Molecular Recognition of Imidazole Derivatives by Co(III)-Porphyrins in Phosphate Buffer (pH = 7.4) and Cetylpyridinium Chloride Containing Solutions
Abstract
:1. Introduction
2. Results and Its Discussion
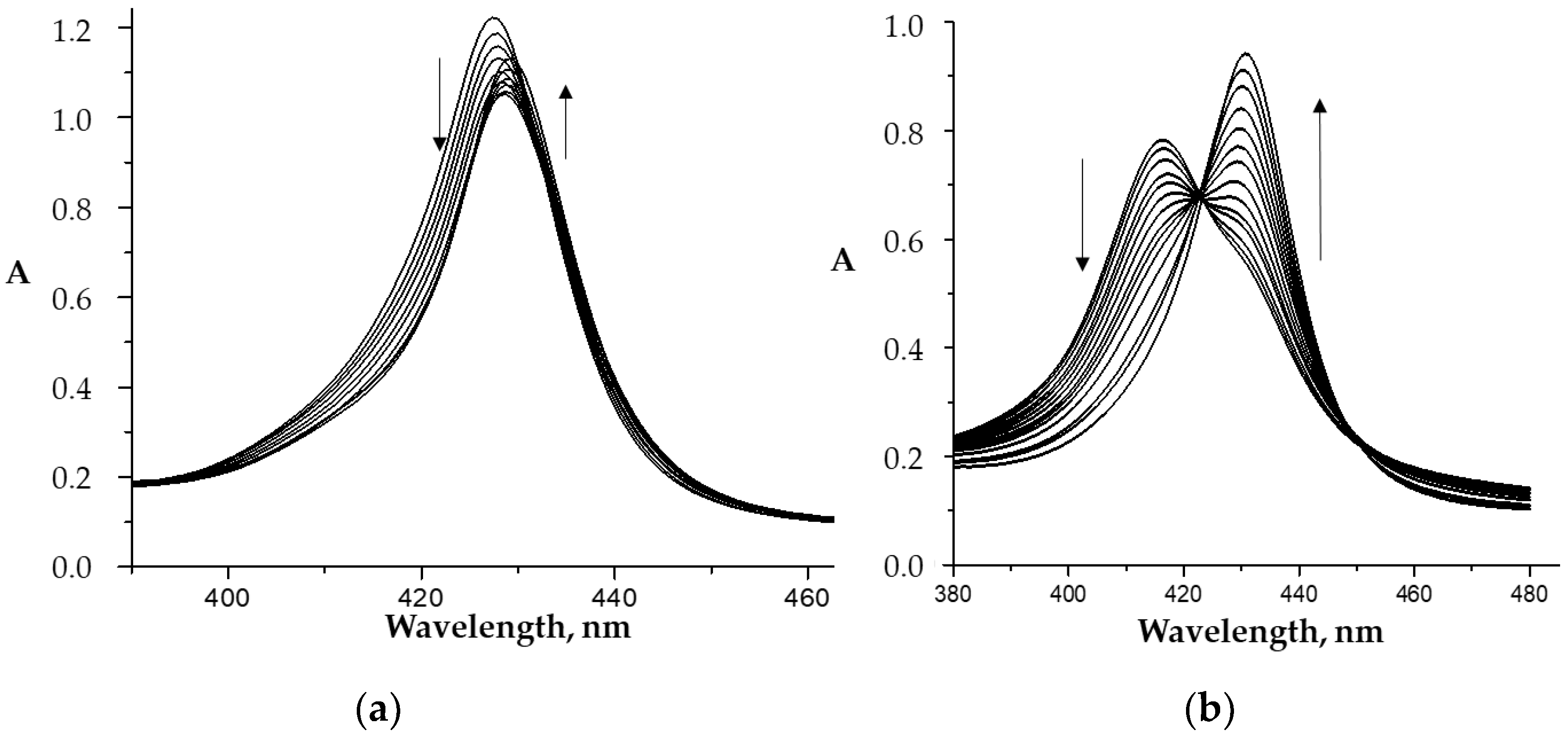
2.1. Formation of the Co(III)P bis-Axial Complexes with Imidazole Derivatives
2.2. Co(III)/Co(II) Red-Ox Processes in the Composition of the Co(III)P in the Presence of CPC
2.3. The Effect of Micelle Formation on the Processes on Axial Coordination of the Organic Ligands by the Co(III)-Porphyrins
3. Materials and Methods
3.1. Spectrophotometric Studies
3.2. NMR Studies
3.3. Quantum-Chemical Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Steed, J.W.; Atwood, J. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Chichester, UK, 2009. [Google Scholar]
- Grunenberg, J. Complexity in molecular recognition. Phys. Chem. Chem. Phys. 2011, 13, 10136–10146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Landry, M.P.; Barone, P.W.; Kim, J.H.; Lin, S.; Ulissi, Z.W.; Lin, D.; Mu, B.; Boghossian, A.A.; Hilmer, A.J.; et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 2013, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Mannige, R.V.; Haxton, T.K.; Proulx, C.; Robertson, E.J.; Battigelli, A.; Butterfoss, G.L.; Zuckermann, R.N.; Whitelam, S. Peptoid nanosheets exhibit a new secondary-structure motif. Nature 2015, 526, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, F.; Schneider, H.J. Experimental Binding Energies in Supramolecular Complexes. Chem. Rev. 2016, 116, 5216–5300. [Google Scholar] [CrossRef]
- Ogoshi, H.; Mizutani, T.; Hayashi, T.; Kuroda, Y. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; Volume 6, Chapter 46; pp. 279–337. [Google Scholar]
- Sanders, J.K.M. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; Volume 3, Chapter 22; pp. 347–368. [Google Scholar]
- Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Supramolecular porphyrin complexes. Russ. Chem. Rev. 2005, 74, 765–780. [Google Scholar] [CrossRef]
- Rauch, V.; Conradt, J.; Takahashi, M.; Kanesato, M.; Wytko, J.A.; Kikkawa, Y.; Kalt, H.; Weiss, J. Self-organized porphyrin arrays on surfaces: The case of hydrophilic side chains and polar surfaces. J. Porphyr. Phthalocyanines 2014, 18, 67–75. [Google Scholar] [CrossRef]
- Higashino, T.; Fujimori, Y.; Sugiura, K.; Tsuji, Y.; Ito, S.; Imahori, H. Synthesis of push-pull porphyrin with two electron-donating and two electron-withdrawing groups and its application to dye-sensitized solar cell. J. Porphyr. Phthalocyanines 2015, 19, 140–149. [Google Scholar] [CrossRef]
- Sharma, G.D.; Zervaki, G.E.; Ladomenou, K.; Koukaras, E.N.; Angaridis, P.P.; Coutsolelos, A.G. Donor-π-acceptor, triazine-linked porphyrin dyads as sensitizers for dye-sensitized solar cells. J. Porphyr. Phthalocyanines 2015, 19, 175–191. [Google Scholar] [CrossRef]
- Shinoda, S.; Ohashi, M.; Tsukube, H. “Pocket dendrimers” as nanoscale receptors for bimolecular guest accommodation. Chem. A Eur. J. 2007, 13, 81–89. [Google Scholar] [CrossRef]
- Mamardashvili, G.; Shaekhov, T.; Gibadullina, E.; Voronina, J.; Burilov, A.; Koifman, O.; Mamardashvili, N.; Dehaen, W. Synthesis and binding ability of mono- and tetrasubstituted aminophosphonate Zn-tetraarylporphyrins towards N- and O-containing organic substrates. Supramol. Chem. 2014, 26, 427–437. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Kulikova, O.M.; Maltseva, O.V.; Koifman, O.I.; Mamardashvili, N.Z. One and two point binding of organic bases molecules by meso-nitro substituted Zn-octaethylporphyrins. J. Porphyr. Phthalocyanines 2014, 18, 1101–1107. [Google Scholar] [CrossRef]
- Tran Nguyen, N.; Mamardashvili, G.M.; Kulikova, O.M.; Scheblykin, I.G.; Mamardashvili, N.Z.; Dehaen, W. Binding ability of first and second generation/carbazolylphenyl dendrimers with Zn(II) tetraphenylporphyrin core towards small heterocyclic substrates. RSC Adv. 2014, 4, 19703–19709. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Maltceva, O.V.; Mamardashvili, N.Z.; Nguyen, N.T.; Dehaen, W. Cation assisted complexation of octacarbazolylphenyl substituted Zn(II)-tetraphenylporphyrin with [2,2,2]cryptand. RSC Adv. 2015, 5, 44557–44562. [Google Scholar] [CrossRef]
- Maltceva, O.; Mamardashvili, G.; Khodov, I.; Lazovskiy, D.; Khodova, V.; Krest’yaninov, M.; Mamardashvili, N.; Dehaen, W. Molecular recognition of nitrogen—Containing bases by Zn[5,15-bis-(2,6-dodecyloxyphenyl)]porphyrin. Supramol. Chem. 2017, 29, 360–369. [Google Scholar] [CrossRef]
- Ashley, K.R.; Leipoldt, J.G. Kinetic and Equilibrium Study of the Reaction of (meso-Tetrakis(p-suIfonatophenyI)porphyrinato)diaquocobaltate(III) with Pyridine in Aqueous Solution. Inorg. Chem. 1981, 20, 2326–2333. [Google Scholar] [CrossRef]
- Hambright, P.; Langley, R. Cyanide scavengers: Kinetics of the reactions of cyanide with a water soluble cobalt(III) porphyrin. J. Inorg. Biochem. 1988, 32, 197–205. [Google Scholar] [CrossRef]
- Kaigorodova, E.Y.; Mamardashvili, G.M.; Mamardashvili, N.Z. Axial Coordination of Pyridine- and Imidazole-Based Drug Molecules to Co(III)-Tetra(4-Carboxyphenyl)porphyrin. Russ. J. Inorg. Chem. 2018, 63, 1192–1198. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Kaigorodova, E.Y.; Khodov, I.A.; Scheblykin, I.; Mamardashvili, N.Z.; Koifman, O.I. Micelles encapsulated Co(III)-tetra(4-sulfophenyl)porphyrin in aqueous CTAB solutions: Micelle formation, imidazole binding and redox Co(III)/Co(II) processes. J. Mol. Liq. 2019, 293, 111471. [Google Scholar] [CrossRef]
- Allen, S.A.; Datta, S.; Sandoval, J.; Tomilov, A.; Sears, T.; Woolard, K.; Angelastro, J.M.; Cortopassi, G.A. Cetylpyridinium chloride is a potent AMP-activated kinase (AMPK) inducer and has therapeutic potential in cancer. Mitochondrion 2020, 50, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sallam, G.; Shaban, S.Y.; Nassar, A.; El-Khouly, M.E. Water soluble porphyrin as optical sensor for the toxic heavy metal ions in an aqueous medium. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 241, 118609. [Google Scholar] [CrossRef]
- Mondal, S.; Banerjee, A.; Das, B. Spectroscopic and interfacial investigation on the interaction of hemoglobin with conventional and ionic liquid surfactants. J. Mol. Liq. 2020, 301, 112450. [Google Scholar] [CrossRef]
- Maaskant, R.V.; Polanco, E.A.; van Lier, R.C.W.; Roelfes, G. Cationic iron porphyrins with sodium dodecyl sulphate for micellar catalysis of cyclopropanation reactions. Org. Biomol. Chem. 2020, 18, 638–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel-Yagui, C.O.; Pessoa, A.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar] [PubMed]
- Khodov, I.A.; Alper, G.A.; Mamardashvili, G.M.; Mamardashvili, N.Z. Hybrid multi-porphyrin supramolecular assemblies: Synthesis and structure elucidation by 2D DOSY NMR studies. J. Mol. Struct. 2015, 1099, 174–180. [Google Scholar] [CrossRef]
- Bichan, N.G.; Tyulyaeva, E.Y.; Khodov, I.A.; Lomova, T.N. Synthesis and spectroscopic characterization of super-stable rhenium(V)porphyrins. J. Mol. Struct. 2014, 1061, 82–89. [Google Scholar] [CrossRef]
- Khodov, I.A.; Nikiforov, M.Y.; Alper, G.A.; Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Synthesis and spectroscopic characterization of Ru(II) and Sn(IV)-porphyrins supramolecular complexes. J. Mol. Struct. 2015, 1081, 426–430. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Maltceva, O.V.; Lazovskiy, D.A.; Khodov, I.A.; Borovkov, V.; Mamardashvili, N.Z.; Koifman, O.I. Medium viscosity effect on fluorescent properties of Sn(IV)-tetra(4-sulfonatophenyl)porphyrin complexes in buffer solutions. J. Mol. Liq. 2019, 277, 1047–1053. [Google Scholar] [CrossRef]
- Lazovskiy, D.A.; Mamardashvili, G.M.; Khodov, I.A.; Mamardashvili, N.Z. Water soluble porphyrin-fluorescein triads: Design, DFT calculation and pH-change-triggered fluorescence response. J. Photochem. Photobiol. A Chem. 2020, 402, 112832. [Google Scholar] [CrossRef]
- Ballester, P.; Claudel, M.; Durot, S.; Kocher, L.; Schoepff, L.; Heitz, V. A Porphyrin Coordination Cage Assembled from Four Silver(I) Triazolyl-Pyridine Complexes. Chem. A Eur. J. 2015, 21, 15339–15348. [Google Scholar] [CrossRef]
- Durot, S.; Taesch, J.; Heitz, V. Multiporphyrinic Cages: Architectures and Functions. Chem. Rev. 2014, 114, 8542–8578. [Google Scholar] [CrossRef]
- Galiullina, L.F.; Rakhmatullin, I.Z.; Klochkova, E.A.; Aganov, A.V.; Klochkov, V.V. Structure of pravastatin and its complex with sodium dodecyl sulfate micelles studied by NMR spectroscopy. Magn. Reson. Chem. 2014, 53, 110–114. [Google Scholar] [CrossRef]
- Rakhmatullin, I.Z.; Galiullina, L.F.; Klochkova, E.A.; Latfullin, I.A.; Aganov, A.V.; Klochkov, V.V. Structural studies of pravastatin and simvastatin and their complexes with SDS micelles by NMR spectroscopy. J. Mol. Struct. 2016, 1105, 25–29. [Google Scholar] [CrossRef]
- Blokhin, D.S.; Fayzullina, A.R.; Filippov, A.V.; Karataeva, F.K.; Klochkov, V.V. Spatial structure of fibrinopeptide B in water solution with DPC micelles by NMR spectroscopy. J. Mol. Struct. 2015, 1102, 91–94. [Google Scholar] [CrossRef]
- Blokhin, D.S.; Filippov, A.V.; Antzutkin, O.N.; Afonin, S.; Klochkov, V.V. Spatial Structures of PAP(262–270) and PAP(274–284), Two Selected Fragments of PAP(248–286), an Enhancer of HIV Infectivity. Appl. Magn. Reson. 2015, 46, 757–769. [Google Scholar] [CrossRef]
- Usachev, K.S.; Efimov, S.V.; Kolosova, O.A.; Klochkova, E.A.; Aganov, A.V.; Klochkov, V.V. Antimicrobial peptide protegrin-3 adopt an antiparallel dimer in the presence of DPC micelles: A high-resolution NMR study. J. Biomol. NMR 2015, 62, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Usachev, K.S.; Efimov, S.V.; Kolosova, O.A.; Filippov, A.V.; Klochkov, V.V. High-resolution NMR structure of the antimicrobial peptide protegrin-2 in the presence of DPC micelles. J. Biomol. NMR 2015, 61, 227–234. [Google Scholar] [CrossRef]
- Khodov, I.A.; Kiselev, M.G.; Efimov, S.V.; Klochkov, V.V. Comment on “conformational analysis of small organic molecules using NOE and RDC data: A discussion of strychnine and α-methylene-γ-butyrolactone.”. J. Magn. Reson. 2016, 266, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-Aggregates of Porphyrin−Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Gandini, S.C.; Yushmanov, V.E.; Tabak, M. Interaction of Fe(III)- and Zn(II)-tetra(4-sulfonatophenyl) porphyrins with ionic and nonionic surfactants: Aggregation and binding. J. Inorg. Biochem. 2001, 85, 263–277. [Google Scholar] [CrossRef]
- Qiu, W.G.; Li, Z.F.; Bai, G.M.; Meng, S.N.; Dai, H.X.; He, H. Interaction of water-soluble cationic porphyrin with anionic surfactant. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.M.R.; Nascimento, B.F.O.; Pineiro, M.; Valente, A.J.M. Thermodynamic study of the interaction between 5,10,15,20-tetrakis-(N-methyl-4-pyridyl)porphyrin tetraiodine and sodium dodecyl sulfate. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 480, 279–286. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Kaigorodova, E.Y.; Simonova, O.R.; Lazovskiy, D.A.; Mamardashvili, N.Z. Interaction of the Sn(IV)-tetra(4-sulfonatophenyl)porphyrin axial complexes with cetyltrimethylammonium bromide: Aggregation and location in micelles, fluorescence properties and photochemical stability. J. Mol. Liq. 2020, 318, 113988. [Google Scholar] [CrossRef]
- Patel, T.; Ghosh, G.; Aswal, V.; Bahadur, P. Structural characteristics of the aqueous mixed nonionic-cationic surfactants: Effect of chain length, head group and temperature. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 333, 145–149. [Google Scholar] [CrossRef]
- Dharaiya, N.; Chavda, S.; Singh, K.; Marangoni, D.G.; Bahadur, P. Spectral and hydrodynamic studies on p-toluidine induced growth in cationic micelle. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 306–312. [Google Scholar] [CrossRef]
- Xu, K.; Ren, H.; Zeng, G.; Ding, L.; Huang, J. Investigation of interaction between phenol and cetylpyridinium chloride micelle in the absence and in the presence of electrolyte by 1H NMR spectroscopy. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 356, 150–155. [Google Scholar] [CrossRef]
- Mamardashvili, G.; Kaigorodova, E.; Simonova, O.; Mamardashvili, N. Influence of the macrocycle structure on the ability of Co(II)-porphyrins to oxidize in the presence of organic bases. J. Coord. Chem. 2018, 71, 4194–4209. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Chizhova, N.V.; Kaigorodova, E.Y.; Mamardashvili, N.Z. Cobalt(III) tetrabenzoporphyrin: Synthesis, spectral and coordination properties. Russ. J. Inorg. Chem. 2017, 62, 301–308. [Google Scholar] [CrossRef]
- Mondal, B.; Saha, S.; Borah, D.; Mazumdar, R.; Mondal, B. Nitric Oxide Dioxygenase Activity of a Nitrosyl Complex of Cobalt(II) Porphyrinate in the Presence of Hydrogen Peroxide via Putative Peroxynitrite Intermediate. Inorg. Chem. 2019, 58, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.A.; Kurtikyan, T.S. Electrocatalytic reactions of dioxygen and nitric oxide with reduced (nitrosyl) cobalt porphyrins—Cyclic voltammetry and computational chemistry. J. Porphyr. Phthalocyanines 2011, 15, 99–105. [Google Scholar] [CrossRef]
- Krupei, W.J.; Chamberlin, T.A.; Kochanny, M. Regiospecific Aryl Nitration of Meso-Substituted Tetraarylporphyrins: A Simple Route to Bifunctional Porphyrins. J. Org. Chem. 1989, 54, 2753–2756. [Google Scholar] [CrossRef]
- Mamardashvili, N.Z.; Golubchikov, O.A. The synthesis of porphyrins from dipyrrolylmethanes. Russ. Chem. Rev. 2000, 69, 307–323. [Google Scholar] [CrossRef]
- Rumyantseva, V.D.; Gorshkova, A.S.; Mironov, A.F. Improved Method of 5,10,15,20-Tetrakis(4-hydroxyphenyl)porphyrins Synthesis. Macroheterocycles 2013, 6, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Chizhova, N.V.; Kumeev, R.S.; Mamardashvili, N.Z. Synthesis and spectral properties of Co(II) and Co(III) tetraarylporphyrins. Russ. J. Inorganic. Chem. 2013, 58, 836–840. [Google Scholar] [CrossRef]
- Meites, L. Introduction to Chemical Equilibrium and Kinetics; Pergamon: Oxford, UK, 1981. [Google Scholar]
- Wu, D.H.; Chen, A.; Johnson, C.S. An Improved Diffusion-Ordered Spectroscopy Experiment Incorporating Bipolar-Gradient Pulses. J. Magn. Reson. Ser. A 1995, 115, 260–264. [Google Scholar] [CrossRef]
- Cano, K.E.; Thrippleton, M.J.; Keeler, J.; Shaka, A.J. Cascaded z-filters for efficient single-scan suppression of zero-quantum coherence. J. Magn. Reson. 2004, 167, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Schaefer, H.F. Applications of Electronic Structure Theory; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–556. [Google Scholar] [CrossRef]
- Weinhold, F. Nature of H-bonding in clusters, liquids, and enzymes: An ab initio, natural bond orbital perspective. J. Mol. Struct. THEOCHEM 1997, 398, 181–197. [Google Scholar] [CrossRef]
- Weinhold, F.; Glendening, E.D. NBO 5.0 Program Manual: Natural Bond Orbital Analysis Programs; Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin: Madison, WI, USA, 2001; Available online: https://nbo7.chem.wisc.edu/nboman.pdf (accessed on 6 February 2021).
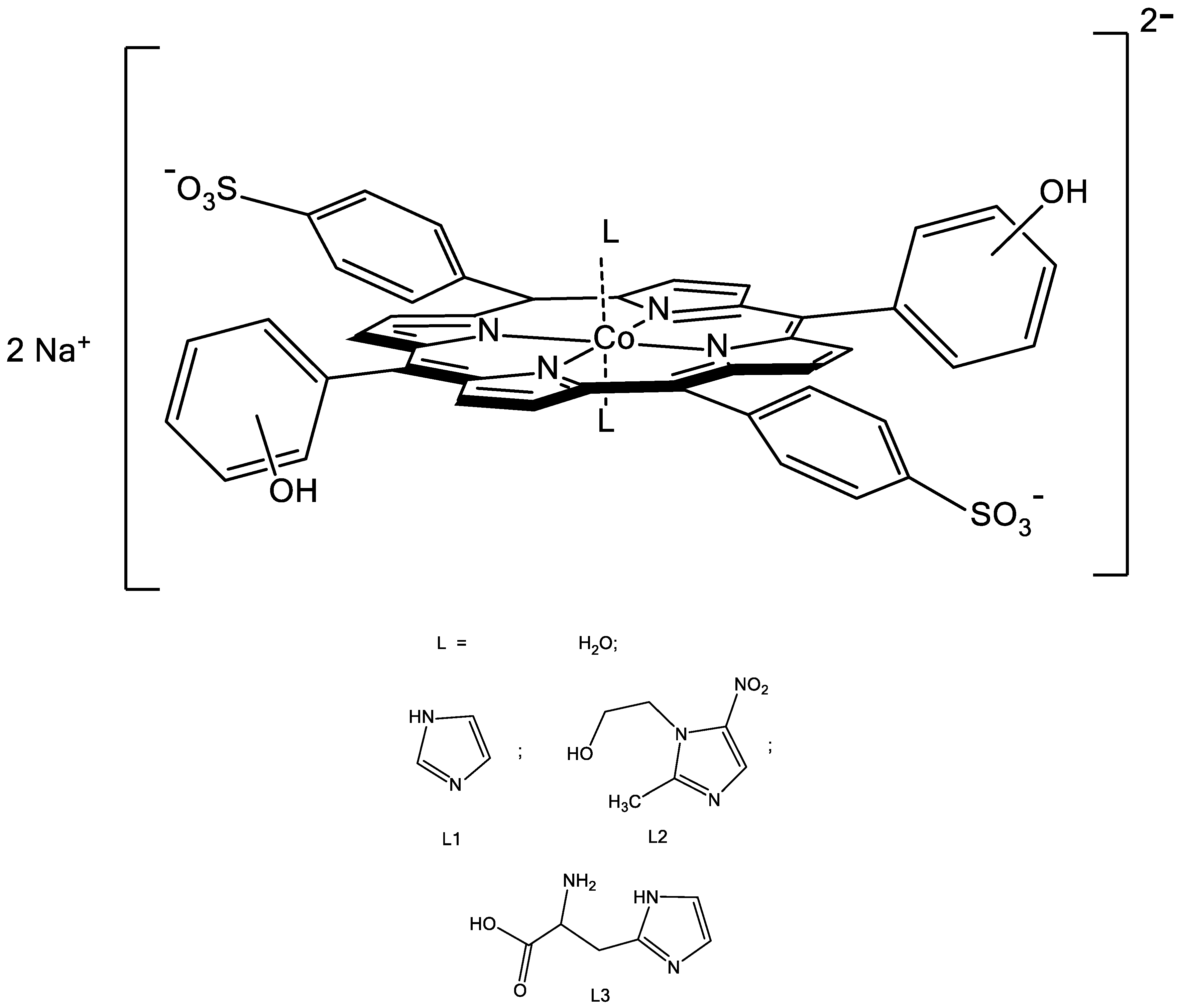












| Co(III)P and L | −ΔG1 a | −ΔG2 b | −ΔGHB c | −ΔGCo(III)P-(L)2 d | r, Å (OH⋅⋅⋅O-L) |
|---|---|---|---|---|---|
| Co(III)P1/L1 | 33.0 | 24.6 | - | 216.2 | - |
| Co(III)P1/L2 | 37.7 | 25.0 | 4.7 | 295.4 | 1.56 |
| Co(III)P1/L3 | 35.8 | 25.2 | 2.8 | 222.6 | 1.70 |
| Co(III)P2/L1 | 33.1 | 24.5 | - | 215.4 | - |
| Co(III)P2/L2 | 38.6 | 24.9 | 5.5 | 309.7 | 1.47 |
| Co(III)P2/L3 | 34.8 | 25.0 | 1.7 | 216.9 | 1.81 |
| System | Co-Np, Ǻ | Co-Nj, Ǻ | r (OH…O = L), ° | Est(Zn-Nj) kJ/mol | qCT, e | −Eint(BSSE) kJ/mol |
|---|---|---|---|---|---|---|
| Co(III)P1-(L2)2 | 1.924 | 1.937 | 1.56 | −216.4 | 0.242 | 295.4 |
| Co(III)P1-(L3)2 | 1.934 | 1.929 | 1.80 | −238.5 | 0.218 | 222.6 |
| Co(III)P2-(L2)2 | 1.929 | 1.930 | 1.47 | −214.6 | 0.259 | 309.7 |
| Co(III)P2-(L3)2 | 1.946 | 1.922 | 1.81 | −242.7 | 0.215 | 216.9 |
| CCPCAgg,M | cmc *, M (Na = CCPC/Cporph.) | |
|---|---|---|
| Co(III)P1-(H2O)2 | 4.61 × 10−5 | 9.43 × 10−4 (82) |
| Co(III)P2-(H2O)2 | 4.67 × 10−5 | 9.55 × 10−4 (83) |
| Co(III)P1-(L1)(H2O) | 4.77 × 10−4 | 1.52 × 10−3 (132) |
| Co(III)P1-(L2)(H2O) | 5.22 × 10−4 | 1.70 × 10−3 (148) |
| Co(III)P1-(L3)(H2O) | 5.84 × 10−4 | 1.77 × 10−3 (154) |
| Co(III)P1-(L1)2 | 1.12 × 10−3 | 2.18 × 10−3 (190) |
| Co(III)P1-(L2)2 | 1.28 × 10−3 | 2.47 × 10−3 (215) |
| Co(III)P1-(L3)2 | 1.32 × 10−3 | 2.58 × 10−3 (224) |
| [Co(III)P] | m- | p- | o- | α-CH2- | β-CH2- | γ-CH2- | (-CH2-)13 | ω-CH3 | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 9.08 | 8.67 | 8.19 | 4.75 | 2.12 | 1.57 | 1.38 | 0.95 | |
| 0.001 | 9.10 | 8.69 | 8.22 | 4.74 | 2.10 | 1.41 | 1.20 | 0.78 | |
| Δδ | 0.02 | 0.02 | 0.03 | −0.01 | −0.02 | −0.16 | −0.18 | −0.17 |
| [Co(III)P-(H2O)2]Mc | [Co(III)P-(L)(H2O)]Mc | [Co(III)P-(L)2]Mc | |
|---|---|---|---|
| Co(III)P1/L1 | 2.75 × 10−4 | 1.84 × 10−4 | <10−5 |
| Co(III)P1/L2 | 5.77 × 10−5 | ||
| Co(III)P1/L3 | 7.92 × 10−5 | ||
| Co(III)P2/L1 | 2.71 × 10−4 | 1.80 × 10−4 | |
| Co(III)P2/L2 | 4.07 × 10−5 | ||
| Co(III)P2/L3 | 7.85 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamardashvili, G.; Kaigorodova, E.; Dmitrieva, O.; Koifman, O.; Mamardashvili, N. Molecular Recognition of Imidazole Derivatives by Co(III)-Porphyrins in Phosphate Buffer (pH = 7.4) and Cetylpyridinium Chloride Containing Solutions. Molecules 2021, 26, 868. https://doi.org/10.3390/molecules26040868
Mamardashvili G, Kaigorodova E, Dmitrieva O, Koifman O, Mamardashvili N. Molecular Recognition of Imidazole Derivatives by Co(III)-Porphyrins in Phosphate Buffer (pH = 7.4) and Cetylpyridinium Chloride Containing Solutions. Molecules. 2021; 26(4):868. https://doi.org/10.3390/molecules26040868
Chicago/Turabian StyleMamardashvili, Galina, Elena Kaigorodova, Olga Dmitrieva, Oscar Koifman, and Nugzar Mamardashvili. 2021. "Molecular Recognition of Imidazole Derivatives by Co(III)-Porphyrins in Phosphate Buffer (pH = 7.4) and Cetylpyridinium Chloride Containing Solutions" Molecules 26, no. 4: 868. https://doi.org/10.3390/molecules26040868
APA StyleMamardashvili, G., Kaigorodova, E., Dmitrieva, O., Koifman, O., & Mamardashvili, N. (2021). Molecular Recognition of Imidazole Derivatives by Co(III)-Porphyrins in Phosphate Buffer (pH = 7.4) and Cetylpyridinium Chloride Containing Solutions. Molecules, 26(4), 868. https://doi.org/10.3390/molecules26040868








