Possibility to Biotransform Anthracyclines by Peroxidases Produced by Bjerkandera adusta CCBAS 930 with Reduction of Geno- and Cytotoxicity and Pro-Oxidative Activity
Abstract
:1. Introduction
2. Results
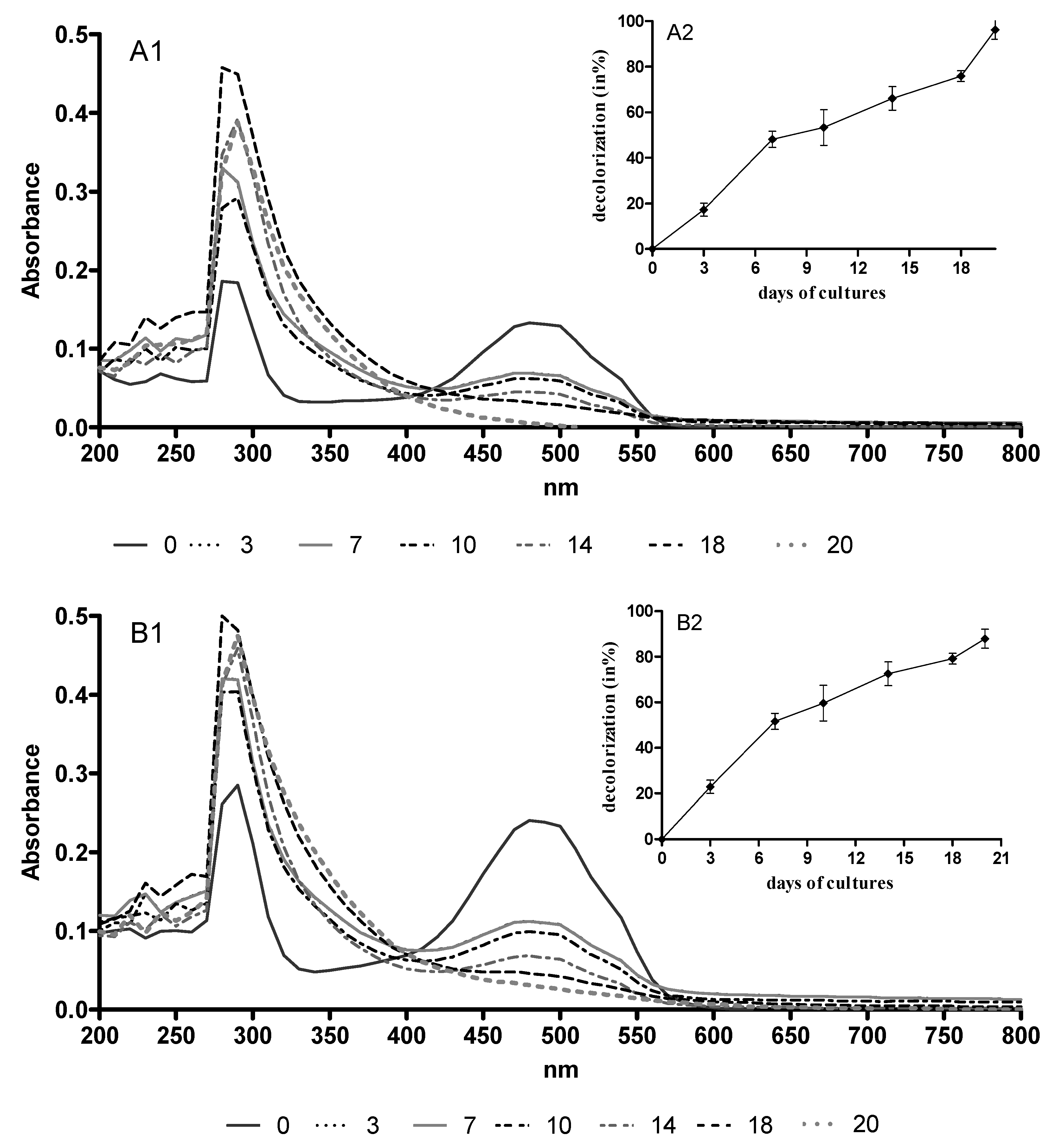
2.1. AQA Bioremoval by B. adusta CCBAS 930
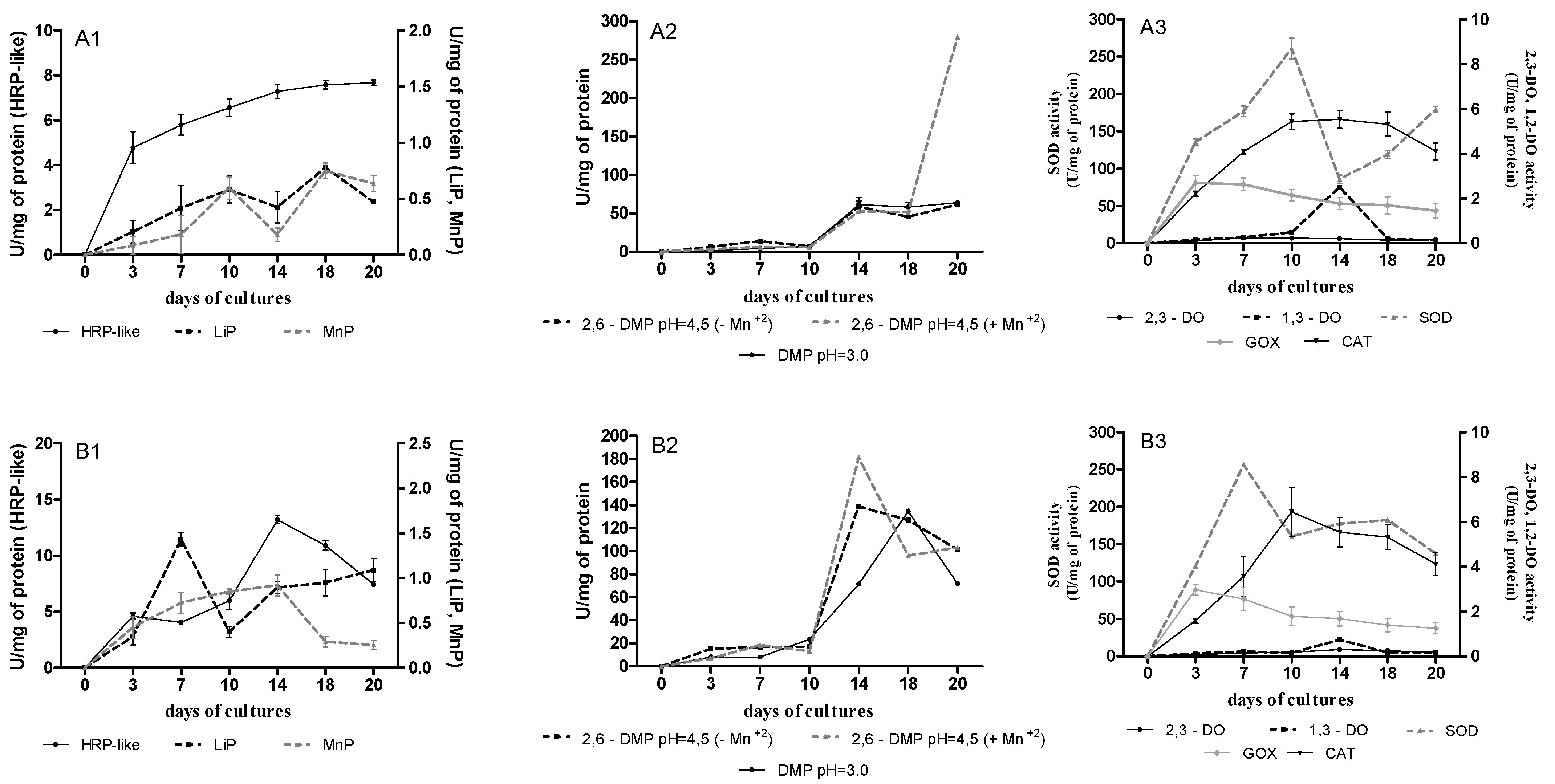
2.2. Extracellular Oxidoreductase Activities during AQA Biotransformation in B. adusta CCBAS 930 Cultures
2.3. Changes of pH during AQA Biotransformation in B. adusta CCBAS 930 Cultures
2.4. PhC and SOR Contents during AQA Biotransformation in B. adusta CCBAS 930 Cultures
2.5. Evaluation of Antioxidative Activity of AQAs during Biological Treatment
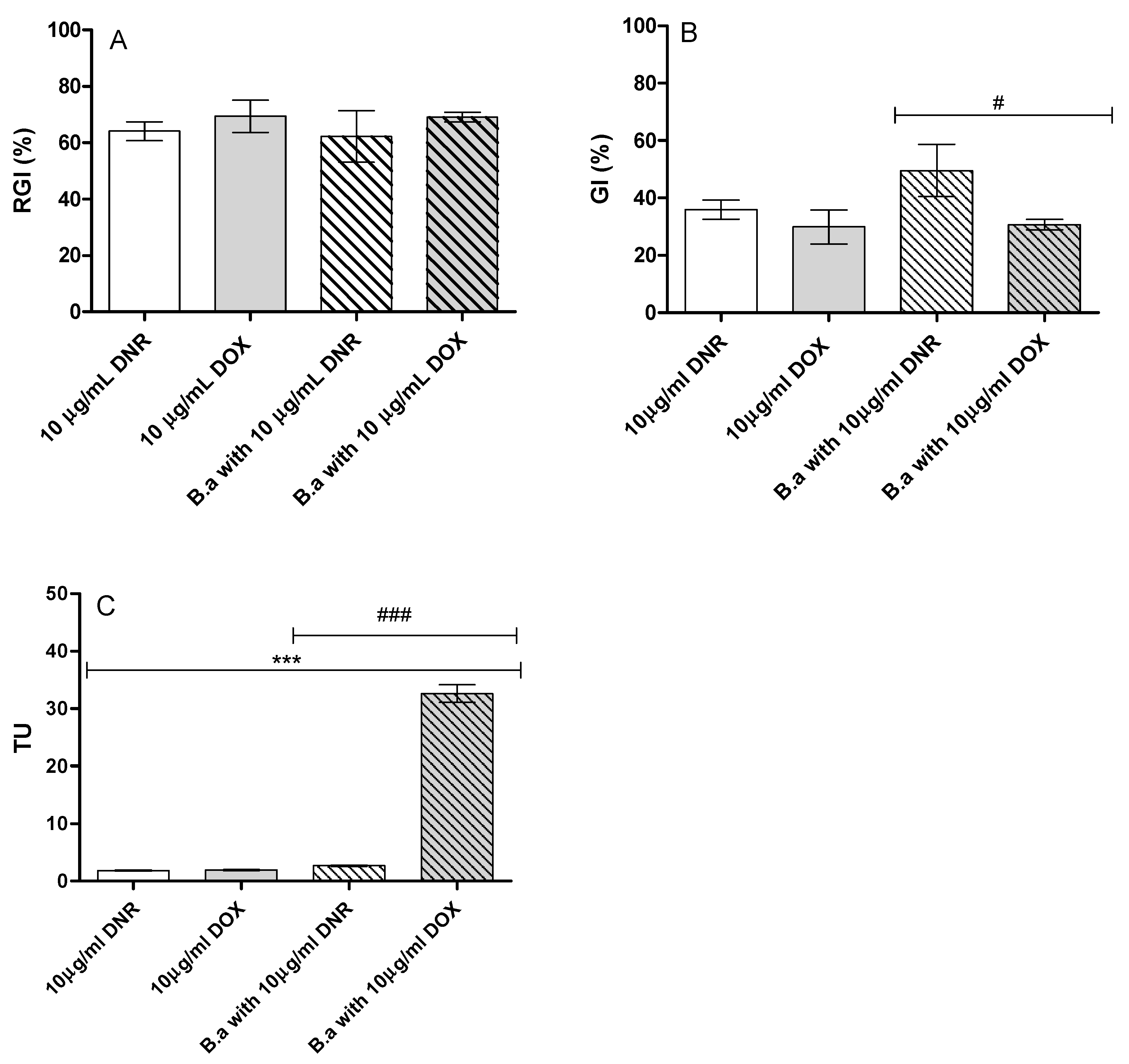
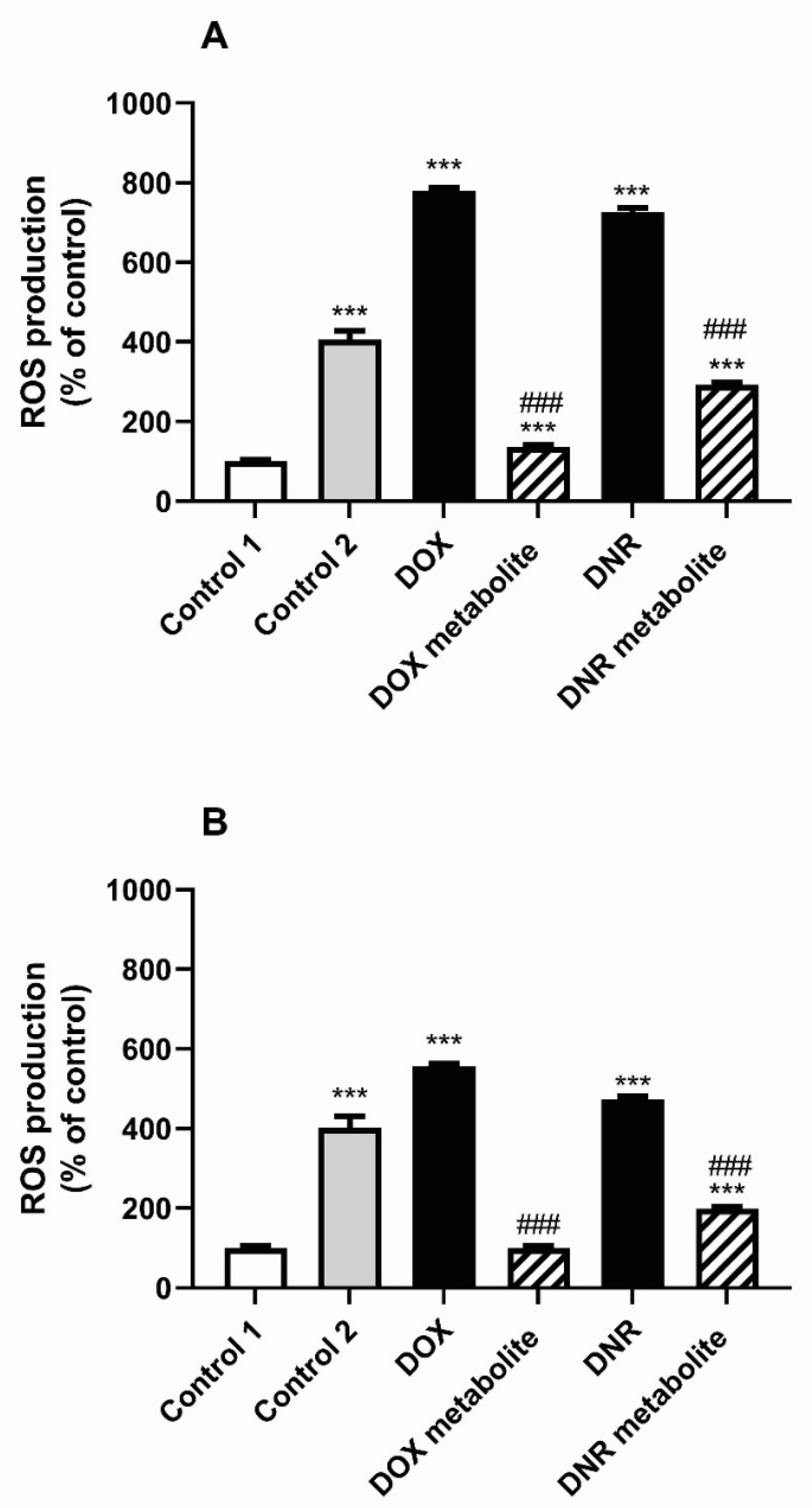
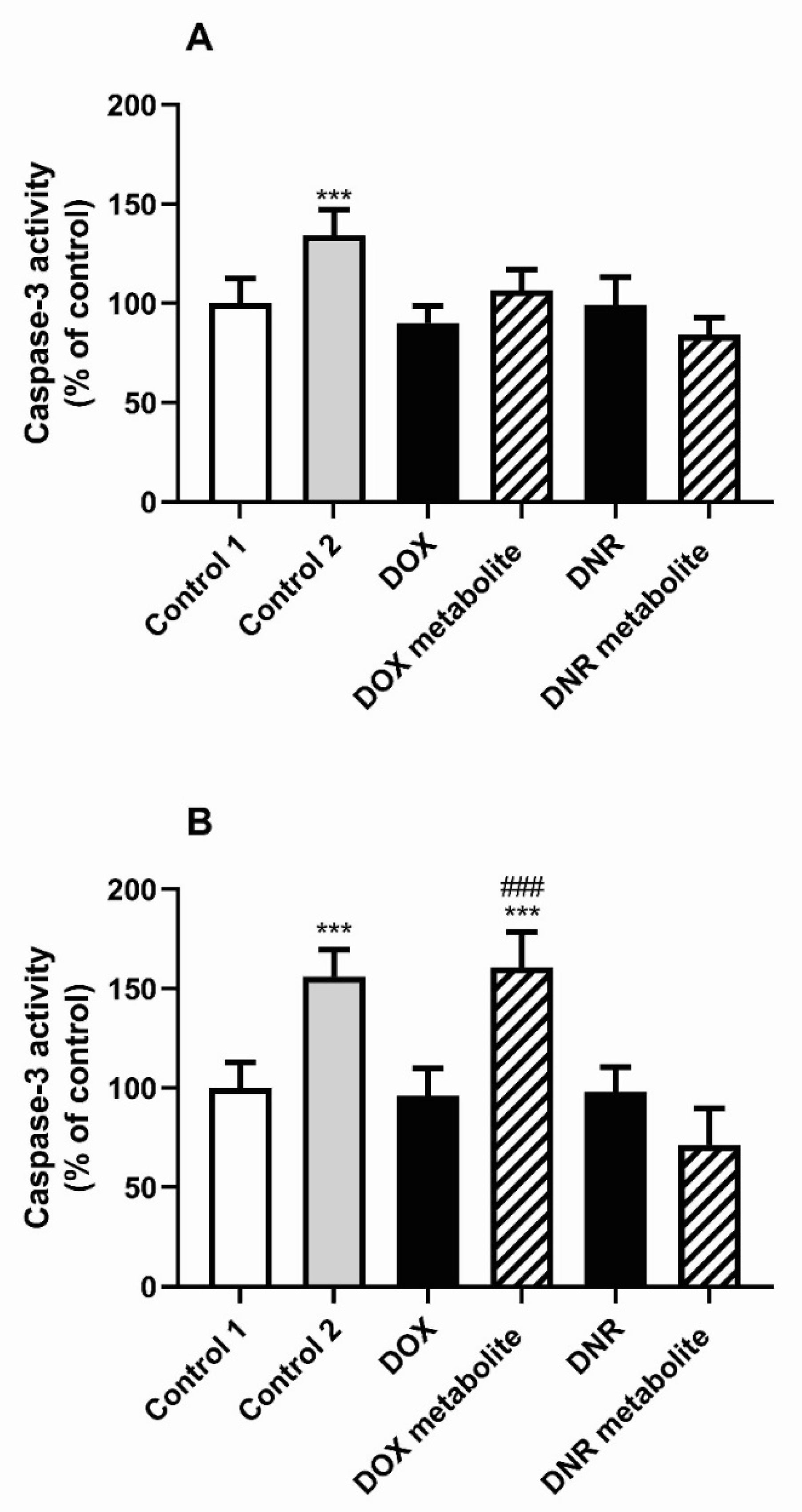
2.6. Comparative Evaluation of AQA Toxicity before and after B. adusta CCBAS 930 Treatment
2.6.1. Phytotoxicity
2.6.2. Ecotoxicity
2.6.3. Genotoxicity
2.6.4. Cytotoxicity
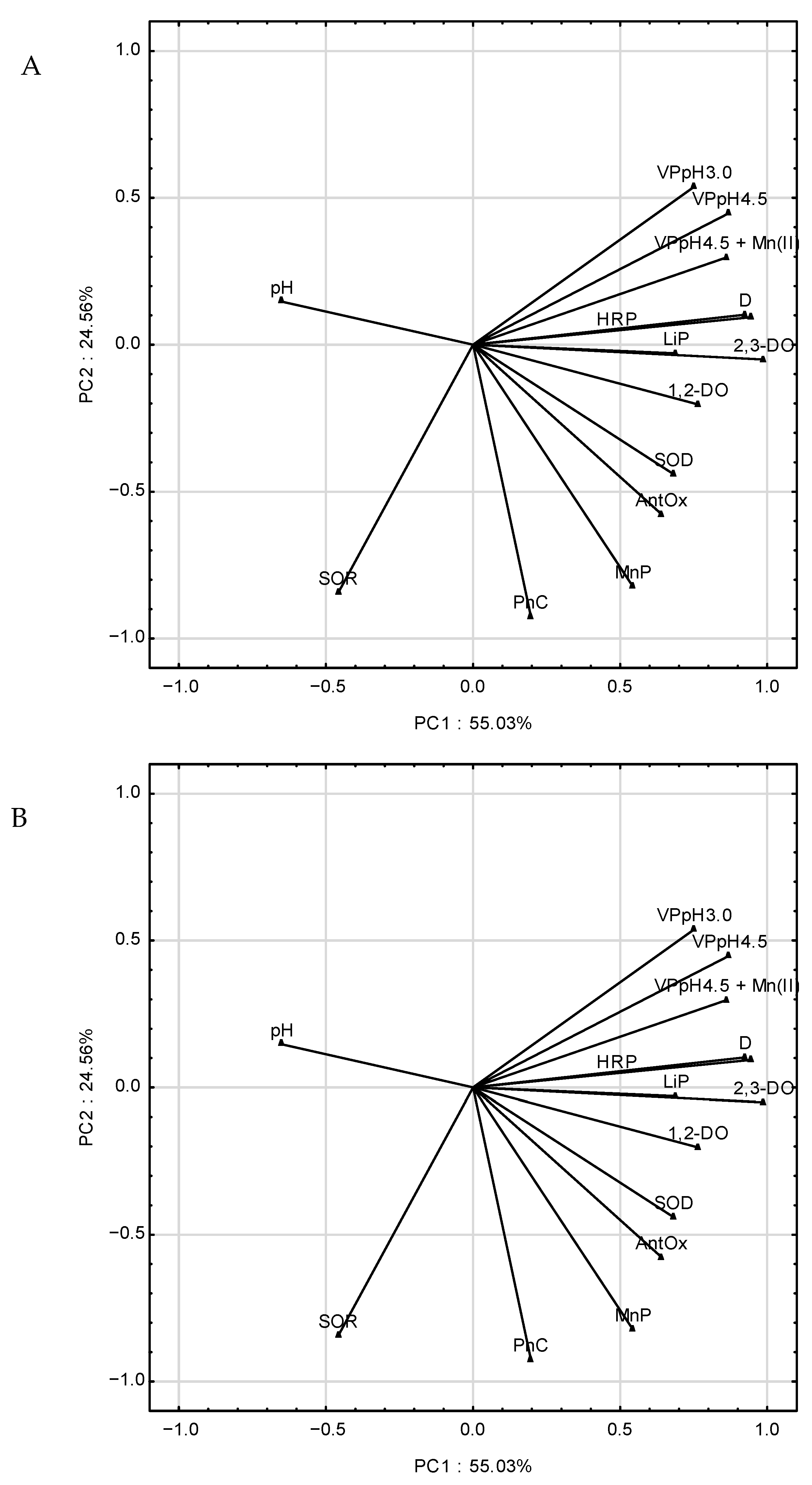
2.7. Determination of Main Principal Components (PC) during AQA Biotransformation in B. adusta CCBAS 930 Cultures
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cultures of Bjerkandera adusta CCBAS 930
4.3. Bioremoval of Anthracyclines by B. adusta CCBAS 930
4.4. Evaluation of Oxidoreductase Activities
4.5. Determination of Phenolic Compounds (PhC) and Free Radical (SOR) Contents
4.6. Determiantion of Antioxidative Activity of Initial Antracycline Solutions and Post-Culture Fluids of B. adusta CCBAS 930
4.7. Determination of pH of Post-Culture Fluids
4.8. Phytotoxicity Assay
4.9. Ecotoxicity Assay
4.10. Genotoxicity Assay
4.11. Cell Culture with Conditioned Medium
4.12. Resazurin Reduction Assay
4.13. Measurement Reactive Oxygen Species (ROS) Production
4.14. Caspase-3 Activity
4.15. Calcein AM Staining
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A


| Samples | C | V | CIF | SD |
|---|---|---|---|---|
| DNR before treatment (initial solution 10 µg mL−1) | 10 | - | 13.42 | 0.54 |
| 5 | 5.68 | 0.11 | ||
| 2.50 | 2.80 | 0.08 | ||
| 1.25 | 1.54 | 0.06 | ||
| 0.62 | 1.21 | 0.08 | ||
| 0.31 | 1.60 | 0.03 | ||
| 0.15 | 2.50 | 0.06 | ||
| DOX before treatment (initial solution 10 µg mL−1) | 10 | - | 9.58 | 0.12 |
| 5 | 3.70 | 0.08 | ||
| 2.50 | 3.27 | 0.08 | ||
| 1.25 | 2.53 | 0.02 | ||
| 0.62 | 1.23 | 0.04 | ||
| 0.31 | 1.21 | 0.08 | ||
| 0.15 | 2.88 | 0.10 | ||
| DNR after treatment by B. adusta CCBAS 930 | - | 100 | 0.98 | 0.02 |
| 50 | 0.46 | 0.05 | ||
| 25 | 0.73 | 0.06 | ||
| 12.50 | 0.11 | 0.02 | ||
| 6.25 | 0.40 | 0.04 | ||
| 3.12 | 0.93 | 0.04 | ||
| 1.56 | 0.72 | 0.08 | ||
| DOX after treatment by B. adusta CCBAS 930 | - | 100 | 1.13 | 0.14 |
| 50 | 1.08 | 0.06 | ||
| 25 | 0.65 | 0.06 | ||
| 12.50 | 0.98 | 0.07 | ||
| 6.25 | 1.01 | 0.09 | ||
| 3.12 | 0.99 | 0.06 | ||
| 1.56 | 1.06 | 0.06 |
References
- Ribes, J.; Clries, R.; BuxóM Ameijide, A.; Valls, J.; Gispert, R. Predictions of cancer incidence and mortality in Catalonia to 2015 by means of Bayesian models. Med. Clin. 2008, 131, 32–41. [Google Scholar] [CrossRef]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.-Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.; Mahnik, S.N.; Weissenbacher, N.; Mader, R.M.; Krenn, P.; Hann, S.; Koellensperger, G.; Uhl, M.; Knasmüller, S.; Ferk, F.; et al. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci. Technol. 2007, 56, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2020, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Mompelat, S.; le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Incidence of anticancer drugs in an aquatic urban system: From hospital effluents through urban wastewater to natural environment. Environ. Pollut. 2014, 193, 216–223. [Google Scholar] [CrossRef]
- Negreira, N.; López de Alda, M.; Barceló, D. Cytostatic drugs and metabolites in municipal and hospital wastewaters in Spain: Filtration, occurrence, and environmental risk. Sci. Total Environ. 2014, 497, 68–77. [Google Scholar] [CrossRef]
- Franquet-Griell, H.; Gomez-Canela, C.; Ventura, F.; Lacorte, S. Anticancer drugs: Consumption trends in Spain, prediction of environmental concentrations and potential risks. Environ. Pollut. 2017, 229, 505–515. [Google Scholar] [CrossRef]
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources— a review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Kümmerer, K. Pharmaceuticals in the environment. Ann. Rev. Environ. Res. 2010, 35, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Minotti, G.; Menna, P.; Salvatorell, E.; Gairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic development in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Belcarz, R.; Ginalska, G.; Korniłłowicz-Kowalska, T. Extracellular enzyme activities of Bjerkandera adusta R59 soil strain, capable for daunomycin and humic acids degradation. Appl. Microbiol. Biotechnol. 2005, 68, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Korniłłowicz-Kowalska, T.; Wrzosek, M.; Ginalska, G.; Iglik, H.; Bancerz, R. Identification and application of a new fungal strain Bjerkandera adusta R59 in decolorization of daunomycin wastes. Enzyme Microb. Technol. 2006, 38, 583–590. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska, K. Decolorization of Remazol Brilliant Blue (RBBR) and Poly R-478 dyes by Bjerkandera adusta CCBAS 930. Cent. Eur. J. Biol. 2012, 7, 948–956. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska, K. Anthraquinone dyes decolorization capacity of anamorphic Bjerkandera adusta CCBAS 930 strain and its HRP-like negative mutants. World J. Microbiol. Biotechnol. 2014, 30, 1725–1736. [Google Scholar] [CrossRef] [Green Version]
- Korniłłowicz-Kowalska, T.; Rybczyńska-Tkaczyk, K. Growth conditions, physiological properties and selection of optimal parameters of biodegradation of anticancer drug daunomycin in industrial effluents by Bjerkandera adusta CCBAS930. Int. Microbiol. 2020, 23, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Rybczyńska-Tkaczyk, K.; Święciło, A.; Szychowki, K.A. Korniłłowicz-Kowalska, T. Comparative study of eco- and cytotoxicity during biotransformation of anthraquinone dye Alizarin Blue Black B in optimized cultures of microscopic fungi. Ecotox. Environ. Safe. 2018, 147, 776–787. [Google Scholar] [CrossRef]
- Akdogan, H.A.; Topuz, M.C.; Urhan, A.A. Studies on decolorization of reactive blue 19 textile dye by Coprinus plicatilis. J. Environ. Health Sci. Eng. 2014, 12, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszka, K.J.; McCormick, M.L.; Britigan, B.E. Pxidation of anthracycline anticancer agents by the peroxidase mimic microperoxidase 11 and hydrogen peroxide. Free Rad. Biol. Med. 2003, 35, 78–93. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T.; Szychowki, K.A.; Gmiński, J. Biotransformation and toxicity mechanism of monoanthraquinone dyes during Bjerkandera adusta CCBAS 930 cultures. Ecotoxicol. Environ. Saf. 2020, 191, 110203. [Google Scholar] [CrossRef] [PubMed]
- Reszka, K.J.; Wagner, B.A.; Burns, C.P.; Britigan, B.E. Effects of peroxidase substrates on the Amplex red/peroxidase assay. Anal. Biochem. 2005, 342, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.; Makarov, O.; Chernyshova, M.; Turkovskaya, O.; Jarosz-Wilkołazka, A. Versatile peroxidase of Bjerkandera fumosa: Substrate and inhibitor specificity. Enzyme Microb. Technol. 2013, 52, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Eibes, G.; Debernardi, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Oxidation of pharmaceutically active compounds by a ligninolytic fungal peroxidase. Biodegradation 2011, 22, 539–550. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Eibes, G.; Lloret, L.; Lu-Chau, T.A.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Fostering the action of versatile peroxidase as a highly efficient biocatalyst for the removal of endocrine disrupting compounds. New Biotechnol. 2016, 33, 187–195. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Lu-Chau, T.A.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Continuous removal of endocrine disruptors by versatile peroxidase using a two-stage system. Biotechnol. Proc. 2015, 31, 908–916. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T. Activities of versatile peroxidase in cultures of Clonostachys rosea f. catenulata and Clonostachys rosea f. rosea during biotransformation of alkali lignin. J. AOAC Int. 2018, 101, 1415–1421. [Google Scholar]
- Hadibarata, T.; Tachibana, S.; Askari, M. Identification of metabolites from phenanthrene oxidation by phenolooxidases and dioxygenases of Pyloporus sp. S133. J. Microbiol. Biotechnol. 2011, 21, 299–304. [Google Scholar] [CrossRef]
- Hadibarata, T.; Rahim, A.; Yusoff, M.; Kristanti, R.A. Acceleration of anthraquinone-type dye removal by white-rot fungus under optimized environmental conditions. Water Air Soil Poll. 2012, 223, 4669–4677. [Google Scholar] [CrossRef]
- Belinky, P.A.; Flikshtein, N.; Lechenko, S.; Gepstein, S.; Dosoretz, C.G. Reactive oxygen species and induction of lignin peroxidase in Phanerochaete chrysosporium. Appl. Environ. Microbiol. 2013, 69, 6500–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohacz, J. Lignocellulose-degrading enzymes, free-radical transformations during composting of lignocellulosic wasteand biothermal phases in small-scale reactors. Sci. Total Environ. 2017, 580, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, A.; Parato, B.; Spina, F.; Tigini, V.; Prigione, V.; Varese, G.C. Decolourisation and detixification in the fungal treatment of textile wastewaters from dyeing processes. New Botechnol. 2011, 29. [Google Scholar] [CrossRef]
- Vanhulle, S.; Trovaslet, M.; Enaud, E.; Lucas, M.; Sonveaux, M.; Decock, C.; Onderwater, R.; Shneider, Y.-J.; Corbisier, A.-M. Cytotoxicity and genotoxicity evaluation during decolorization of dyes by White Rot Fungi. World J. Microbiol. Biotechnol. 2008, 24, 337–344. [Google Scholar] [CrossRef]
- Anastasi, A.; Spina, F.; Prigione, V.; Tigini, V.; Giansanti, P. Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta. Bioresour. Technol. 2010, 101, 3067–3075. [Google Scholar] [CrossRef]
- Anastasi, A.; Spina, F.; Romagnolo, A.; Prigione, V.; Tigini, V.; Varese, G.C. Integrated fungal biomass and activated sludge treatment for textile wastewaters bioremediation. Bioresour. Technol. 2012, 123, 106–111. [Google Scholar] [CrossRef]
- Hatvani, N. Antibacterial effect of the culture fluid of Lentinus edodes mycelium grown in submerged liquid culture. Int. J. Antimicrob. Agents. 2010, 17, 71–74. [Google Scholar] [CrossRef]
- Gerasimenya, V.P.; Efremenkova, O.V.; Kamzolkina, O.V.; Bogush, T.A.; Tolstych, I.V.; Zenkova, V.A. Antimicrobial and antitoxical action of edible and medicinal mushroom Pleurotus ostreatus (Jacq.: Fr.) Kumm. extracts. Int. J. Med. Mushrooms 2020, 4, 6. [Google Scholar] [CrossRef]
- Novotny, C.; Dias, N.; Kapanen, A.; Malachova, K.; Vandrovcova, K.; Itavaara, M.; Lima, N. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes. Chemosphere 2006, 63, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agri. Water Manage. 2015, 155, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Jureczko, A.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866. [Google Scholar] [CrossRef] [PubMed]
- Zounková, R.; Odráška, P.; Doležalová, L.; Hilscherová, K.; Maršálek, B.; Bláha, L. Ecotoxicity and genotoxicity assessment of cytostatic pharmaceuticals. Environ. Toxicol. Chem. 2007, 26, 2208. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Krętowski, R.; Kalinowska, M.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Possible mechanisms of the prevention of doxorubicin toxicity by cichoric acid—antioxidant nutrient. Nutrients 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T. Biosorption optimization and equilibrium isotherm of industrial dye compounds in novel strains of microscopic fungi. Int. J. Environ. Sci. Technol. 2016, 13, 2837–2846. [Google Scholar] [CrossRef] [Green Version]
- Korniłłowicz-Kowalska, T.; Rybczyńska, K. Screening of microscopic fungi and their enzyme activities for decolorization and biotransformation of some aromatic compounds. Int. J. Environ. Sci. Technol. 2015, 12, 2673–2686. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Święciło, A.; Rybczyńska-Tkaczyk, K.; Najda, A.; Krzepiłko, A.; Prażak, R.; Zawiślak, G. Application of growth tests employing a ∆sod1 mutant of Saccharomyces cerevisiae to study the antioxidant activity of berry fruit extracts. LWT Food Sci. Technol. 2018, 94, 96–102. [Google Scholar] [CrossRef]
- Paździoch-Czochra, M.; Malarczyk, E.; Sielewiesiuk, J. Relationship of demethylation processes to veratric acid concentration and cell density in cultures of Rhodococcus erythropolis. Cell Biol. Int. 2003, 27, 325–336. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Leja, M.L.; Kaminskyy, D.V.; Binduga, U.E.; Pinyazhko, O.R.; Lesyk, R.B.; Gmiński, J. Study of novel anticancer 4-thiazolidinone derivatives. Chem. Biol. Interact. 2017, 262, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Wójtowicz, A.K. TBBPA causes neurotoxic and the apoptotic responses in cultured mouse hippocampal neurons in vitro. Pharmacol. Rep. 2016, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A.; et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Rybczyńska, K.; Korniłłowicz-Kowalska, T. Evaluation of dye compounds’ decolorization capacity of selected H. haematococca and T. harzianum strains by Principal Component Analysis (PCA). Water Air Soil Poll. 2015, 226, 228. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T.; Szychowski, K.A. Possibility to Biotransform Anthracyclines by Peroxidases Produced by Bjerkandera adusta CCBAS 930 with Reduction of Geno- and Cytotoxicity and Pro-Oxidative Activity. Molecules 2021, 26, 462. https://doi.org/10.3390/molecules26020462
Rybczyńska-Tkaczyk K, Korniłłowicz-Kowalska T, Szychowski KA. Possibility to Biotransform Anthracyclines by Peroxidases Produced by Bjerkandera adusta CCBAS 930 with Reduction of Geno- and Cytotoxicity and Pro-Oxidative Activity. Molecules. 2021; 26(2):462. https://doi.org/10.3390/molecules26020462
Chicago/Turabian StyleRybczyńska-Tkaczyk, Kamila, Teresa Korniłłowicz-Kowalska, and Konrad A. Szychowski. 2021. "Possibility to Biotransform Anthracyclines by Peroxidases Produced by Bjerkandera adusta CCBAS 930 with Reduction of Geno- and Cytotoxicity and Pro-Oxidative Activity" Molecules 26, no. 2: 462. https://doi.org/10.3390/molecules26020462







