Abstract
In this study, the essential oil (EO) from Laurelia sempervirens was analyzed by GC/MS and safrole (1) was identified as the major metabolite 1, was subjected to direct reactions on the oxygenated groups in the aromatic ring and in the side chain, and eight compounds (4 to 12) were obtained by the process. EO and compounds 4–12 were subjected to biological assays on 24 strains of the genus Saprolegnia, specifically of the species 12 S. parasitica and 12 S. australis. EO showed a significant effect against Saprolegnia strains. Compound 6 presents the highest activity against two resistant strains, with minimum inhibitory concentration (MIC) and minimum oomyceticidal concentration (MOC) values of 25 to 100 and 75 to 125 µg/mL, respectively. The results show that compound 6 exhibited superior activities compared to the commercial controls bronopol and azoxystrobin used to combat these pathogens.
1. Introduction
Over the last decades, the production of fish, crustaceans, shellfish, and amphibians through aquaculture has become the fastest growing food sector in the world. However, the growing business of aquaculture often suffers from heavy financial losses due to the development of infections caused by microbial pathogens, particularly by different species of the genus Saprolegnia [1]. The infection caused by this genus of pathogens is known as saprolegniosis; this infection occurs in all freshwater aquatic ecosystems and significantly attacks fish and eggs, mainly when certain changes occur in the culture environment, which causes the appearance of these oomycetes [2]. The mechanism of infection begins by means of its spores, which infect the epidermal tissues of fish, generally starting from the head and fins, spreading over the entire surface of the body, causing cell necrosis with cutaneous and epidermal damage. The oomycete penetrates the membrane affecting the ova, and proliferation occurs through the water to other closer ones, causing death [3]. Until 2002, Saprolegnia sp. was kept under control through the use of Malachite green; however, due to its carcinogenic and toxicological effects, treatment with this chemical has been banned internationally [4]. Formalin is another of the most potent fish oomycide, but with the drawback of having an acute impact on aquatic ecosystems [5]. Other chemical alternatives have been used to control the disease effectively, including 8-quinolinol, dichlorophen, copper sulfate pentahydrate, sodium chloride or bronopol [6,7,8]. However, to date, most of the compounds described for the control of Saprolegnia are ineffective or have negative effects on the health of fish, operators or the environment [9]. Hence, an economical, effective and friendly alternative is required.
Not insignificant is the recent increased interest in using natural substances derived from plants for different industrial purposes, not only as food seasoning or natural medicine, but also for pest management in agriculture, livestock, and aquaculture. The plant-derived products most commonly used to control diseases are the essential oils. These are complex mixtures of hydrocarbons and oxygenated hydrocarbons arising from the isoprenoid pathways, including terpenoids and phenylpropanoids [10]. These compounds have served as the starting point for the discovery and development of new antimicrobial agents.
The genus Laurelia belongs to the family Atherospermataceae, is represented by only two species, and is endemic to the southern hemisphere. Both species have a high content of alkaloids, and other compounds include terpenes, phenolics, phenylpropanoids, and flavonoids, which usually have a wide range of pharmacological activities [11,12].
Laurelia sempervirens (Ruiz and Pav.) Tul. is an evergreen endemic tree of the temperate rainforests of southern Chile [13]. Numerous pharmacological and phytochemical studies have reported repellent, insecticidal, antibacterial, antioxidant, antitumoral and antifungal activity potential of essential oil from L. sempervirens [14,15,16,17].
Safrole is a major component of Chilean laurel oil and a component of several other essential oils [18,19]. It has differential biological activities, such as cytotoxic, analgesic and antimicrobial activities [20], which can be used to participate in numerous chemical reactions.
Therefore, our working group has developed safrole derivatives, by direct reactions on oxygenated groups in the aromatic ring and the side chain, in search of new biological activities such as antioomycete.
The objective of this study was to evaluate the antioomycete activity of the essential oil of L. sempervirens, its main compound safrole, and its synthetic derivatives against strains of S. parasitica and S. australis, both pathogenic oomycetes of economic importance for salmon farming.
2. Results and Discussion
2.1. Yield and Chemical Constituents of EO
The extraction yield of L. sempervirens EO was 2.78% (v/dry weight) and the density was 1.15 ± 0.01 g/mL. The results of the gas chromatographic analysis of L. sempervirens EO is summarized in Table 1.

Table 1.
EO composition of leaves of L. sempervirens.
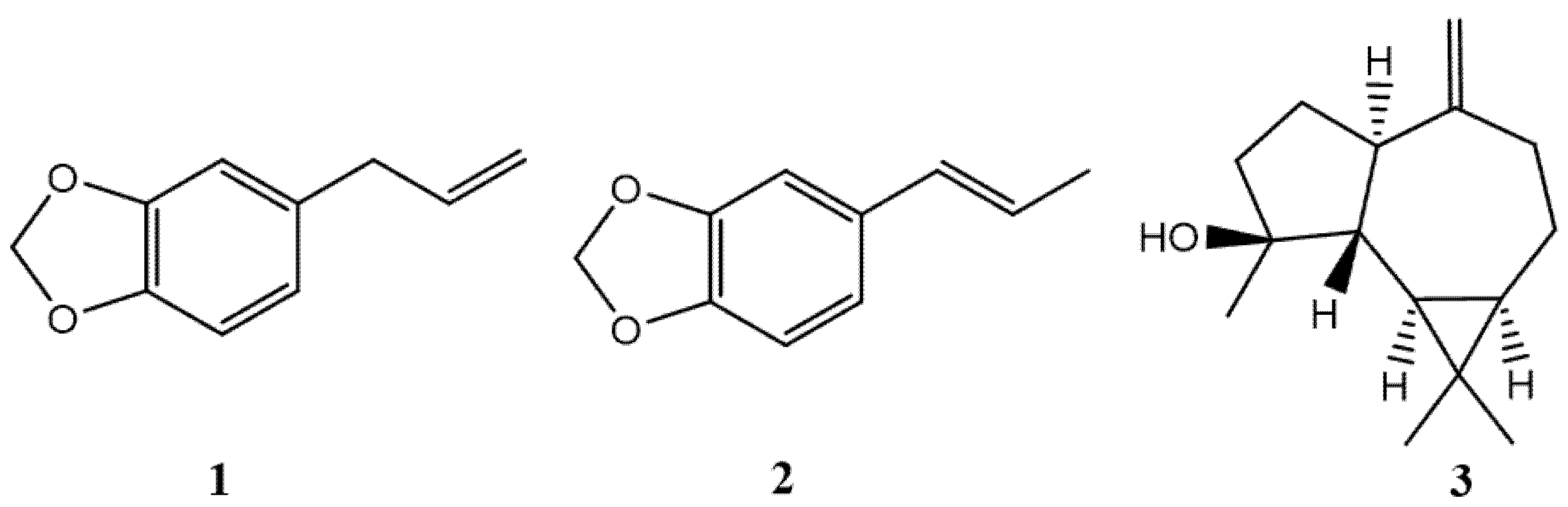
Sixteen components were identified in the leaf EO: 78.06% were phenylpropanoids, 3.08% terpenes and 0.37% benzaldehydes. Leaf EO was mainly characterized by safrole (1) (65.03%), isosafrole (2) (11.90%), and spathulenol (3) (11.16%) (see Figure 1).
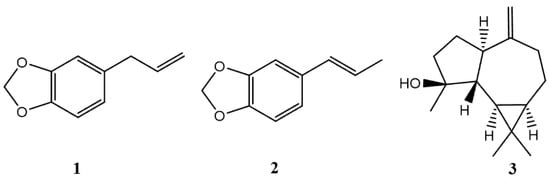
Figure 1.
Structures of the main compounds present in the EO of L. sempervirens.
Some studies of leaf EOs from L. sempervirens showed different chemical compositions according to the region. The results of this work show that the chemical composition of this EO was different with those obtained from plants collected in the Metropolitan, Ñuble, Bio-Bío and La Araucanía regions [14,16,17,22]. The most important differences are found in the absence of spatulenol content, the lower isosafrole content, and a higher safrole content. In this context, the variability in the essential oil content is influenced by the geographical origin of the species, as well as by the drying methods, the extraction time and the type of organ studied [23].
Safrole derivatives can be synthesized by chemical modification, and some of these derivatives have been proved to have better antimicrobial activity; indeed, in the last decade, this research group reported some safrole derivatives obtained by traditional organic chemistry protocols with a series of modifications [24,25].
In this work, however, the preparation of catechol from the methylenedioxy ring safrole cleavage reaction was performed with TiCl4 to shorten the reaction time and improve the yield, in comparison with the previously reported method [25].
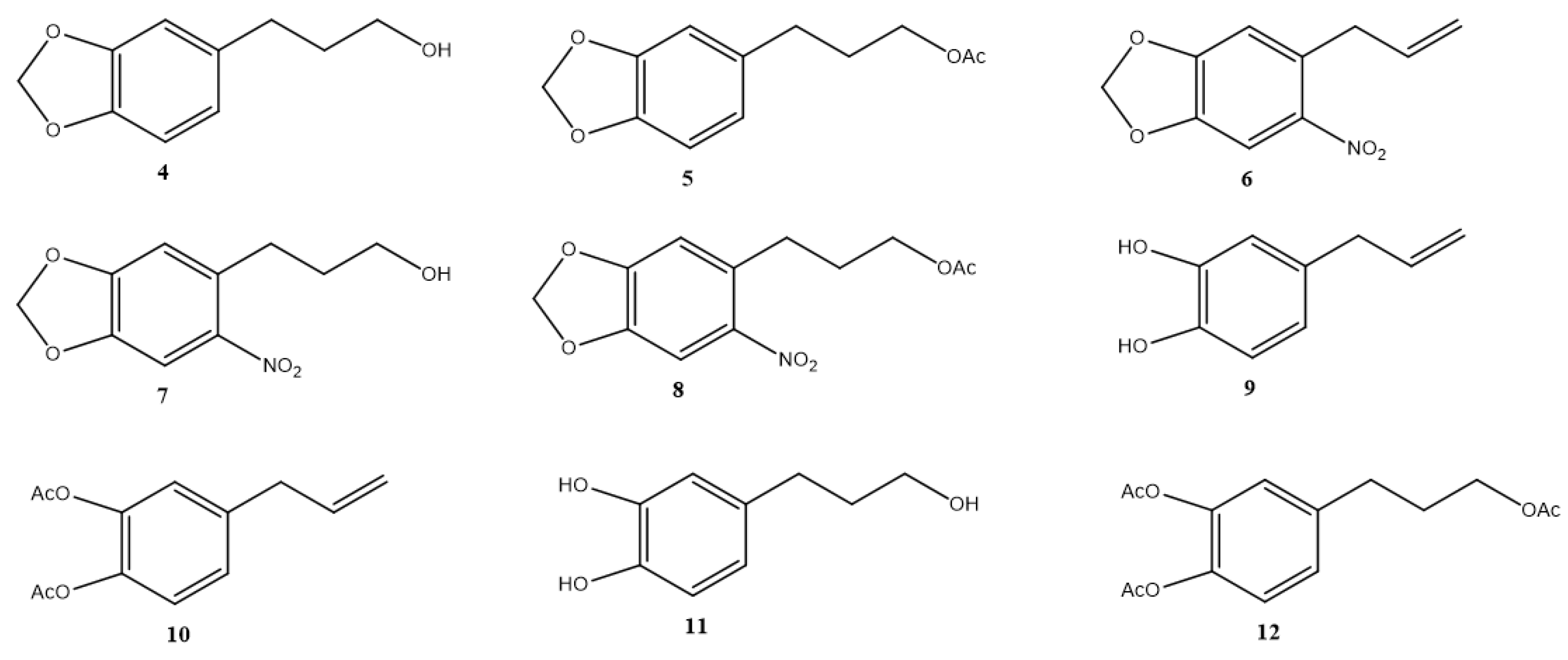
Safrole derivatives 4–12 were obtained in moderate to good yields (40.0–95.0%) (Figure 2). NMR data of 4–12 were consistent with what we had previously reported (see Supplementary Materials).
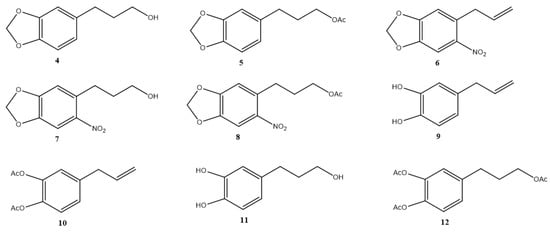
Figure 2.
Structure of safrole derivatives 4–12.
2.2. Biological Assays
The in vitro antimycotic activity of L. sempervirens EO, safrole and its derivatives 4–12 against twenty-four strains of S. parasitica and S. australis, twelve of each.
First, the minimum inhibitory concentration (MIC) at 48 h of EO, safrole and its derivatives 4–7 (Table 2) and for compounds 8–12 (Table 3) was evaluated against 22 strains of Saprolegnia (S. parasitica 1–11 and S. australis 12–22, respectively).

Table 2.
MIC values a of leaf EO, safrole and compounds 4–7 against mycelium at 48 h.

Table 3.
MIC values a of compounds 8–12 against mycelium at 48 h.
EOs used in this study presented activity against the 22 isolates tested. The highest MIC value observed for EO was against strain 1 of S. parasitica and strain 18 of S. australis, with 50 µg/mL, while the minimum value was of 175 µg/mL for strain 15 of S. australis. When comparing the effectiveness of the oil against the strains, it was observed that EOs were very efficient against the pathogenic strain of S. parasitica on the strains of S. australis. EO showed bronopol-equivalent effectiveness against strains 1, 11, 17 and 20, while azoxystrobin showed an equivalence in the action on the strains 6, 7 and 11.
However, the EO showed higher anti-oomycete activity against strains 21 and 22 and 1, 2, 3, 4, and 8, for bronopol and azoxystrobin, respectively, but both controls showed lower efficiency than EO against strains 18 and 19 of S. australis. As an aside, previous studies have reported that EOs from Laureliopsis philippianna, Tymus vulgaris and Origanum vulgare present similar anti-oomycidal activity against S. parasitica, due to their high content of aromatic compounds [23,26].
For safrole and its derivatives, the results show that 6 and 7 were the most active compounds compared to the controls against the twenty-two strains tested. However, the MIC values of compound 6 for the growth inhibition of S. parasitica and S. australis were 3.125 to 6.25 and 6.25 to 75 µg/mL, respectively, while for compound 7, which differs in the presence of a primary alcohol positioned on the side chain of the aromatic ring with 6, these values decreased from 3.125 to 6.25 and 50 to 125 µg/mL for the strains, respectively. These data confirm what has been suggested in previous studies about the importance that exists between nitro groups and primary alcohols in the sense that they can generate intra-molecular hydrogen bridges that would diminish the antimicrobial action of this type of molecule [27].
Subsequently, the oil and all the compounds were evaluated against strains 23 and 24, corresponding to S. parasitica and S. australis, respectively, which were less sensitive to the commercial products tested, such us fluconazole and ketoconazole.
Table 4 summarizes the MIC, the minimum oomyceticidal concentration (MOC) and membrane damage values for the EO, safrole and its derivatives 4–12 against strains 23 and 24.

Table 4.
Summary of the anti-oomycete activity of EO, safrole and derivatives against resistant strains of S. parasitica and S. australis.
Our results show that nitrosylated compounds can cause greater damage to Saprolegnia strains when compared to commercial antifungal compounds (bronopol and azoxystrobin) used as controls.
In a biological system, nitro groups can be enzymatically reduced, causing very unstable nitro radicals that can be re-oxidized under aerobic conditions, generating reactive superoxide anions. However, a reduction in the NO2 groups can also originate nitroso and hydroxylamine intermediates that react with biomolecules to produce toxic effects [28], including protein cysteine thiols [29]. On the other hand, we can consider binding interactions between nitroaromatics compounds and biomacromolecules such as proteins, because nitrobenzene compounds can act as enzymatic inhibitor by interaction with tryptophan and tyrosine residues [30]. This supports the importance of specific interactions of nitro aromatic compounds to cause cell membrane proteins’ malfunction and cell membrane damage.
Nitrosylation increased the efficiency of safrole derivatives, improving their antifungal activity reflected in the damage to cell membranes. These good results for safrole-derived compounds open the possibility that they could be considered as effective control agents, including animal and plant pathogenic oomycetes, because previous studies demonstrated their low toxicity in in vitro models of healthy epithelial cells, red blood cells and Artemia salina [25,31].
3. Materials and Methods
3.1. General
Standard pure compounds for co-injection in GC/MS, α-pinene, β-phellandrene, safrole, isosafrole, and piperonal were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The separation and identification of the synthesized compounds was carried out by previously performed methods [24,25].
3.2. Plant Material
The leaves of L. sempervirens were collected in Antilhue, Los Ríos Region, Chile, in December 2019. The plant material was identified by Forestry Engineer Patricio Novoa (National Botanical Garden, Viña del Mar, Chile), and a voucher specimen (LS-1219) was deposited at the Natural Products and Organic Synthesis Laboratory of Universidad de Playa Ancha, Valparaíso, Chile.
3.3. Essential Oil Isolation and Analysis
The fresh leaves (500 g) of L. sempervirens were submitted to hydrodistillation for 4 h. The distilled leaves were extracted using ethyl acetate, dried over anhydrous sodium sulfate, and the solvent evaporated. The EO was analyzed and its components identified by gas chromatography/mass spectrometry (GC/MS) using previously standardized instrumentation and methodology [32].
3.4. Isolation and Identification of the Major Constituent
EO obtained as described above (5.0 g) was chromatographed on a column of silica gel and eluted with a gradient of hexane/ethyl acetate (100:0 to 90:10). One hundred and fifty fractions were collected and analyzed by TLC. The fractions 18–78 were purified by flash chromatography using a mixture of hexane/ethyl acetate (95:5) as eluent. Thirty fractions were collected. Fractions 13–25 contained the active compound (2.1 g) identified as safrole (1).
3.5. Synthesis of Safrole Derivatives
Safrole derivatives (Figure 2) obtained from the natural compound 1 were synthesized and characterized by standard methods [24,25], except for compound 9, 4-allylbenzene-1,2-diol.
The natural compound 1 (100 mg, 0.62 mmol) was added to a stirred solution of TiCl4 (0.3 mL, 0.098 mmol) in dry dichloromethane (10 mL) under nitrogen atmosphere. The mixture was stirred for 8 h at room temperature, diluted with water (20 mL), extracted with ethyl acetate (2 × 50 mL), dried (Na2SO4), and concentrated. The separation and identification of the compound 9 was carried out by the previously performed method [25].
3.6. Oomycete Strain
Twelve Saprolegnia parasitica strains and twelve S. australis strains (the Cell Biology Laboratory, Faculty of medicine, Universidad de Valparaíso) were used in this study. These were isolated from Salmo salar carp eggs and biochemically and molecularly characterized in previous studies [23].
3.7. Minimum Inhibitory Concentration Evaluation
Minimum inhibitory concentration (MIC) was determined by a serial dilution technique using 96-well microtiter plates. The EO, safrole (1) and compounds 4–12 were dissolved in 0.1% DMSO solution and added to a Gypsum (G-Y) medium with inoculum. Samples were evaluated at a series of previously defined concentrations [23]. The microplates were incubated in a rotary agitator (160 rpm) for 48 h at 20 °C. The lowest concentrations without visible mycelia growth under an optical microscope were defined as the concentrations that completely inhibited oomycete growth according to the above standardized method [23].
3.8. Spores Germination Inhibition Assay
The minimum oomyceticidal concentration (MOC) was determined by the agar dilution method [33]. Briefly, 10 μL serial sub-cultivation of the tested compound was dissolved in a cultivation medium and inoculated during 72 h in microtiter plates containing 100 μL of broth per well and with incubation for 72 h at 25 °C. The lowest concentration without visible growth or germination of spores was defined as MOC, indicating the death of 99.5% of the original. The commercial oomycides bronopol and azoxystrobin were used as positive controls.
3.9. Cell Membrane Damage Measurement (Cellular Leakage Assay)
Cell leakages were measured in order to determine the effectiveness of the EO, safrole and compounds 4–12 on membrane integrity. This method was assessed according to Flores [34].
3.10. Statistical Analysis
The statistical data of recovery rates were performed by comparison within isolates and between culturing media following a standard method [34].
4. Conclusions
In summary, sixteen compounds were identified from the EO of L. sempervirens. Safrole (1) is the major metabolite detected by GC/mass spectrometry. Compounds 4–12 were biologically active against Saprolegnia. Significant antioomycete activities of novel compounds 5, 6, 7 and 8 were observed against S. parasitica and S. australis. This study provides baseline information on the potential application of EOs as botanical fungicides in fish farms.
Supplementary Materials
The following are available online. NMR data of known compounds 1, 4–12.
Author Contributions
A.M. supervised the whole study; M.M. collected the plant and performed the isolation of the essential oil; A.L.M. and B.S. performed the synthesis of all compounds; A.M. and I.M. conceived and designed the synthesis and biologic experiments; P.G. performed the identification and isolation of Saprolegnia strains; N.C. and V.S. performed the biological assays; A.M.; M.A.C.; E.W. and I.M. collaborated in the discussion and interpretation of the results; A.M. and I.M. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study received no funding support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available for the scientific community.
Acknowledgments
The authors thank Dirección General de Investigación de la Universidad de Playa Ancha (Decreto Exento N 510/2021) and to the staff of the laboratory LPNSO, UPLA.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the essential oil and compounds are available from the authors.
References
- Banfield, M.J.; Kamoun, S. Hooked and Cooked: A Fish Killer Genome Exposed. PLoS Genet. 2013, 9, e1003590. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Donnelly, T.M. Saprolegniasis. In Clinical Veterinary Advisor. Birds and Exotic Pets; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 65–66. [Google Scholar]
- Lamour, K.; Kamoun, S. Oomycete Genetics and Genomics: Diversity, Interactions, and Research Tools; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–24. [Google Scholar]
- Torto-Alalibo, T.; Tian, M.; Gajendran, K.; Waugh, M.E.; van West, P.; Kamoun, S. Expressed Sequence Tags from the Oomycete Fish Pathogen Saprolegnia parasitica Reveal Putative Virulence Factors. BMC Microb. 2005, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Gío, J.J.; Rodríguez-Canul, R.P.; Vidal-Martínez, V.M.; Fájer-Ávila, E.J.; Améndola-Pimenta, M. Formalin Toxicity to Oreochromis niloticus; its Effectiveness against Cichlidogyrus spp. and Host Stress Response. Lat. Am. J. Aquat. Res. 2019, 47, 34–41. [Google Scholar] [CrossRef]
- Hussein, M.M.A.; Wada, S.; Hatai, K.; Yamamoto, A. Antimycotic Activity of Eugenol against Selected Water Molds. J. Aquat. Anim. Health 2000, 12, 224–229. [Google Scholar] [CrossRef]
- Caruana, S.; Yoon, G.H.; Freeman, M.A.; Mackie, J.A.; Shinn, A.P. The Efficacy of Selected Plant Extracts and Bioflavonoids in Controlling Infections of Saprolegnia australis (Saprolegniales; Oomycetes). Aquaculture 2012, 358, 146–154. [Google Scholar] [CrossRef]
- Earle, G.; Hintz, W. New Approaches for Controlling Saprolegnia parasitica, the Causal Agent of a Devastating Fish Disease. Trop Life Sci. Res. 2014, 25, 101–109. [Google Scholar] [PubMed]
- Fregeneda-Grandes, J.M.; Rodríguez-Cadenas, F.; Aller-Gancedo, J.M. Fungi Isolated from Cultured Eggs, Alevins and Broodfish of Brown Trout in a Hatchery Affected by Saprolegniosis. J. Fish. Biol. 2007, 71, 510–518. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Berry, E.W. The Monimiaceae and a new Laurelia. Bot. Gaz. 1935, 96, 751–754. [Google Scholar]
- Cassels, B.K.; Urzüa, A. Bisbenzylisoquinoline Alkaloids of Laurelia sempervirens. J. Nat. Prod. 1985, 48, 671. [Google Scholar] [CrossRef]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Kiessling, A.; Mihoc, M.; Pauchard, A.; Ruiz, E.; et al. Catálogo de las Plantas Vasculares de Chile. Gayana Bot. 2018, 75, 1–430. [Google Scholar] [CrossRef]
- Bittner, M.; Aguilera, M.A.; Hernández, V.; Arbert, C.; Becerra, J.; Casanueva, M.E. Fungistatic activity of essential oils Extracted from Peumus boldus Mol., Laureliopsis philippiana (Looser) Schodde and Laurelia sempervirens (Ruiz & Pav.) Tul. (Chilean Monimiaceae). Chil. J. Agric. Res. 2009, 69, 30–37. [Google Scholar] [CrossRef]
- Montenegro, I.; Madrid, A.; Zaror, L.; Martínez, R.; Werner, E.; Carrasco-Altamirano, H.; Cuellar, M.; Palma, H. Antimicrobial Activity of Ethyl Acetate Extract and Essential Oil from Bark of Laurelia sempervirens against Multiresistant Bacteria. Bol. Latinoam. Caribe Plant. Med. Aromat. 2012, 11, 306–315. [Google Scholar]
- Torres, C.; Silva, G.; Tapia, M.; Rodríguez, J.C.; Urbina, A.; Figueroa, I.; Santillan, C.; Aguilar, S.; Robles, A.; Lagunes, A. Propiedades Insecticidas del Laurelia sempervirens Polvo a Motschulsky Sitophilus zeamais: Control (Coleoptera Curculionidae). Bol. Latinoam. Caribe Plant. Med. Aromat. 2015, 14, 48–59. [Google Scholar]
- Touma, J.; Navarro, M.; Sepúlveda, B.; Pavon, A.; Corsini, G.; Fernández, K.; Quezada, C.; Torres, A.; Larrazabal-Fuentes, M.J.; Paredes, A.; et al. The Chemical Compositions of Essential Oils Derived from Cryptocarya alba and Laurelia sempervirens Possess Antioxidant, Antibacterial and Antitumoral Activity Potential. Molecules 2020, 25, 5600. [Google Scholar] [CrossRef]
- Montes, M.; Valenzuela, L.; Wilkomirsky, T. Safrole: Main Component of Essential Oil of Laurelia sempervirens (R. et P.) Tul. from the Bio-Bio Area (Chile). An. Real Acad. Farm. 1990, 56, 49–54. [Google Scholar]
- Tisserand, R.; Young, R. Essential oil composition. In Essential Oil Safety. A Guide for Health Care Professionals, 2nd ed.; Tisserand, R., Young, R., Eds.; Churchill Livingstone Elsevier: St. Louis, MO, USA, 2014; pp. 5–22. [Google Scholar]
- Eid, A.M.; Hawash, M. Biological Evaluation of Safrole Oil and Safrole Oil Nanoemulgel as Antioxidant, Antidiabetic, Antibacterial, Antifungal and Anticancer. BMC Complement. Med. Ther. 2021, 21, 159. [Google Scholar] [CrossRef]
- Adams, P.R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectromety, 4.1th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2017; pp. 20–45. [Google Scholar]
- Zapata, N.; Vargas, M.; Latorre, E.; Roudergue, X.; Ceballos, R. The Essential Oil of Laurelia sempervirens is Toxic to Trialeurodes vaporariorum and Encarsia formosa. Ind. Crop. Prod. 2016, 84, 418–422. [Google Scholar] [CrossRef]
- Madrid, A.; Godoy, P.; González, S.; Zaror, L.; Moller, A.; Werner, E.; Cuellar, M.; Villena, J.; Montenegro, I. Chemical Characterization and Anti-Oomycete Activity of Laureliopsis philippianna Essential Oils against Saprolegnia parasitica and S. australis. Molecules 2015, 20, 8033–8047. [Google Scholar] [CrossRef]
- Espinoza, L.; Madrid, A.; Taborga, L.; Villena, J.; Cuellar, M.; Carrasco, H. Synthesis of Nine Safrole Derivatives and Their Antiproliferative Activity Towards Human Cancer Cells. J. Chil. Chem. Soc. 2010, 55, 219–222. [Google Scholar] [CrossRef][Green Version]
- Madrid Villegas, A.; Espinoza Catalán, L.; Montenegro Venegas, I.; Villena García, J.; Carrasco Altamirano, H. New Catechol Derivatives of Safrole and Their Antiproliferative Activity towards Breast Cancer Cells. Molecules 2011, 16, 4632–4641. [Google Scholar] [CrossRef]
- Nardoni, S.; Najar, B.; Fronte, B.; Pistelli, L.; Mancianti, F. In Vitro Activity of Essential Oils against Saprolegnia parasitica. Molecules 2019, 24, 1270. [Google Scholar] [CrossRef]
- Giordanetto, F.; Tyrchan, C.; Ulander, J. Intramolecular Hydrogen Bond Expectations in Medicinal Chemistry. ACS Med. Chem. Lett. 2017, 8, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Olender, D.; Żwawiak, J.; Zaprutko, L. Multidirectional Efficacy of Biologically Active Nitro Compounds Included in Medicines. Pharmaceuticals 2018, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Andres, T.; Eckmann, L.; Smith, D. Voltammetry of Nitrobenzene with Cysteine and other Acids in DMSO. Implications for the Biological Reactivity of Reduced Nitroaromatics with Thiols. Electrochim. Acta 2013, 92, 257–268. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Fu, H.; Qu, X.; Zhang, Z.; Kang, F.; Zhu, D. Spectroscopic and Molecular Modeling Investigation on Inhibition Effect of Nitroaromatic Compounds on Acetylcholinesterase Activity. Chemosphere 2019, 236, 124365. [Google Scholar] [CrossRef] [PubMed]
- Madrid, A.; Espinoza, L.; Pavéz, C.; Carrasco, H.; Hidalgo, M.E. Antioxidant and Toxicity Activity In Vitro of Twelve Safrole Derivatives. J. Chil. Chem. Soc. 2014, 59, 2598–2601. [Google Scholar] [CrossRef]
- Moller, A.C.; Parra, C.; Said, B.; Werner, E.; Flores, S.; Villena, J.; Russo, A.; Caro, N.; Montenegro, I.; Madrid, A. Antioxidant and Anti-Proliferative Activity of Essential Oil and Main Components from Leaves of Aloysia polystachya Harvested in Central Chile. Molecules 2021, 26, 131. [Google Scholar] [CrossRef] [PubMed]
- Escobar, B.; Montenegro, I.; Villena, J.; Werner, E.; Godoy, P.; Olguín, Y.; Madrid, A. Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin. Molecules 2017, 22, 968. [Google Scholar] [CrossRef]
- Flores, S.; Montenegro, I.; Villena, J.; Cuellar, M.; Werner, E.; Godoy, P.; Madrid, A. Synthesis and Evaluation of Novel Oxyalkylated Derivatives of 2′,4′-Dihydroxychalcone as Anti-Oomycete Agents against Bronopol Resistant Strains of Saprolegnia sp. Int. J. Mol. Sci. 2016, 17, 1366. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).