
- Yan, F.; Zang, Y.; Sun, J.; Sun, Z.; Zhang, H. Sensing mechanism of reactive oxygen species optical detection. TrAC Trend. Anal. Chem. 2020, 131, 116009. [Google Scholar] [CrossRef]
- Yu, F.; Song, P.; Li, P.; Wang, B.; Han, K. Development of reversible fluorescence probes based on redox oxoammonium cation for hypobromous acid detection in living cells. Chem. Commun. 2012, 48, 7735–7737. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Yu, F.; Chen, J.; Qu, Z.; Han, K. A near-infrared reversible and ratiometric fluorescent probe based on Se-BODIPY for the redox cycle mediated by hypobromous acid and hydrogen sulfide in living cells. Chem. Commun. 2013, 49, 5790–5792. [Google Scholar] [CrossRef]
- Hoover, G.C.; Seferos, D.S. Photoactivity and optical applications of organic materials containing selenium and tellurium. Chem. Sci. 2019, 10, 9182–9188. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-H.; Liu, C.; Mei, Y.; Song, Q.-H. BODIPY-based selenides as fluorescent probes for rapid, sensitive and mitochondria-specific detection of hypochlorous acid. J. Maters Chem. B 2019, 7, 6861–6867. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Kwon, N.; Yoon, J. Fluorescent Probes Containing Selenium as a Guest or Host. Chem 2016, 1, 674–698. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Panda, A.; Zade, S.S. Organoselenium compounds as fluorescent probes. Coordin. Chem. Rev. 2015, 300, 86–100. [Google Scholar] [CrossRef]
- Manjare, S.T.; Kim, Y.; Churchill, D.G. Selenium- and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Wang, X.; Yang, R.; Li, Z.; Sun, Y.; Qu, L.; Zeng, H. A novel fluorescent probe for highly selective and sensitive detection of hypobromous acid in arthritis model mice. Sens. Actuators B Chem. 2020, 315, 128125. [Google Scholar] [CrossRef]
- Xu, K.; Luan, D.; Wang, X.; Hu, B.; Liu, X.; Kong, F.; Tang, B. An Ultrasensitive Cyclization-Based Fluorescent Probe for Imaging Native HOBr in Live Cells and Zebrafish. Angew. Chem. Int. Ed. 2016, 55, 12751–12754. [Google Scholar] [CrossRef]
- Luan, D.; Gao, X.; Kong, F.; Song, X.; Zheng, A.; Liu, X.; Xu, K.; Tang, B. Cyclic Regulation of the Sulfilimine Bond in Peptides and NC1 Hexamers via the HOBr/H2Se Conjugated System. Anal. Chem. 2018, 90, 9523–9528. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Yao, Q.; Fan, J.; Sun, W.; Long, S.; Shao, K.; Du, J.; Wang, J.; Peng, X. In situ imaging of aminopeptidase N activity in hepatocellular carcinoma: A migration model for tumour using an activatable two-photon NIR fluorescent probe. Chem. Sci. 2019, 10, 1619–1625. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Liu, R.; Zhan, Z.; Lv, Y. Fast response near-infrared fluorescent probe for hydrogen sulfide in natural waters. Talanta 2019, 202, 159–164. [Google Scholar] [CrossRef]
- Lv, H.; Yuan, G.; Zhang, G.; Ren, Z.; He, H.; Sun, Q.; Zhang, X.; Wang, S. A novel benzopyran-based colorimetric and near-infrared fluorescent sensor for Hg2+ and its imaging in living cell and zebrafish. Dyes Pigment. 2020, 172, 107658. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Shen, Y.; Zhang, M.; Liu, T.; Cao, H.; Chen, L.; Li, D.; Tian, Y.; Tian, X. Aggregation-induced emission with enhanced two-photon absorption of dicyanomethylene-benzopyran with polyether chain for blood-brain barrier penetration. Dyes Pigment. 2020, 172, 107827. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Ma, Y.; Yu, F.; Liu, H. A novel near-infrared fluorescent probe for detection of hypobromous acid and its bioimaging applications. Spectrochim. Acta A 2019, 222, 117240. [Google Scholar] [CrossRef]
- Jia, P.; Liu, D.; Zhuang, Z.; Qu, L.; Liu, C.; Zhang, Y.; Li, Z.; Zhu, H.; Yu, Y.; Zhang, X.; et al. A highly selective ratiometric fluorescence probe for bioimaging of hypobromous acid in living cells and zebrafish. Sens. Actuators B Chem. 2020, 320, 128583. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef]
- Wen, Y.; Huo, F.; Yin, C. Organelle targetable fluorescent probes for hydrogen peroxide. Chin. Chem. Lett. 2019, 30, 1834–1842. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinker, A.E.; Vivian, C.J.; Beadnell, T.C.; Koestler, D.C.; Teoh, S.T.; Lunt, S.Y.; Welch, D.R. Mitochondrial Haplotype of the Host Stromal Microenvironment Alters Metastasis in a Non-cell Autonomous Manner. Cancer Res. 2020, 80, 1118–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Kim, J. Mitochondrial Retrograde Signalling and Metabolic Alterations in the Tumour Microenvironment. Cells 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Yan, Q.; Ai, S.; Qi, P.; Yang, H.; Zhang, Y.; Qing, Z.; Zhang, L.; Feng, F.; Yang, R. Real-Time Visualizing Mitophagy-Specific Viscosity Dynamic by Mitochondria-Anchored Molecular Rotor. Anal. Chem. 2019, 91, 8574–8581. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, A.; Luan, D.; Wang, X.; Kong, F.; Tong, L.; Xu, K.; Tang, B. High-Quantum-Yield Mitochondria-Targeting Near-Infrared Fluorescent Probe for Imaging Native Hypobromous Acid in Living Cells and in Vivo. Anal. Chem. 2017, 89, 1787–1792. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Q.; Huang, Z.; Huang, L.; Xiao, Y. Immobilizable fluorescent probes for monitoring the mitochondria microenvironment: A next step from the classic. J. Mater. Chem. B 2019, 7, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tian, Y. A ratiometric fluorescent probe for bioimaging and biosensing of HBrO in mitochondria upon oxidative stress. Chem. Commun. 2018, 54, 12198–12201. [Google Scholar] [CrossRef] [PubMed]
- Lüllmann-Rauch, R. History and morphology of the lysosome. In Lysosomes; Springer: Boston, MA, USA, 2005; pp. 1–16. [Google Scholar]
- Qiu, K.; Zhu, H.; Rees, T.W.; Ji, L.; Zhang, Q.; Chao, H. Recent advances in lysosome-targeting luminescent transition metal complexes. Coordin. Chem. Rev. 2019, 398, 113010. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Zhang, W.; Liu, J.; Wang, Y.L.; Du, Z.; Song, B.; Xu, Z.P.; Yuan, J. “Dual-Key-and-Lock” Ruthenium Complex Probe for Lysosomal Formaldehyde in Cancer Cells and Tumors. J. Am. Chem.Soc. 2019, 141, 8462–8472. [Google Scholar] [CrossRef]
- Gao, P.; Wang, J.; Zheng, M.; Xie, Z. Lysosome targeting carbon dots-based fluorescent probe for monitoring pH changes in vitro and in vivo. Chem. Eng. J. 2020, 381, 122665. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, K.; He, L.; Lin, W. Mitochondria and lysosome-targetable fluorescent probes for HOCl: Recent advances and perspectives. J. Mater. Chem. B 2018, 6, 1716–1733. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.; Zhang, Y.; Zhu, X.; Zhou, L.; Fang, R.; Liu, X.; Zhang, H. Lysosome-targeted two-photon fluorescent probe for detection of hypobromous acid in vitro and in vivo. Spectrochim. Acta A 2019, 212, 48–54. [Google Scholar] [CrossRef]
- Qu, W.; Li, K.; Han, D.; Zhong, X.; Chen, C.; Liang, X.; Liu, H. Lysosome-targetable red-emitting ratiometric fluorescent probe for hypobromous acid imaging in living cells. Sens. Actuators B Chem. 2019, 297, 126826. [Google Scholar] [CrossRef]
- Bekdeşer, B.; Zeytünlü, G.; Özyürek, M.; Apak, M.R. A novel hypobromous acid scavenging activity assay using p-cresol as a spectrofluorometric probe. Turk. J. Chem. 2018, 42, 429–438. [Google Scholar] [CrossRef]
- Kim, S.; Bouffard, J.; Kim, Y. Tailoring the Solid-State Fluorescence Emission of BODIPY Dyes by meso Substitution. Chem. Eur. J. 2015, 21, 17459–17465. [Google Scholar] [CrossRef]
- Kim, T.I.; Hwang, B.; Lee, B.; Bae, J.; Kim, Y. Selective Monitoring and Imaging of Eosinophil Peroxidase Activity with a J-Aggregating Probe. J. Am. Chem. Soc. 2018, 140, 11771–11776. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhuang, Z.; Liu, D.; Chen, Y.; Tian, B.; Liu, C.; Li, Z.; Zhu, H.; Yu, Y.; Zhang, X.; et al. A novel highly selective fluorescent probe for imaging endogenous hypobromous acid in living cells and zebrafish. Sens. Actuators B Chem. 2020, 305, 127460. [Google Scholar] [CrossRef]

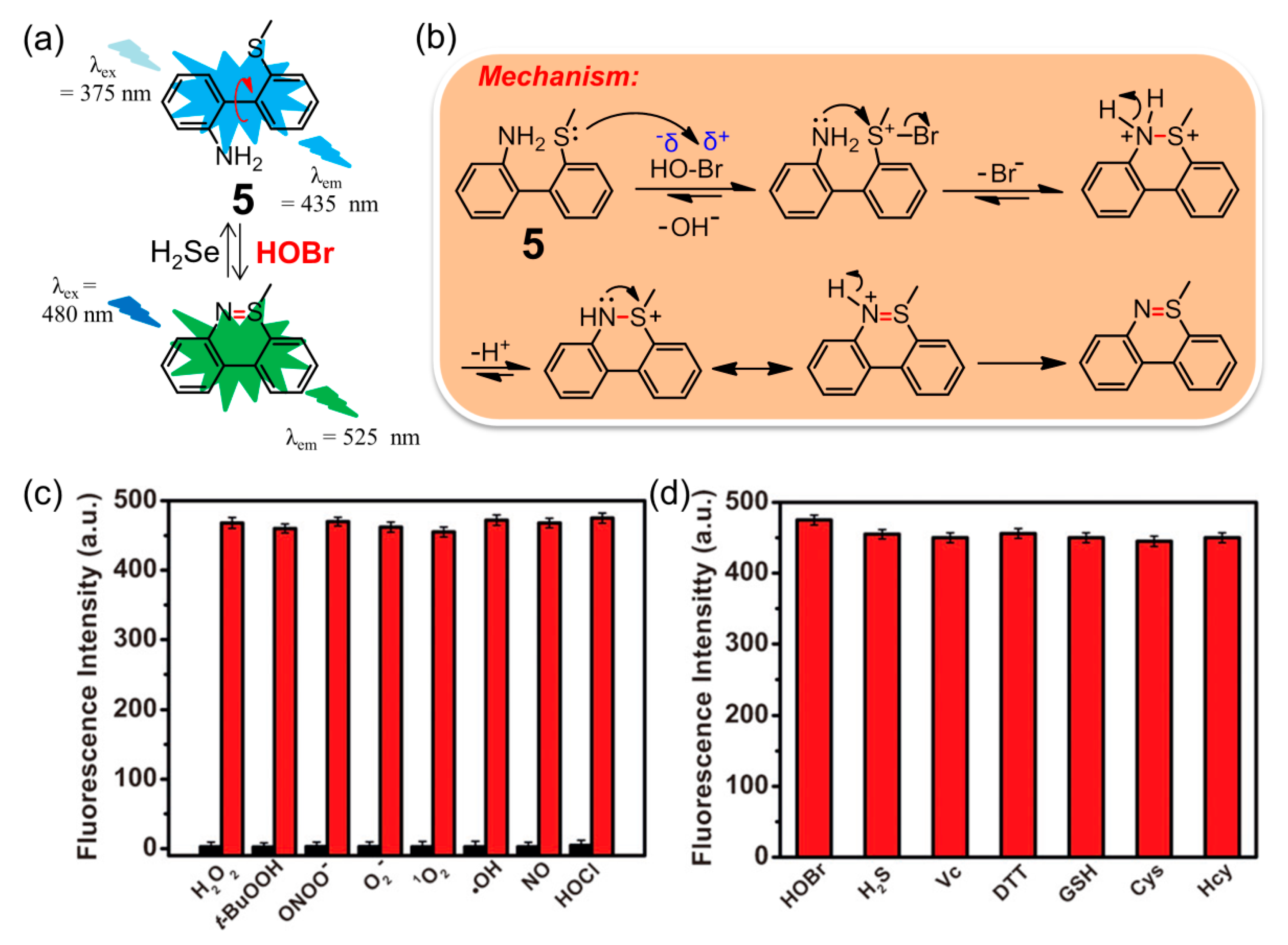
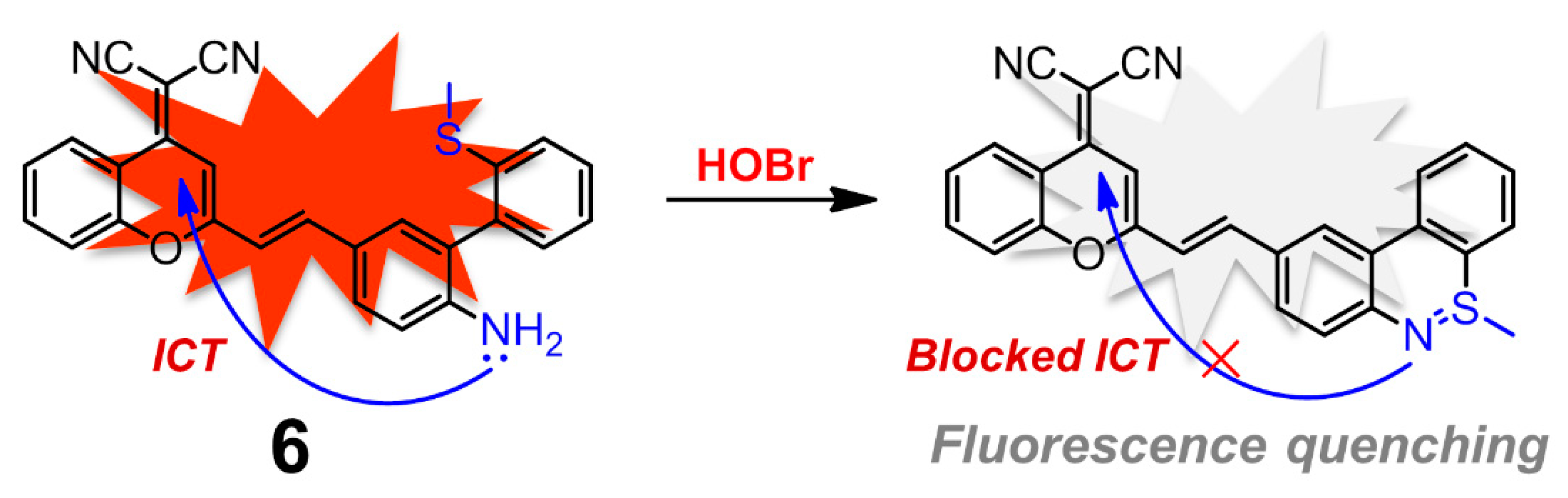
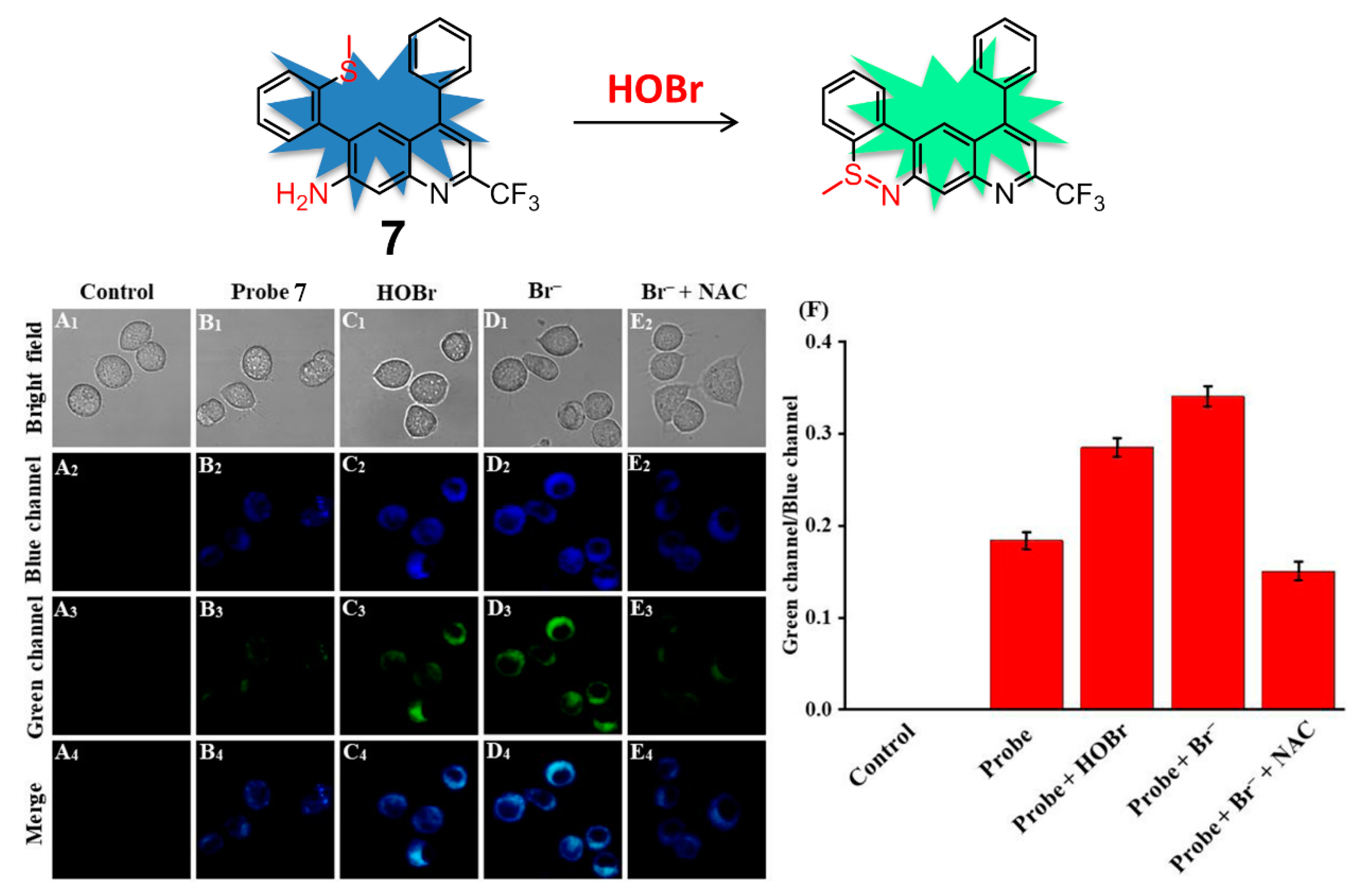
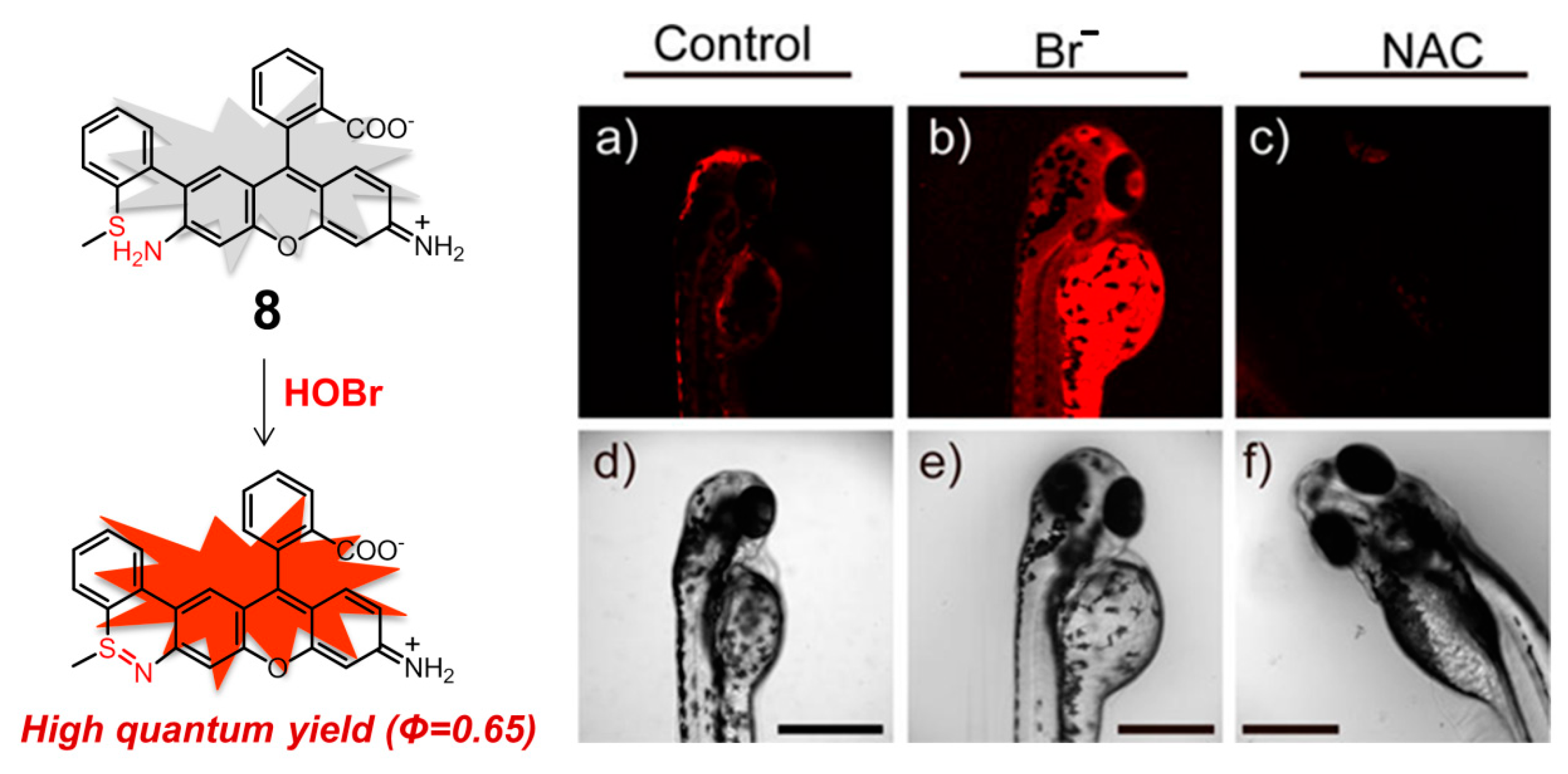


| Entry | Probe | Solvent System | Signal Type | λex/λem (nm) | Response Time | Applications | Detection Limit | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.2 M PBS (pH = 7.4) | ratiometric | 445/550; 610/632 | 900 s | HOBr-imaging in RAW264.7 cells | n.d. a | [68] |
| 2 | 2 | 0.2 M PBS (pH = 7.4) | turn-off | 702/755 | 900 s | HOBr-imaging in RAW264.7 cells | n.d. a | [68] |
| 3 | 3 | 20 mM PBS containing 20% CH3CN (pH = 7.4) | ratiometric | 610/635, 711 | 3.0 min | HOBr-imaging in RAW264.7 cells | 50 nM | [69] |
| 4 | 4 | 10 mM PBS (pH = 7.4) | turn-on | 395/460 | 30 s | monitoring HOBr in arthritis model mice and real-time evaluating the development of arthritis | 30.6 nM | [75] |
| 5 | 5 | 10 mM PBS containing 0.5% CH3CN (pH = 7.4) | turn-on | 480/525 | ca. 3.0 min | imaging endogenous HOBr in HepG2 cells and zebrafish | 17 pM | [76] |
| 6 | 6 | 10 mM PBS -CH3CN (3: 2, v/v, pH = 7.4) | turn-off | 488/655 | 8.0 min | monitoring HOBr in MCF-7 cells | 660 nM | [82] |
| 7 | 7 | 10 mM PBS-EtOH (6:4, v/v, pH = 7.4). | ratiometric | 460/505, 545 | 50 s | tracking the changes of HOBr in RAW 264.7 cells and zebrafish | 92 nM | [83] |
| 8 | 8 | 10 mM HEPES containing 0.3% DMSO (pH = 7.4) | turn-on | 624/663 | ca. 3.0 min | imaging native HOBr in mitochondria of HepG2 cells and zebrafish | 20 pM | [91] |
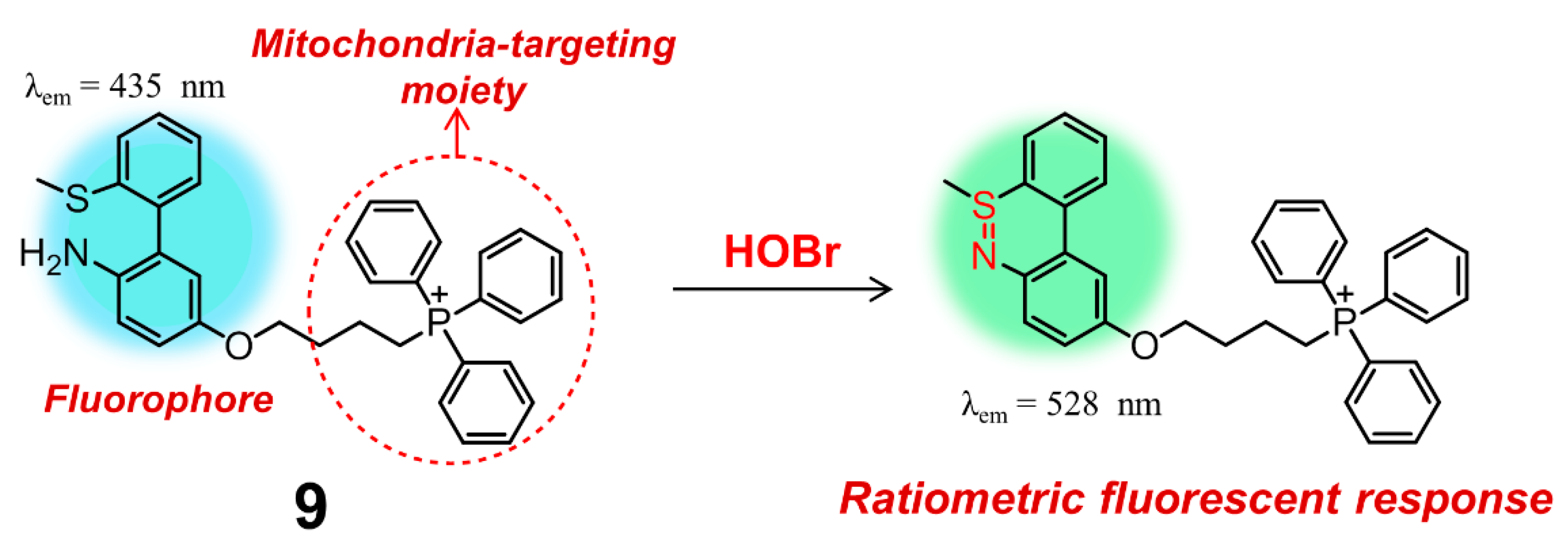
| 9 | 9 | 10 mM PBS containing 0.5% DMSO (pH = 7.4) | ratiometric | 405/437, 528 | 30 s | imaging of HOBr in mitochondria of RAW264.7 cells | 18 nM | [93] |
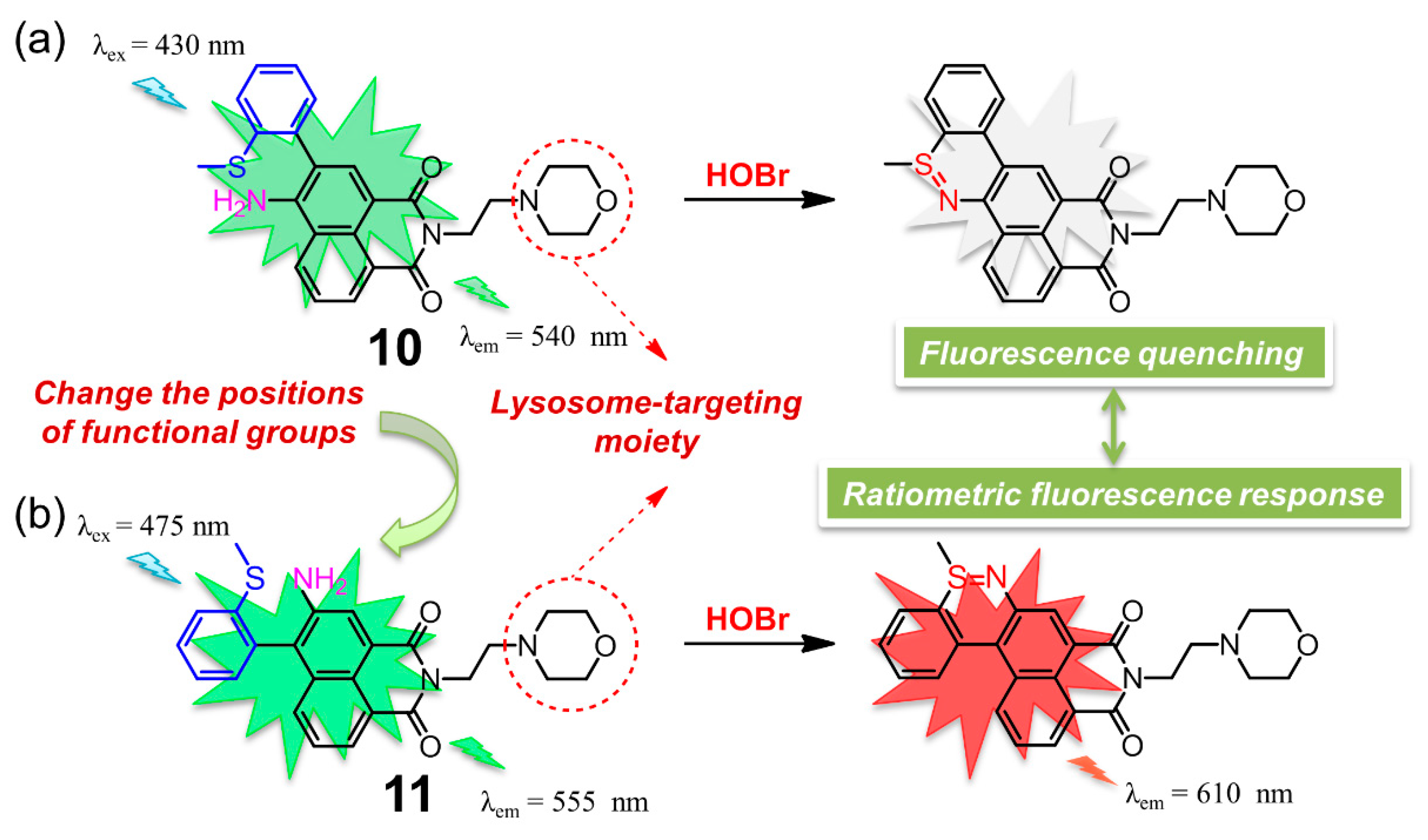
| 10 | 10 | 10 mM HEPES containing 0.1% DMSO (pH = 7.4) | turn-off | 430/540 | immediately | imaging of exogenous and endogenous HOBr in Hela cells and mice | 33.5 nM | [99] |
| 11 | 11 | 10mM PBS-CH3CN (3:2, v/v, pH = 7.4) | ratiometric | 475/555, 610 | 12 s | imaging of exogenous and endogenous HOBr in HeLa cells | 99 nM | [100] |
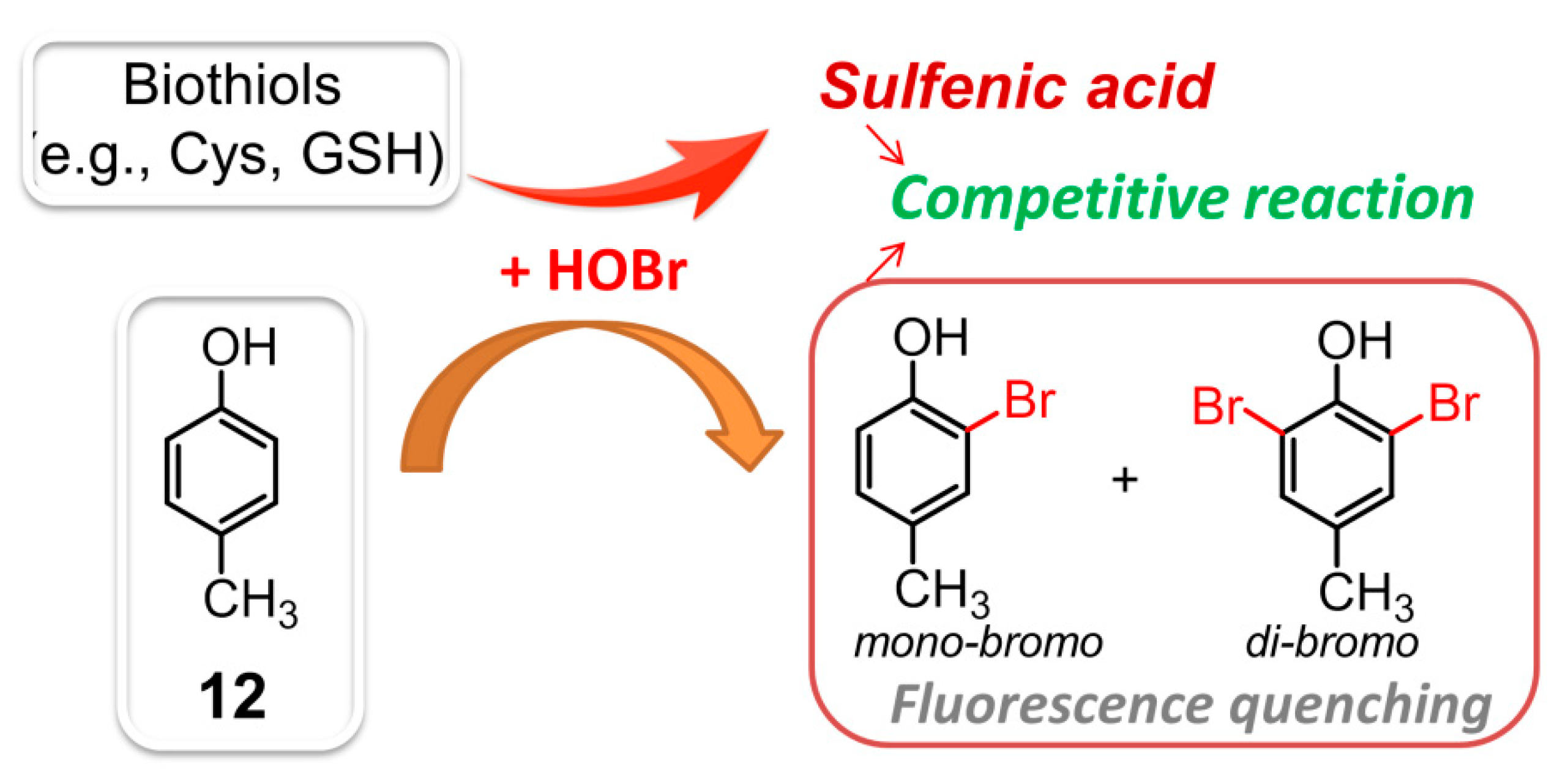
| 12 | 12 | distilled water | turn-off | 260/305 | n.d. a | determination of the HOBr scavenging activity of biothiols and some pharmaceutical samples | 0.37 μM | [101] |
| 13 | 13 | 100 mM acetate buffer containing 0.1% CH3CN (pH = 5.0) | ratiometric | 480/581, 616 | ≤2 s | monitoring EPO activity and fluorescence assays of oxidative stress in cancer cells (HCT116 and A549) as well as immune response detection in live mice. | 3.8 nM | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Dehaen, W. Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives. Molecules 2021, 26, 363. https://doi.org/10.3390/molecules26020363
Fang Y, Dehaen W. Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives. Molecules. 2021; 26(2):363. https://doi.org/10.3390/molecules26020363
Chicago/Turabian StyleFang, Yuyu, and Wim Dehaen. 2021. "Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives" Molecules 26, no. 2: 363. https://doi.org/10.3390/molecules26020363
APA StyleFang, Y., & Dehaen, W. (2021). Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives. Molecules, 26(2), 363. https://doi.org/10.3390/molecules26020363






