Ocular Delivery of Polyphenols: Meeting the Unmet Needs
Abstract
:1. Introduction: Polyphenols a Remedy from Nature
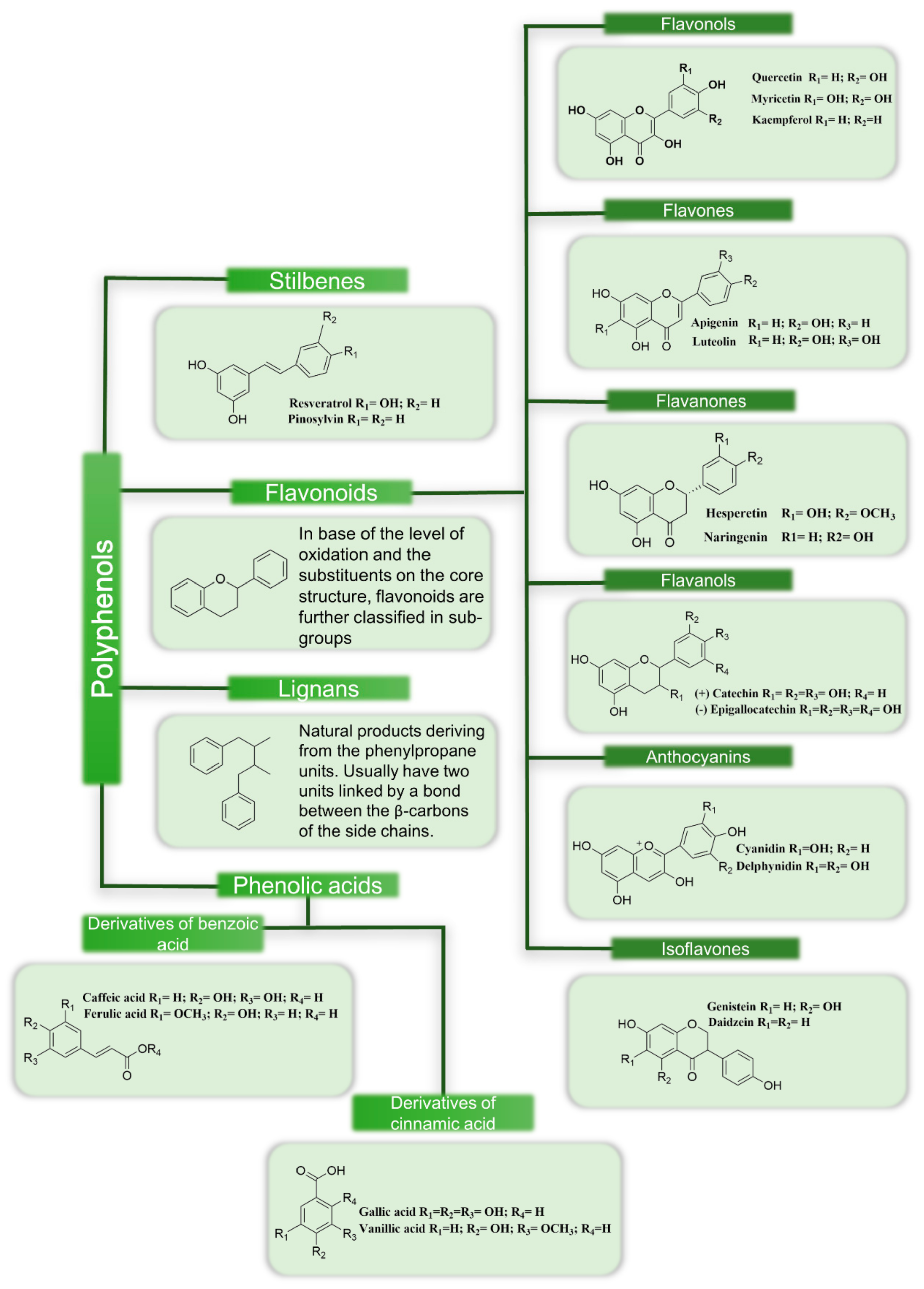
1.1. Classification of Polyphenols
1.2. Natural Sources of Polyphenols
2. The Eye: A Sensory Organ Susceptible to External Stress
2.1. Oxidative Stress
2.2. Inflammation
3. Ocular Pathologies Related to Oxidative Stress and Inflammation
3.1. Dry Eye Disease
3.2. Cataracts
3.3. Glaucoma
3.4. Age-Related Macular Degeneration
3.5. Diabetic Retinopathy
4. An Overview of Ocular Delivery Strategies for Polyphenols
4.1. Resveratrol
4.2. Quercetin
4.3. Epigallocatechin Gallate
4.4. Curcumin
4.5. Catechin
4.6. Naringenin
4.7. Cyanidin
4.8. Myricetin
4.9. Kaempferol
4.10. Hesperetin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP-1 | Activator protein-1 |
| ATP | Adenosine triphosphate |
| AGEs | Advanced glycation end products |
| AMD | Age-Related Macular Degeneration |
| HCE | Corneal epithelial cells |
| CNV | Corneal neovascularization |
| COX-2 | Cyclooxygenase-2 |
| JNK | c-Jun N-terminal kinases |
| DR | Diabetic retinopathy |
| DED | Dry Eye Disease |
| EGCG | Epigallocatechin gallate |
| ERK | Extracellular signal-regulated kinases |
| HREC | Human Retinal Endothelial Cells |
| HUVEC | Human umbilical vein endothelial cells |
| HA | Hyaluronic acid |
| HP-β-Cyclodextrin | Hydroxypropyl-β-cyclodextrin |
| HIF-1 | Hypoxia inducible factor-1 |
| IOP | Increased intraocular pressure |
| iNOS | Inducible nitric oxide synthase |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IP-10 | Interferon gamma-induced protein 10 |
| IL-1 | Interleukin-1 |
| IL-1RA | Interleukin-1 receptor antagonist |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| JAK | Janus-activated kinases |
| LPS | Lipopolysaccharide |
| LFA-1 | Lymphocyte function-associated antigen |
| MAPK | Mitogen-activated kinases |
| MDRP | Multi drug resistance protein |
| Nox | NADPH oxidases |
| NZW | New Zealand White rabbits |
| MNU | N-methyl-N-nitrosourea |
| TMC | N-trimethyl chitosan |
| NPs | Nanoparticles |
| NO | Nitric oxide |
| NOS | Nitric oxide synthases |
| Nrf2 | Nuclear factor erythroid-2 related factor 2 |
| NFAT | Nuclear factor of activated T cells |
| NF-Kb | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PEG | Poly(ethylene glycol) |
| PEG-DSPE | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine Poly(ethylene glycol) |
| PVCL-PVA-PEG | Polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol |
| PVP | Polyvinylpyrrolidone |
| PKC | Protein kinase C |
| RM-β-cyclodextrin | Randomly methylated beta-cyclodextrin |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| SBE-β-cyclodextrin | Sulfolbutylether-β-cyclodextrin |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| VCAM-1 | Vascular Cell Adhesion Molecule |
References
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kondratyuk, T.P.; Pezzuto, J.M. Natural product polyphenols of relevance to human health. Pharm. Biol. 2004, 42, 46–63. [Google Scholar] [CrossRef] [Green Version]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects Phenolic Compdpounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Stewart, A.J.; Stewart, R.F. Phenols. In Reference Module in Earth Systems and Environmental Sciences, Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherland, 2008; pp. 2682–2689. [Google Scholar]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Ghidouche, S.; Ducrot, P.H. Flavonoids: Hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 2007, 12, 2228–2258. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Franke, A.A.; Hankin, J.H.; Yu, M.C.; Maskarinec, G.; Low, S.H.; Custer, L.J. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J. Agric. Food Chem. 1999, 47, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Van De Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, R.G.; Bhange, M.A.; Oulkar, D.P.; Sharma, A.K.; Ahammed Shabeer, T.P. Estimation of polyphenols by using HPLC–DAD in red and white wine grape varieties grown under tropical conditions of India. J. Food Sci. Technol. 2018, 55, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Espuny, A.C.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced formulation approaches for ocular drug delivery: State-of-the-art and recent patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Awwad, S.; Mohamed Ahmed, A.H.A.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef]
- Gipson, I.K. The Ocular Surface: The Challenge to Enable and Protect Vision. Ocul. Surf. 2010, 48, 4390–4398. [Google Scholar] [CrossRef] [Green Version]
- Saccà, S.C.; Cutolo, C.A.; Ferrari, D.; Corazza, P.; Traverso, C.E. The eye, oxidative damage and polyunsaturated fatty acids. Nutrients 2018, 10, 668. [Google Scholar] [CrossRef] [Green Version]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of daylight vision in retinal degeneration: Are oxidative stress and metabolic dysregulation to blame? J. Biol. Chem. 2012, 287, 1642–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [Green Version]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H. Bin Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Moles, M.L.; López-Riquelme, G.O. Relationship between oxidative stress, circadian rhythms, and AMD. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrou, A.L.; Petrou, P.L.; Ntanos, T.; Liapis, A. A possible role for singlet oxygen in the degradation of various antioxidants. A meta-analysis and review of literature data. Antioxidants 2018, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umapathy, A.; Donaldson, P.; Lim, J. Antioxidant delivery pathways in the anterior eye. Biomed Res. Int. 2013, 2013, 207250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H. Bin Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Hawiger, J.; Zienkiewicz, J. Decoding inflammation, its causes, genomic responses, and emerging countermeasures. Scand. J. Immunol. 2019, 90, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Aranguez, A.; Gasull, X.; Diebold, Y.; Pintor, J. Purinergic receptors in ocular inflammation. Mediators Inflamm. 2014, 2014, 320906. [Google Scholar] [CrossRef]
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent advances in the design of topical ophthalmic delivery systems in the treatment of ocular surface inflammation and their biopharmaceutical evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef]
- Comstock, T.L.; Decory, H.H. Advances in corticosteroid therapy for ocular inflammation: Loteprednol etabonate. Int. J. Inflam. 2012, 2012, 789623. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H. A novel elastin-like polypeptide drug carrier for cyclosporine A improves tear flow in a mouse model of Sjögren’s Syndrome. J Control Release 2018, 292, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- O’Neil, E. Advances in Dry Eye Disease Treatment. Curr Opin Ophthalmol. 2019, 30, 166–178. [Google Scholar] [CrossRef]
- Chung, J.K.; Spencer, E.; Hunt, M.; McCauley, T.; Welty, D. Ocular distribution and pharmacokinetics of lifitegrast in pigmented rabbits and mass balance in beagle dogs. J. Ocul. Pharmacol. Ther. 2018, 34, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Lollett, I.V.; Galor, A. Dry eye syndrome: Developments and lifitegrast in perspective. Clin. Ophthalmol. 2018, 12, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Senni, C.; Bernabei, F.; Cicero, A.F.G.; Vagge, A.; Maestri, A.; Scorcia, V.; Giannaccare, G. The Role of Nutrition and Nutritional Supplements in Ocular Surface Diseases. Nutrients 2020, 12, 952. [Google Scholar] [CrossRef] [Green Version]
- Krajčíková, K.; Suváková, M.; Glinská, G.; Ohlasová, J.; Tomečková, V. Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops. Open Chem. 2020, 18, 325–332. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Donaldson, C.I.; Lim, J.C.; Donaldson, P.J. Nutritional strategies to prevent lens cataract: Current status and future strategies. Nutrients 2019, 11, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weikel, K.A.; Garber, C.; Baburins, A.; Taylor, A. Nutritional modulation of cataract. Nutr. Rev. 2014, 72, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Samoila, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal plants and natural products used in cataract management. Front. Pharmacol. 2019, 10, 1–22. [Google Scholar]
- Heruye, S.H.; Nkenyi, L.N.M.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.F.; Ohia, S.E.; Opere, C.A. Current trends in the pharmacotherapy of cataracts. Pharmaceuticals 2020, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R. The Pathophysiology and Treatment of Glaucoma: A Review Robert. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Benoist d’Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and anti-oxidative stress markers in chronic glaucoma: A systematic review and meta-analysis. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Shoaie-Nia, K.; Zanon-Moreno, V.; Sanz-Gonzalez, S.M.; del Castillo, J.B.; Garcia-Medina, J.J. Strategies to Reduce Oxidative Stress in Glaucoma Patients. Curr. Neuropharmacol. 2017, 16, 903–918. [Google Scholar] [CrossRef]
- Aslan, M.; Dogan, S.; Kucuksayan, E. Oxidative stress and potential applications of free radical scavengers in glaucoma. Redox Rep. 2013, 18, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in glaucoma: Old and new promising treatments. Adv. Pharmacol. Sci. 2017, 2017, 4320408. [Google Scholar] [CrossRef]
- Saccà, S.C.; Izzotti, A.; Vernazza, S.; Tirendi, S.; Scarfì, S.; Gandolfi, S.; Bassi, A.M. Can Polyphenols in Eye Drops Be Useful for Trabecular Protection from Oxidative Damage? J. Clin. Med. 2020, 9, 3584. [Google Scholar] [CrossRef]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; To, C.H.; Lam, T.T.; Tse, D.Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxid. Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N. Risk Factors and Biomarkers of Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2016, 54, 64–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftery, J.; Clegg, A.; Jones, J.; Seng, C.T.; Lotery, A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): Modelling cost effectiveness. Br. J. Ophthalmol. 2007, 91, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muangnoi, C.; Sharif, U.; Bhuket, P.R.N.; Rojsitthisak, P.; Paraoan, L. Protective effects of curcumin ester prodrug, curcumin diethyl disuccinate against H2O2-induced oxidative stress in human retinal pigment epithelial cells: Potential therapeutic avenues for age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar]
- Frank, R. Diabetic retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef]
- Mahajan, N.; Arora, P.; Sandhir, R. Perturbed biochemical pathways and associated oxidative stress lead to vascular dysfunctions in diabetic retinopathy. Oxid. Med. Cell. Longev. 2019, 2019, 8458472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of inflammation in diabetic retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Miao, X.; Li, F.; Wang, S.; Liu, Q.; Wang, Y.; Sun, J. Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2017, 2017, 9702820. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W. Treatment of diabetic retinopathy: Recent advances and unresolved challenges. World J. Diabetes 2016, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Kim, J.H.; Oh, B.G.; Subedi, L.; Khan, Z.; Kim, S.Y. Phytochemicals: Target-based therapeutic strategies for diabetic retinopathy. Molecules 2018, 23, 1519. [Google Scholar] [CrossRef] [Green Version]
- Simo, R.; Ballarini, S.; Cunha-Vaz, J.; Ji, L.; Haller, H.; Zimmet, P.; Wong, T. Non-Traditional Systemic Treatments for Diabetic Retinopathy: An Evidence-Based Review. Curr. Med. Chem. 2015, 22, 2580–2589. [Google Scholar] [CrossRef] [Green Version]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Rossino, M.G.; Casini, G. Nutraceuticals for the treatment of diabetic retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Janagam, D. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2015, 176, 139–148. [Google Scholar] [CrossRef]
- Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic drug delivery systems for antibiotherapy—A review. Pharmaceutics 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhani, P.; Patil, A.; Majumdar, S. Recent advances in topical nano drug-delivery systems for the anterior ocular segment. Ther. Deliv. 2018, 9, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in biodegradable nano-sized polymer-based ocular drug delivery. Polymers 2019, 11, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojzer, E.B.; Hrncic, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M. V Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B Biol. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol encapsulation in high molecular weight chitosan-based nanogels for applications in ocular treatments: Impact on human ARPE-19 culture cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef]
- Bhatt, P.; Fnu, G.; Bhatia, D.; Shahid, A.; Sutariya, V. Nanodelivery of Resveratrol-Loaded PLGA Nanoparticles for Age-Related Macular Degeneration. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Abengózar-Vela, A.; Schaumburg, C.S.; Stern, M.E.; Calonge, M.; Enríquez-de-Salamanca, A.; González-García, M.J. Topical Quercetin and Resveratrol Protect the Ocular Surface in Experimental Dry Eye Disease. Ocul. Immunol. Inflamm. 2019, 27, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Wang, M.C.; Chen, Z.Y.; Chiu, W.Y.; Chen, K.H.; Lin, I.C.; Yang, W.C.V.; Wu, C.C.; Tseng, C.L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine–Glycine–aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef]
- Luo, L.J.; Lai, J.Y. Epigallocatechin Gallate-Loaded Gelatin-g-Poly(N-Isopropylacrylamide) as a New Ophthalmic Pharmaceutical Formulation for Topical Use in the Treatment of Dry Eye Syndrome. Sci. Rep. 2017, 7, 1–14. [Google Scholar]
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.M.L. Potential Eye Drop Based on a Calix [4] arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability, and Anti-Inflammatory Effect. Mol. Pharm. 2017, 14, 1610–1622. [Google Scholar] [CrossRef]
- Alshamrani, M.; Sikder, S.; Coulibaly, F.; Mandal, A.; Pal, D.; Mitra, A.K. Self-Assembling Topical Nanomicellar Formulation to Improve Curcumin Absorption Across Ocular Tissues. AAPS PharmSciTech 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Sai, N.; Dong, X.; Huang, P.; You, L.; Yang, C.; Liu, Y.; Wang, W.; Wu, H.; Yu, Y.; Du, Y.; et al. A novel gel-forming solution based on PEG-DSPE/Solutol HS 15 mixed micelles and gellan gum for ophthalmic delivery of curcumin. Molecules 2020, 25, 81. [Google Scholar] [CrossRef] [Green Version]
- Lou, J.; Hu, W.; Tian, R.; Zhang, H.; Jia, Y.; Zhang, J.; Zhang, L. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int. J. Nanomed. 2014, 9, 2517–2525. [Google Scholar]
- Shim, W.; Kim, C.E.; Lee, M.; Lee, S.H.; Park, J.; Do, M.; Yang, J.; Lee, H. Catechin solubilization by spontaneous hydrogen bonding with poly(ethylene glycol) for dry eye therapeutics. J. Control. Release 2019, 307, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-β-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun, J.; Wang, Y.; Ma, Y.; Chen, W.; Zhang, Z.; Gui, G.; Lin, B. Ocular pharmacokinetics of naringenin eye drops following topical administration to rabbits. J. Ocul. Pharmacol. Ther. 2015, 31, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liang, X.; Li, X.; Guan, Z.; Liao, Z.; Luo, Y.; Luo, Y. Ocular delivery of cyanidin-3-glycoside in liposomes and its prevention of selenite-induced oxidative stress. Drug Dev. Ind. Pharm. 2016, 42, 546–553. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, F.; Lan, J.; Sun, F.; Li, J.; Li, M.; Song, K.; Wu, X. Ultra-small micelles based on polyoxyl 15 hydroxystearate for ocular delivery of myricetin: Optimization, in vitro, and in vivo evaluation. Drug Deliv. 2019, 26, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Zheng, Z.; Lan, J.; Li, X.; Li, M.; Song, K.; Wu, X. New micelle myricetin formulation for ocular delivery: Improved stability, solubility, and ocular anti-inflammatory treatment. Drug Deliv. 2019, 26, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Chuang, Y.L.; Fang, H.W.; Ajitsaria, A.; Chen, K.H.; Su, C.Y.; Liu, G.S.; Tseng, C.L. Development of Kaempferol-loaded gelatin nanoparticles for the treatment of corneal neovascularization in mice. Pharmaceutics 2019, 11, 635. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Li, R.; Yan, M.; Li, Q.; Li, Y.; Wu, X. Ultra-small nanocomplexes based on polyvinylpyrrolidone K-17PF: A potential nanoplatform for the ocular delivery of kaempferol. Eur. J. Pharm. Sci. 2020, 147, 105289. [Google Scholar] [CrossRef]
- Srirangam, R.; Hippalgaonkar, K.; Avula, B.; Khan, I.A.; Majumdar, S. Evaluation of the intravenous and topical routes for ocular delivery of hesperidin and hesperetin. J. Ocul. Pharmacol. Ther. 2012, 28, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Adelli, G.R.; Hingorani, T.; Punyamurthula, N.; Balguri, S.P.; Majumdar, S. Evaluation of Topical Hesperetin Matrix film for Back-of-the-Eye Delivery. Eur. J. Pharm. Biopharm. 2015, 92, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzuto, J.M. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Lei, X.P.; Dong, W. Bin Resveratrol as a potential therapeutic drug for respiratory system diseases. Drug Des. Devel. Ther. 2017, 11, 3591–3598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazué, F.; Ghiringhelli, F.; Latruffe, N. Transport, stability, and biological activity of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.I.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, H.Y.; Lee, M.S.; Chang, E.; Kim, S.Y.; Kang, B.; Ko, H.; Kim, I.H.; Zhong, Q.; Jo, Y.H.; Kim, C.T.; et al. Formulation and characterization of quercetin-loaded oil in water nanoemulsion and evaluation of hypocholesterolemic activity in rats. Nutrients 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakran, M.; Sahoo, N.G.; Li, L. Dissolution enhancement of quercetin through nanofabrication, complexation, and solid dispersion. Colloids Surf. B Biointerfaces 2011, 88, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.S. Application of bioactive quercetin in oncotherapy: From nutrition to nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic effects of plant flavonoids. BioMed Res. Int. 2014, 2014, 480258. [Google Scholar] [CrossRef] [Green Version]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Sahpazidou, D.; Geromichalos, G.D.; Stagos, D.; Apostolou, A.; Haroutounian, S.A.; Tsatsakis, A.M.; Tzanakakis, G.N.; Hayes, A.W.; Kouretas, D. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol. Lett. 2014, 230, 218–224. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Time-course regulation of quercetin on cell survival/proliferation pathways in human hepatoma cells. Mol. Nutr. Food Res. 2008, 52, 457–464. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis—Inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [Green Version]
- McKay, T.B.; Karamichos, D. Quercetin and the ocular surface: What we know and where we are going. Exp. Biol. Med. 2017, 242, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abengózar-Vela, A.; Calonge, M.; Stern, M.E.; González-García, M.J.; Enríquez-De-Salamanca, A. Quercetin and resveratrol decrease the inflammatory and oxidative responses in human ocular surface epithelial cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2709–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef] [PubMed]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Keske, M.; Ng, H.; Premilovac, D.; Rattigan, S.; Kim, J.; Munir, K.; Yang, P.; Quon, M. Vascular and Metabolic Actions of the Green Tea Polyphenol Epigallocatechin Gallate. Curr. Med. Chem. 2014, 22, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin—Gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [Green Version]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Su, Y.; Wei, D.; Qian, L.; Xing, D.; Pan, J.; Chen, Y.; Huang, M. Catechin relieves hypoxia/reoxygenation-induced myocardial cell apoptosis via down-regulating lncRNA MIAT. J. Cell. Mol. Med. 2020, 24, 2356–2368. [Google Scholar] [CrossRef]
- Lee, S.M.; Ko, I.G.; Kim, S.E.; Kim, D.H.; Kang, B.N. Protective effect of catechin on apoptosis of the lens epithelium in rats with N-methyl-N-nitrosourea-induced cataracts. Korean J. Ophthalmol. 2010, 24, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [Green Version]
- Hartogh, D.J.D.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De La Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, P.; Yan, L.J.; Li, Y.Q.; Yang, H.T.; Duan, H.Y.; Wu, J.T.; Fan, X.W.; Wang, S.L. Cyanidin ameliorates cisplatin-induced cardiotoxicity via inhibition of ROS-mediated apoptosis. Exp. Ther. Med. 2018, 15, 1959–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhu, J.; Deng, F.; Wu, W.; Zheng, Z.; Lv, C.; Li, Y.; Xiang, W.; Lu, X.; Qin, S. Microarray Based Functional Analysis of Myricetin and Proteomic Study on Its Anti-Inflammatory Property. BioMed Res. Int. 2019, 2019, 3746326. [Google Scholar] [CrossRef] [Green Version]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Luo, L. Effect of myricetin on primary open-angle glaucoma. Transl. Neurosci. 2018, 9, 132–141. [Google Scholar] [CrossRef]
- He, Y.; Leung, K.W.; Zhang, Y.H.; Duan, S.; Zhong, X.F.; Jiang, R.Z.; Peng, Z.; Tombran-Tink, J.; Ge, J. Mitochondrial complex i defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: Protection by antioxidants. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderioa, J.; Constance Lay-Lay Saw, A.-N.K. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2013, 30, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid. Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Zhao, C.; Peng, Q.; Xie, P.; Liu, Q.H. Kaempferol inhibited VEGF and PGF expression and in vitro angiogenesis of hrecs under diabetic-like environment. Brazilian J. Med. Biol. Res. 2017, 50, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.H.; Garg, N.K.; Lunde, E.; Han, K.Y.; Jain, S.; Azar, D.T. Corneal Neovascularization: An Anti-VEGF Therapy Review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, J.; Scott, B.; Boom, K.; Shen, J.; Borsoi, C.; Suri, K.; Grande, R.; Fresta, M.; Celia, C.; Zhao, Y.; et al. Hesperetin Liposomes for Cancer Therapy. Curr. Drug Deliv. 2016, 13, 711–719. [Google Scholar] [CrossRef]
- Morin., B.; Nichols, L.; Zalasky, K.; Davis, J.D.; Manthey, J.A.; Holland, L.J. The Citrus Flavonoids Hesperetin and Nobiletin Differentially Regulate Low Density Lipoprotein Receptor Gene Transcription in HepG2 Liver Cells. J. Nutr. 2008, 23, 1274–1281. [Google Scholar] [CrossRef]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar]
- Park, S.; Sorenson, C.M.; Sheibani, N. PECAM-1 isoforms, eNOS and endoglin axis in regulation of angiogenesis. Clin. Sci. 2015, 129, 217–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Shibasaki, F. Hypoxia-Inducible Factor as an Angiogenic Master Switch. Front. Pediatr. 2015, 3, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srirangam, R.; Hippalgaonkar, K.; Majumdar, S. Intravitreal Kinetics of Hesperidin, Hesperetin and Hesperidin G: Effect of Dose and Physicochemical Properties. J. Pharm. Sci. 2012, 23, 1631–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Polyphenol | Type of Formulation | Pathology | Size (nm) | Entrapment Efficacy | ζ Potential (mV) | In Vitro/In Vivo Results | Reference |
|---|---|---|---|---|---|---|---|
| Resveratrol | Gold NPs | DR | 10 | N/A | N/A | Reduced retinal expression of VEGF-1, ICAM-1, IL-6, and IL-1β in diabetic rats | [90] |
| Nanogel based on chitosan with a high molecular weight | Diseases of the posterior segment | 144 | 59 ± 1 | +32 ± 1 | No cytotoxicity in ARPE-19 cells; no significant production of IL-6 and IL-8 after an inflammatory stimulus | [91] | |
| PLGA NPs | AMD | 102.7 ± 2.8 | 65.2 ± 2.2 | −47.3 ± 0.9 | No cytotoxicity in ARPE-19 cells; reduction of VEGF secretion in the same cell line | [92] | |
| Quercetin alone and in combination with resveratrol | β-cyclodextrin | Dry eye disease | N/A | N/A | N/A | Decrease of the clinical sign and inflammatory response in a murine model of DED | [93] |
| EGCG | Gelatin NPs coated with hyaluronic acid | Dry eye disease | 253.4 ± 7.3 | 97.8 ± 0.5 | +9.2 ± 1.8 | No cytotoxicity in Human Corneal Epithelial Cells/improvement of tear secretion and reduced levels of inflammatory cytokines in rabbits with DED | [94] |
| Gelatin NPs coated with HA and RGD sequence | Corneal neovascularization | 168.8 ± 22.5 | 97.1 ± 0.55 | +19.7 ± 2.0 | Inhibition of HUVEC migration rate/inhibition of vessel formation in mice with corneal NV | [95] | |
| Lipid NPs | Various ocular diseases | 183.9 ± 0.6 | 98.9 ± 0.1 | +28.8 ± 0.8 | Good ocular tolerance in HET-CAM test/no signs of irritability in NZW rabbits | [96,97] | |
| In situ gelling system made from gelatin-g-poly(N-isopropylacrylamid) | Dry eye disease | N/A | N/A | N/A | Good biocompatibility, no cytotoxicity, ↓ of the expression of inflammatory cytokines in HCE cells/improved corneal thickness in DED rabbit models | [98] | |
| Curcumin | Derivative of calix [4] arene | Uveitis | 82 | N/A | +24.3 | ↓ clinical inflammatory score; inflammatory cytokines in rats | [99] |
| Nanomicellar formulation (hydrogenated castor oil-40 and octoxynol-40) | AMD | 17.9 | 82.6 ± 0.5 | Slightly- | Doses of 5–10 μM show no cytotoxicity in D407 cells; inhibition of VEGF production under oxidative stress | [100] | |
| PEG-DSPE/Solutol HS 15 with gellan gum | Diseases related to the ocular surface | 13.4 ± 0.1 | 97.2 ± 2.4 | −4.6 ± 0.3 | No ocular irritation and no changes in the appearance of the cornea, iris and conjunctiva were observed in NZW rabbits; additionally, ocular retention ↑ | [101] | |
| Gel matrix made from Pluronic F127 and Pluronic F68 in combination with albumin NPs | DR | 221.2 | 85.4 ± 1 | N/A | Nonirritating, ↑ corneal permeation, ↑ aqueous humor concentration with respect to the control in NZW rabbits | [102] | |
| Catechin | Complex with PEG | DED | two distinct size distribution of ~5 and ~200 nm | N/A | N/A | Recovery of the density of goblet cells in DED induced NOD.B10-H2b mice; repression of different anti-inflammatory indicators | [103] |
| Naringenin | SBE-β-CD/Chitosan NPs | AMD | 446.4 ± 112.8 | N/A | +22.5 ± 4.9 | Nonirritating to NZW rabbits’ eyes; after topical application ↑ concentration was achieved in aqueous humor than with the control form. | [104] |
| Eyedrop formulation containing HP-β-CD, poloxamer 407, polycarbophil, disodium edentate, BAK, sodium chloride | AMD/retinitis pigmentosa | N/A | N/A | N/A | Consistent quantity of drug was found in the posterior part of the eye after topical administration in NZW rabbits | [105] | |
| Cyanidin | N-trimethyl chitosan (TMC) decorated liposome | Cataract | 158.3 ± 2.8 | 53.7 ± 0.2 | +31.7 ± 1 | ↑ corneal residence time and permeation/restoration of the levels of antioxidant enzymes in cataract induced Sprague Dawley rats | [106] |
| Myricetin | polyoxyl 15 hydroxystearate micelles | Ocular anti-inflammatory treatment | 12.1 ± 0.7 | 96.1 ± 0.3 | −4.2 ± 0.4 | Good tolerability in healthy rabbits; decent anti-inflammatory activity | [107] |
| PVCL–PVA–PEG micelles | Ocular anti-inflammatory treatment | 60.7 ± 1 | 99.5 ± 0.5 | −2.2 ± 0.3 | No cytotoxicity observed in HCECs cells/good corneal permeation in NZ albino rabbits; dosage-related anti-inflammatory activity was observed | [108] | |
| Kaempferol | Gelatin NPs cross-linked with glutaraldehyde | CNV | 85 ± 8 | 95± 1 | +25.6 ± 2.1 | Inhibition of cell migration in HUVECs cells/↓ of the growth of corneal blood vessels in mice with CNV | [109] |
| PVP nanocomplex | Various ocular diseases | 8.6 | 93.10 | −5.31 ± 0.2 | Nontoxic to HCECs cells/good ocular tolerability and anti-inflammatory activity was observed in NZW rabbits | [110] | |
| Hesperetin | hydroxylpropyl beta-cyclodextrin (HP-β-CD), randomly methylated beta-cyclodextrin | Diabetic retinopathy and diabetic macular edema | N/A | N/A | N/A | A significant concentration of the drug was observed in ocular tissues after topical administration in NZW rabbits | [111] |
| Film matrix made from PolyOx® WSR N-10 | Posterior segment diseases | N/A | N/A | N/A | No damage on corneal tissues was observed; significant levels of the drug after topical instillation detected in the ocular tissue of NZW rabbits | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstić, L.; González-García, M.J.; Diebold, Y. Ocular Delivery of Polyphenols: Meeting the Unmet Needs. Molecules 2021, 26, 370. https://doi.org/10.3390/molecules26020370
Krstić L, González-García MJ, Diebold Y. Ocular Delivery of Polyphenols: Meeting the Unmet Needs. Molecules. 2021; 26(2):370. https://doi.org/10.3390/molecules26020370
Chicago/Turabian StyleKrstić, Luna, María J. González-García, and Yolanda Diebold. 2021. "Ocular Delivery of Polyphenols: Meeting the Unmet Needs" Molecules 26, no. 2: 370. https://doi.org/10.3390/molecules26020370
APA StyleKrstić, L., González-García, M. J., & Diebold, Y. (2021). Ocular Delivery of Polyphenols: Meeting the Unmet Needs. Molecules, 26(2), 370. https://doi.org/10.3390/molecules26020370







