Pulse Duration Dependent Asymmetry in Molecular Transmembrane Transport Due to Electroporation in H9c2 Rat Cardiac Myoblast Cells In Vitro
Abstract
:1. Introduction
2. Results
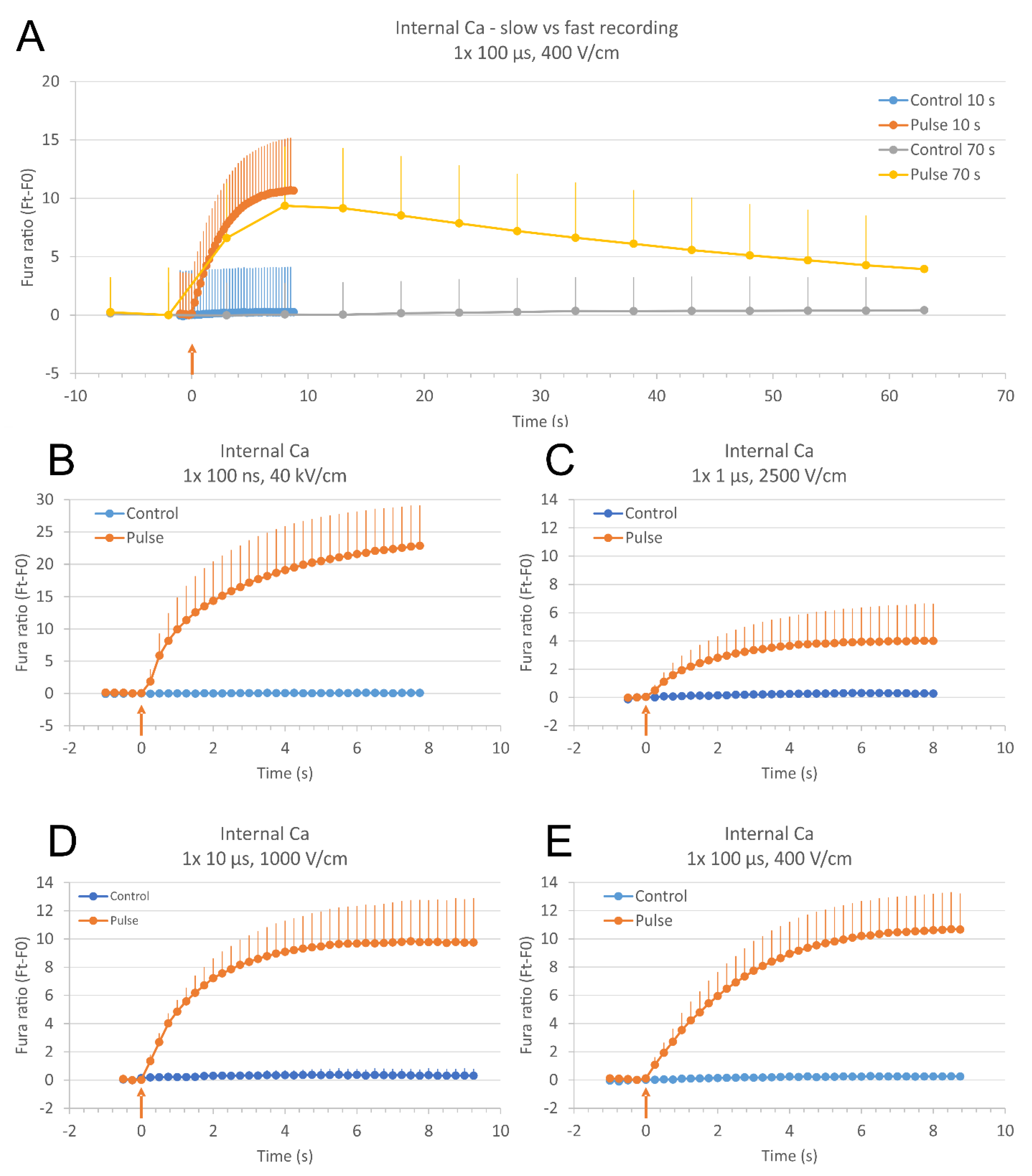
2.1. Comparing Fast and Slow Image Acquisition Modes to Monitor the Changes in Relative Calcium Concentration in Cells after Pulse Exposure
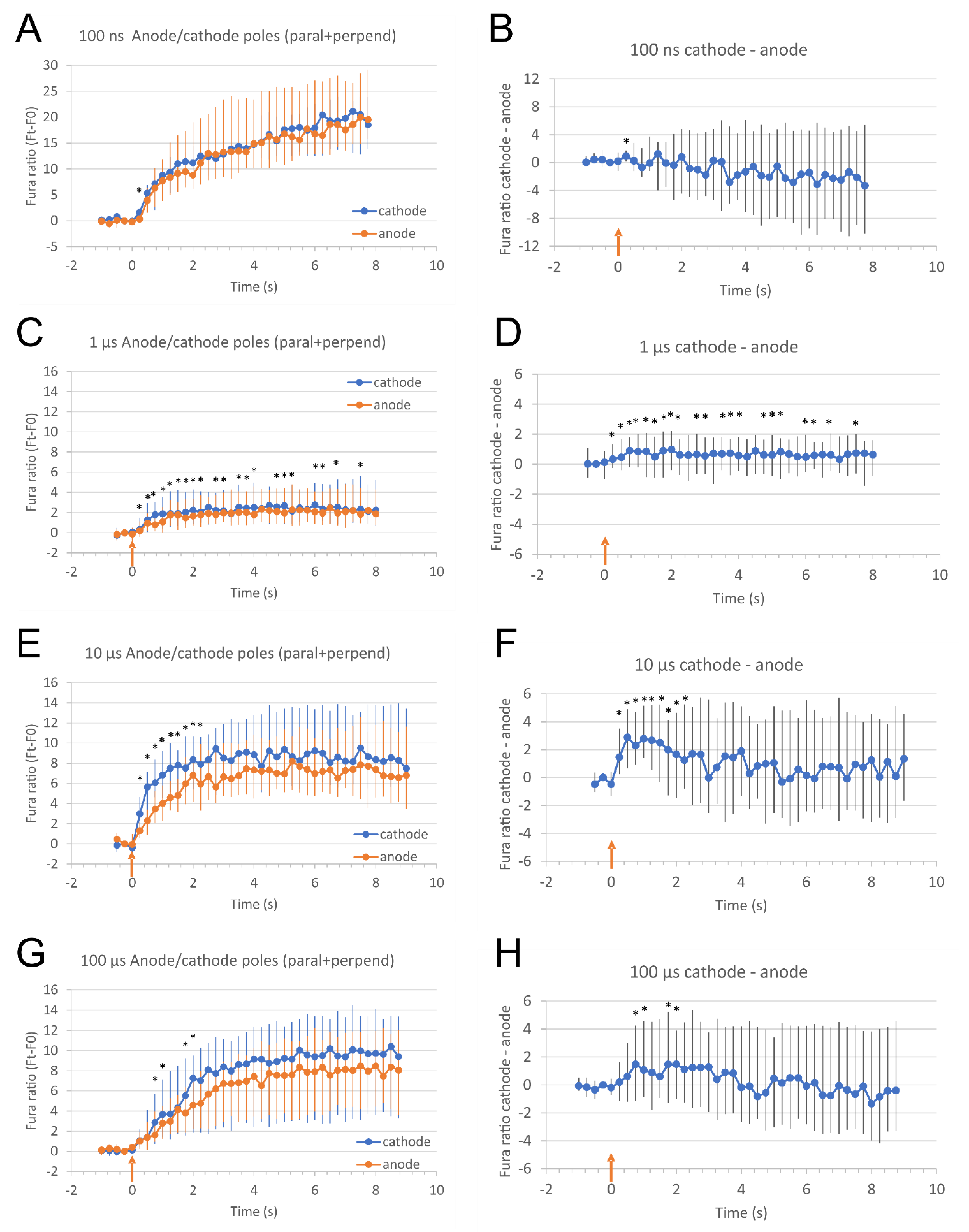
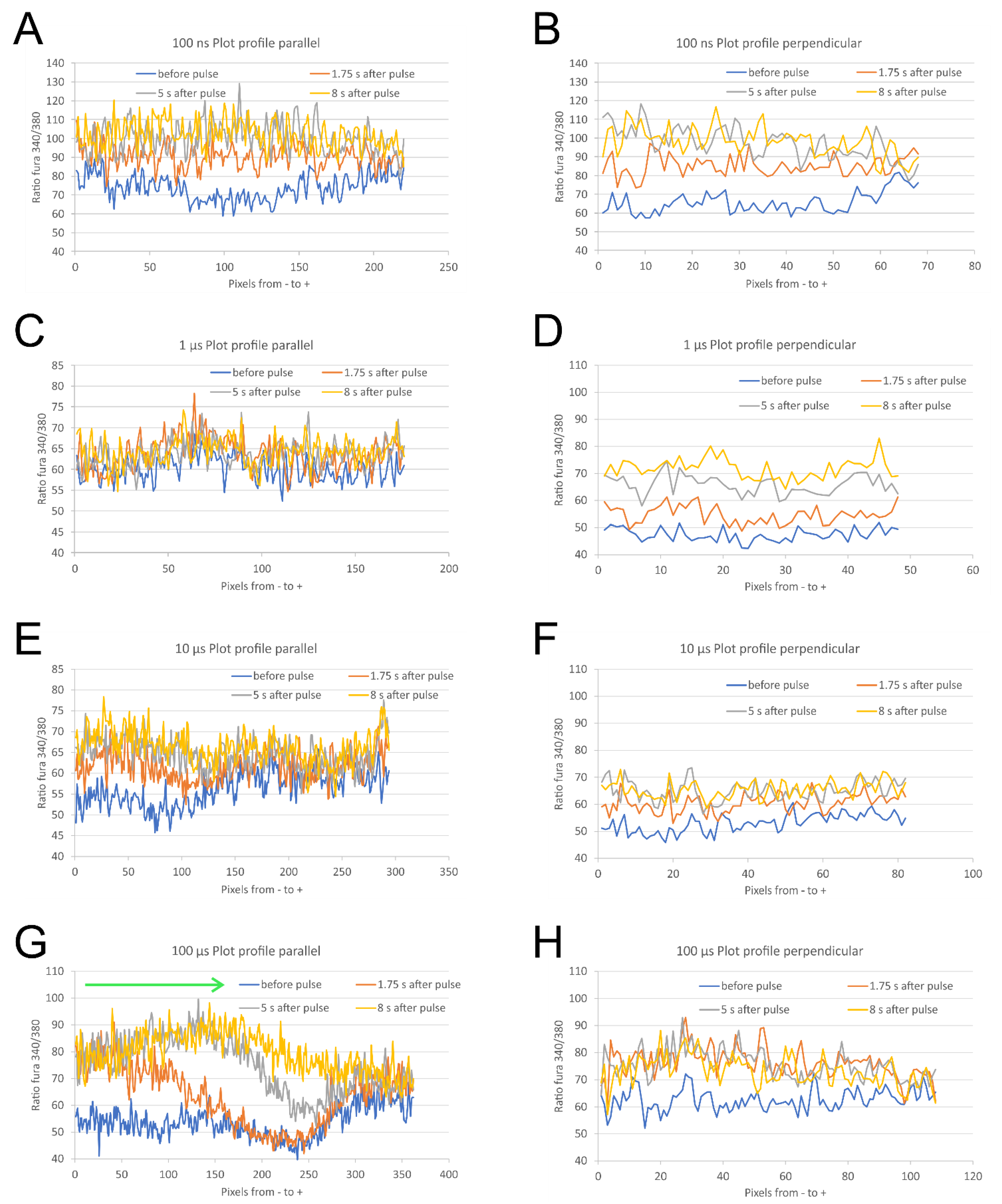
2.2. The Direction of Calcium Ions Uptake after Exposure to Pulse with Respect to the Direction of Electric Field
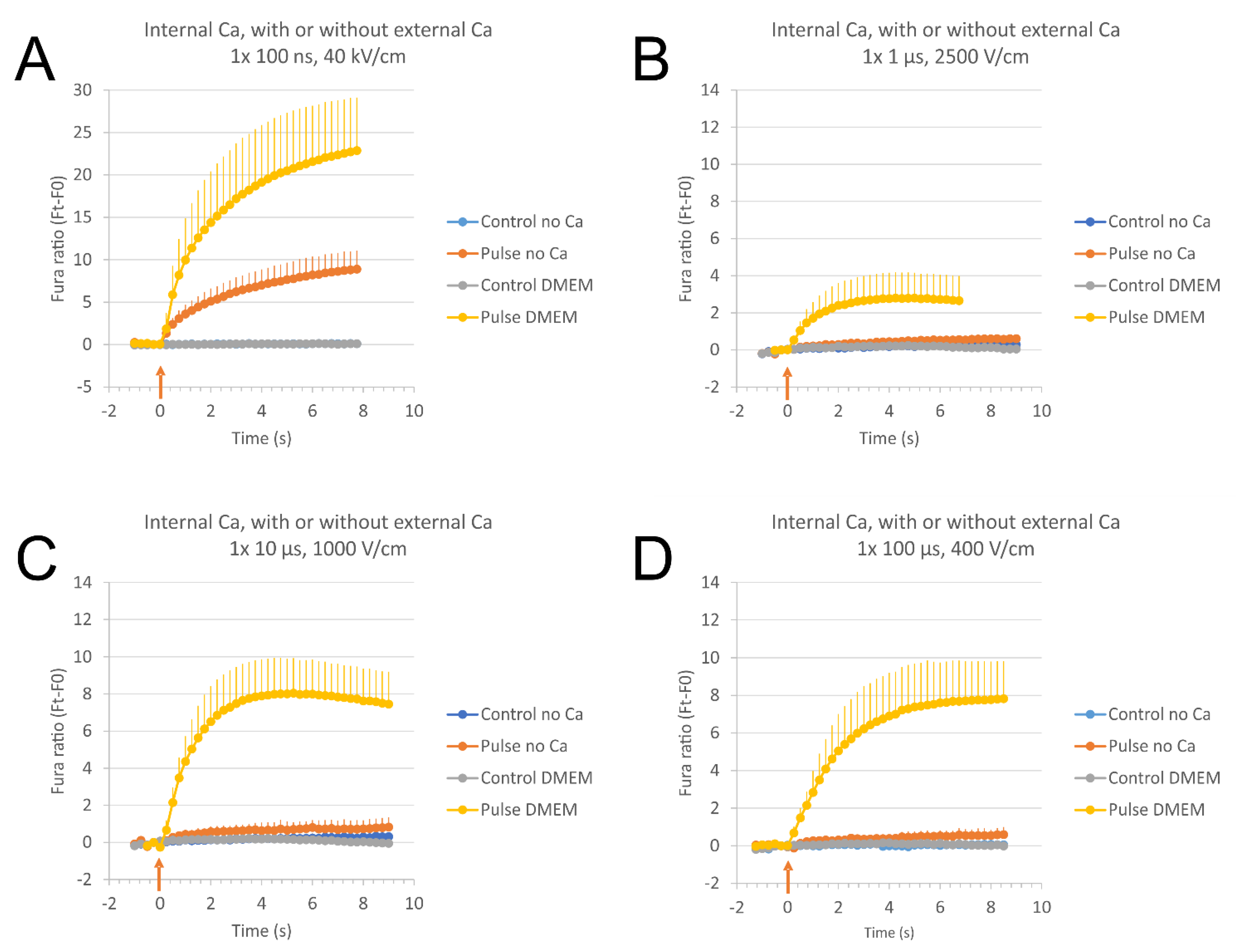
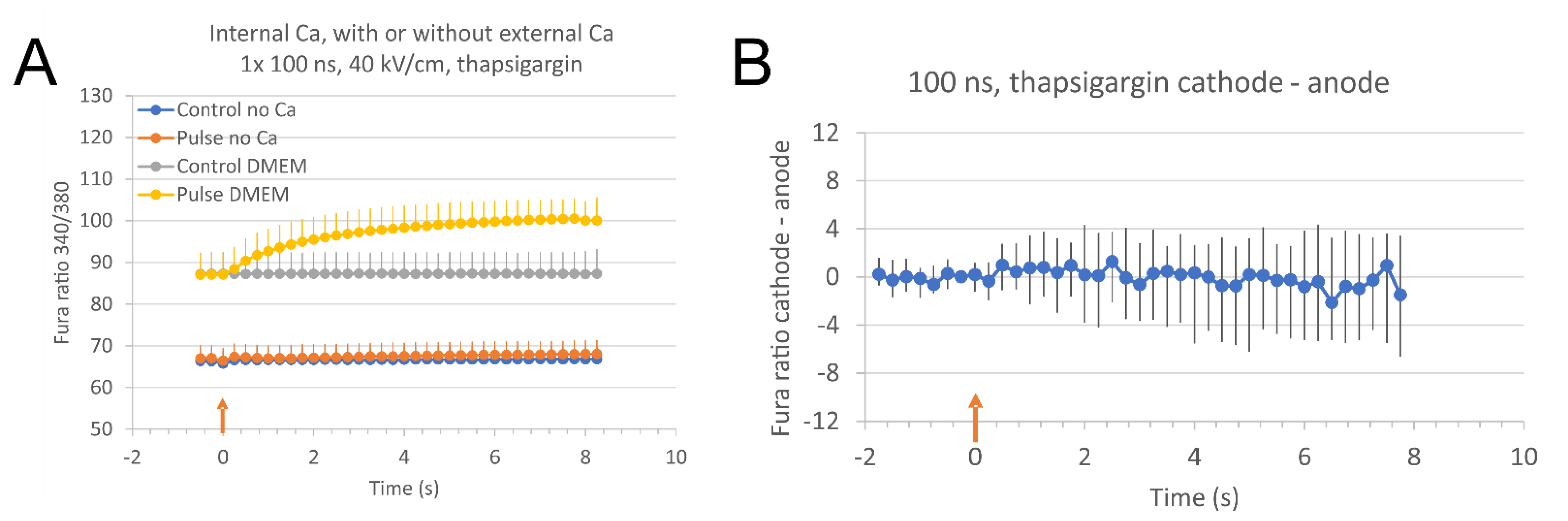
2.3. The Source of Ca2+ for Calcium Concentration Elevation after Electroporation
2.4. The Direction of Calcium Ions Uptake after Pulse Exposure to Low Electric Field and Reversed Polarity 100 μs Pulses
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Detection of Cell Relative Calcium Concentration with Fura-2 AM
4.3. Exposure of Cells to Electric Pulses
4.4. Image Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| EP | electroporation |
| PI | propidium iodide |
| VGCC | voltage-gated calcium channels |
| nsEP | electroporation with nanosecond electric pulses |
| AM | acetoxymethyl |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| PS | phosphatidylserine |
| ER | endoplasmic reticulum |
| ITV | induced transmembrane voltage |
| SOCE | store-operated (capacitive) Ca2+ entry |
| CICR | Ca-induced Ca2+ release |
References
- Kotnik, T.; Frey, W.; Sack, M.; Haberl Meglič, S.; Peterka, M.; Miklavčič, D. Electroporation-Based Applications in Biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- McBride, S.; Avazzadeh, S.; Wheatley, A.M.; O’Brien, B.; Coffey, K.; Elahi, A.; O’Halloran, M.; Quinlan, L.R. Ablation Modalities for Therapeutic Intervention in Arrhythmia-Related Cardiovascular Disease: Focus on Electroporation. J. Clin. Med. 2021, 10, 2657. [Google Scholar] [CrossRef]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Campana, L.G.; Edhemović, I.; Soden, D.; Perrone, A.M.; Scarpa, M.; Campanacci, L.; Čemažar, M.; Valpione, S.; Miklavčič, D.; Mocellin, S.; et al. Electrochemotherapy - Emerging Applications Technical Advances, New Indications, Combined Approaches, and Multi-Institutional Collaboration. Eur. J. Surg. Oncol. 2019, 45, 92–102. [Google Scholar] [CrossRef]
- Zorec, B.; Préat, V.; Miklavčič, D.; Pavšelj, N. Active Enhancement Methods for Intra- and Transdermal Drug Delivery: A Review. Slov. Med. J. 2013, 82, 339–356. [Google Scholar]
- Lambricht, L.; Lopes, A.; Kos, Š.; Serša, G.; Préat, V.; Vandermeulen, G. Clinical Potential of Electroporation for Gene Therapy and DNA Vaccine Delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering CRISPR: A Review of the Challenges and Approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef] [Green Version]
- Gehl, J. Electroporation: Theory and Methods, Perspectives for Drug Delivery, Gene Therapy and Research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Kinosita, K.; Tsong, T.Y. Formation and Resealing of Pores of Controlled Sizes in Human Erythrocyte Membrane. Nature 1977, 268, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Guionet, A.; Moosavi Nejad, S.; Teissié, J.; Sakugawa, T.; Katsuki, S.; Akiyama, H.; Hosseini, H. Spatio-Temporal Dynamics of Calcium Electrotransfer during Cell Membrane Permeabilization. Drug Deliv. Transl. Res. 2018, 8, 1152–1161. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Corbett, J.D.; Gimm, J.A.; Golan, D.E.; Langer, R.; Weaver, J.C. Millisecond Measurement of Transport during and after an Electroporation Pulse. Biophys. J. 1995, 68, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Puc, M.; Kotnik, T.; Mir, L.M.; Miklavcic, D. Quantitative Model of Small Molecules Uptake after in Vitro Cell Electropermeabilization. Bioelectrochemistry 2003, 60, 1–10. [Google Scholar] [CrossRef]
- Rols, M.P.; Teissié, J. Electropermeabilization of Mammalian Cells. Quantitative Analysis of the Phenomenon. Biophys. J. 1990, 58, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.C.; Weaver, J.C. Transmembrane Molecular Transport during versus after Extremely Large, Nanosecond Electric Pulses. Biochem. Biophys. Res. Commun. 2011, 412, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista Napotnik, T.; Miklavčič, D. In Vitro Electroporation Detection Methods—An Overview. Bioelectrochemistry 2018, 120, 166–182. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beebe, S.J.; Chen, Y.-J.; Sain, N.M.; Schoenbach, K.H.; Xiao, S. Transient Features in Nanosecond Pulsed Electric Fields Differentially Modulate Mitochondria and Viability. PLoS ONE 2012, 7, e51349. [Google Scholar] [CrossRef] [PubMed]
- Pucihar, G.; Krmelj, J.; Reberšek, M.; Napotnik, T.B.; Miklavčič, D. Equivalent Pulse Parameters for Electroporation. IEEE Trans. Biomed. Eng. 2011, 58, 3279–3288. [Google Scholar] [CrossRef] [Green Version]
- Semenov, I.; Zemlin, C.; Pakhomova, O.N.; Xiao, S.; Pakhomov, A.G. Diffuse, Non-Polar Electropermeabilization and Reduced Propidium Uptake Distinguish the Effect of Nanosecond Electric Pulses. Biochim. Biophys. Acta 2015, 1848, 2118–2125. [Google Scholar] [CrossRef] [Green Version]
- Craviso, G.L.; Choe, S.; Chatterjee, P.; Chatterjee, I.; Vernier, P.T. Nanosecond Electric Pulses: A Novel Stimulus for Triggering Ca2+ Influx into Chromaffin Cells via Voltage-Gated Ca2+ Channels. Cell. Mol. Neurobiol. 2010, 30, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Xiao, S.; Kang, D.; Schoenbach, K.H.; Pakhomov, A.G. Cell Stimulation and Calcium Mobilization by Picosecond Electric Pulses. Bioelectrochemistry 2015, 105, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Vernier, P.T.; Sun, Y.; Chen, M.-T.; Gundersen, M.A.; Craviso, G.L. Nanosecond Electric Pulse-Induced Calcium Entry into Chromaffin Cells. Bioelectrochemistry 2008, 73, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Beier, H.T.; Roth, C.C.; Tolstykh, G.P.; Ibey, B.L. Resolving the Spatial Kinetics of Electric Pulse-Induced Ion Release. Biochem. Biophys. Res. Commun. 2012, 423, 863–866. [Google Scholar] [CrossRef]
- Hanna, H.; Denzi, A.; Liberti, M.; André, F.M.; Mir, L.M. Electropermeabilization of Inner and Outer Cell Membranes with Microsecond Pulsed Electric Fields: Quantitative Study with Calcium Ions. Sci. Rep. 2017, 7, 13079. [Google Scholar] [CrossRef]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Salemi, S.; Craft, C.M.; Gundersen, M.A. Calcium Bursts Induced by Nanosecond Electric Pulses. Biochem. Biophys. Res. Commun. 2003, 310, 286–295. [Google Scholar] [CrossRef]
- Semenov, I.; Xiao, S.; Pakhomov, A.G. Primary Pathways of Intracellular Ca(2+) Mobilization by Nanosecond Pulsed Electric Field. Biochim. Biophys. Acta 2013, 1828, 981–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenov, I.; Xiao, S.; Pakhomova, O.N.; Pakhomov, A.G. Recruitment of the Intracellular Ca2+ by Ultrashort Electric Stimuli: The Impact of Pulse Duration. Cell Calcium 2013, 54, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.A.; Blackmore, P.F.; Schoenbach, K.H.; Beebe, S.J. Stimulation of Capacitative Calcium Entry in HL-60 Cells by Nanosecond Pulsed Electric Fields. J. Biol. Chem. 2004, 279, 22964–22972. [Google Scholar] [CrossRef] [Green Version]
- Dermol-Černe, J.; Batista Napotnik, T.; Reberšek, M.; Miklavčič, D. Short Microsecond Pulses Achieve Homogeneous Electroporation of Elongated Biological Cells Irrespective of Their Orientation in Electric Field. Sci. Rep. 2020, 10, 9149. [Google Scholar] [CrossRef]
- Kotnik, T.; Pucihar, G.; Miklavcic, D. Induced Transmembrane Voltage and Its Correlation with Electroporation-Mediated Molecular Transport. J. Membr. Biol. 2010, 236, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Rems, L.; Miklavčič, D. Tutorial: Electroporation of Cells in Complex Materials and Tissue. J. Appl. Phys. 2016, 119, 201101. [Google Scholar] [CrossRef]
- Hibino, M.; Itoh, H.; Kinosita, K. Time Courses of Cell Electroporation as Revealed by Submicrosecond Imaging of Transmembrane Potential. Biophys. J. 1993, 64, 1789–1800. [Google Scholar] [CrossRef] [Green Version]
- Hibino, M.; Shigemori, M.; Itoh, H.; Nagayama, K.; Kinosita, K. Membrane Conductance of an Electroporated Cell Analyzed by Submicrosecond Imaging of Transmembrane Potential. Biophys. J. 1991, 59, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Sowers, A.E. Fusion Events and Nonfusion Contents Mixing Events Induced in Erythrocyte Ghosts by an Electric Pulse. Biophys. J. 1988, 54, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Sowers, A.E.; Lieber, M.R. Electropore Diameters, Lifetimes, Numbers, and Locations in Individual Erythrocyte Ghosts. FEBS Lett. 1986, 205, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Sözer, E.B.; Pocetti, C.F.; Vernier, P.T. Asymmetric Patterns of Small Molecule Transport After Nanosecond and Microsecond Electropermeabilization. J. Membr. Biol. 2018, 251, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Tekle, E.; Astumian, R.D.; Chock, P.B. Selective and Asymmetric Molecular Transport across Electroporated Cell Membranes. Proc. Natl. Acad. Sci. USA 1994, 91, 11512–11516. [Google Scholar] [CrossRef] [Green Version]
- Bo, W.; Silkunas, M.; Mangalanathan, U.; Novickij, V.; Casciola, M.; Semenov, I.; Xiao, S.; Pakhomova, O.N.; Pakhomov, A.G. Probing Nanoelectroporation and Resealing of the Cell Membrane by the Entry of Ca2+ and Ba2+ Ions. Int. J. Mol. Sci. 2020, 21, 3386. [Google Scholar] [CrossRef]
- Cheek, E.R.; Fast, V.G. Nonlinear Changes of Transmembrane Potential During Electrical Shocks: Role of Membrane Electroporation. Circ. Res. 2004, 94, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djuzenova, C.S.; Zimmermann, U.; Frank, H.; Sukhorukov, V.L.; Richter, E.; Fuhr, G. Effect of Medium Conductivity and Composition on the Uptake of Propidium Iodide into Electropermeabilized Myeloma Cells. Biochim. Biophys. Acta 1996, 1284, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, B.; Teissié, J. Time Courses of Mammalian Cell Electropermeabilization Observed by Millisecond Imaging of Membrane Property Changes during the Pulse. Biophys. J. 1999, 76, 2158–2165. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, B.; Teissié, J. Direct Observation in the Millisecond Time Range of Fluorescent Molecule Asymmetrical Interaction with the Electropermeabilized Cell Membrane. Biophys. J. 1997, 73, 2630–2637. [Google Scholar] [CrossRef] [Green Version]
- Klauke, N.; Smith, G.; Cooper, J.M. Regional Electroporation of Single Cardiac Myocytes in a Focused Electric Field. Anal. Chem. 2010, 82, 585–592. [Google Scholar] [CrossRef]
- Mehrle, W.; Zimmermann, U.; Hampp, R. Evidence for a Symmetrical Uptake of Fluorescent Dyes through Electro-Permeabilized Membranes of Avena Mesophyll Protoplasts. FEBS Lett. 1985, 185, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Michel, O.; Pakhomov, A.G.; Casciola, M.; Saczko, J.; Kulbacka, J.; Pakhomova, O.N. Electropermeabilization Does Not Correlate with Plasma Membrane Lipid Oxidation. Bioelectrochemistry 2020, 132, 107433. [Google Scholar] [CrossRef]
- Sun, Y.H.; Vernier, P.T.; Behrend, M.; Wang, J.J.; Thu, M.M.; Gundersen, M.; Marcu, L. Fluorescence Microscopy Imaging of Electroperturbation in Mammalian Cells. J. Biomed. Opt. 2006, 11, 024010. [Google Scholar] [CrossRef]
- Sweeney, D.C.; Reberšek, M.; Dermol, J.; Rems, L.; Miklavčič, D.; Davalos, R.V. Quantification of Cell Membrane Permeability Induced by Monopolar and High-Frequency Bipolar Bursts of Electrical Pulses. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2689–2698. [Google Scholar] [CrossRef]
- Teruel, M.N.; Meyer, T. Electroporation-Induced Formation of Individual Calcium Entry Sites in the Cell Body and Processes of Adherent Cells. Biophys. J. 1997, 73, 1785–1796. [Google Scholar] [CrossRef] [Green Version]
- Vernier, P.T.; Sun, Y.; Gundersen, M.A. Nanoelectropulse-Driven Membrane Perturbation and Small Molecule Permeabilization. BMC Cell Biol. 2006, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, J.; Chen, M.-T.; Vernier, P.T.; Gundersen, M.A.; Valderrábano, M. Cardiac Myocyte Excitation by Ultrashort High-Field Pulses. Biophys. J. 2009, 96, 1640–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saulis, G. Cell Electroporation: Part 3. Theoretical Investigation of the Appearance of Asymmetric Distribution of Pores on the Cell and Their Further Evolution. Bioelectrochem. Bioenerg. 1993, 32, 249–265. [Google Scholar] [CrossRef]
- Tekle, E.; Astumian, R.D.; Chock, P.B. Electro-Permeabilization of Cell Membranes: Effect of the Resting Membrane Potential. Biochem. Biophys. Res. Commun. 1990, 172, 282–287. [Google Scholar] [CrossRef]
- Kinosita, K.; Itoh, H.; Ishiwata, S.; Hirano, K.; Nishizaka, T.; Hayakawa, T. Dual-View Microscopy with a Single Camera: Real-Time Imaging of Molecular Orientations and Calcium. J. Cell Biol. 1991, 115, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucihar, G.; Kotnik, T.; Teissié, J.; Miklavčič, D. Electropermeabilization of Dense Cell Suspensions. Eur. Biophys. J. 2007, 36, 173–185. [Google Scholar] [CrossRef]
- Camello, C.; Lomax, R.; Petersen, O.H.; Tepikin, A.V. Calcium Leak from Intracellular Stores—the Enigma of Calcium Signalling. Cell Calcium 2002, 32, 355–361. [Google Scholar] [CrossRef]
- Michelangeli, F.; East, J.M. A Diversity of SERCA Ca2+ Pump Inhibitors. Biochem. Soc. Trans. 2011, 39, 789–797. [Google Scholar] [CrossRef]
- Antigny, F.; Jousset, H.; König, S.; Frieden, M. Thapsigargin Activates Ca2+ Entry Both by Store-Dependent, STIM1/Orai1-Mediated, and Store-Independent, TRPC3/PLC/PKC-Mediated Pathways in Human Endothelial Cells. Cell Calcium 2011, 49, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Reberšek, M.; Faurie, C.; Kandušer, M.; Čorović, S.; Teissié, J.; Rols, M.-P.; Miklavčič, D. Electroporator with Automatic Change of Electric Field Direction Improves Gene Electrotransfer In-Vitro. Biomed. Eng. Online 2007, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Knisley, S.B.; Grant, A.O. Asymmetrical Electrically Induced Injury of Rabbit Ventricular Myocytes. J. Mol. Cell. Cardiol. 1995, 27, 1111–1122. [Google Scholar] [CrossRef]
- Li, J.; Lin, H. Numerical Simulation of Molecular Uptake via Electroporation. Bioelectrochemistry 2011, 82, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Sözer, E.B.; Pocetti, C.F.; Vernier, P.T. Transport of Charged Small Molecules after Electropermeabilization — Drift and Diffusion. BMC Biophys. 2018, 11, 4. [Google Scholar] [CrossRef]
- Cheng, D.K.-L.; Tung, L.; Sobie, E.A. Nonuniform Responses of Transmembrane Potential during Electric Field Stimulation of Single Cardiac Cells. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H351–H362. [Google Scholar] [CrossRef]
- Hescheler, J.; Meyer, R.; Plant, S.; Krautwurst, D.; Rosenthal, W.; Schultz, G. Morphological, Biochemical, and Electrophysiological Characterization of a Clonal Cell (H9c2) Line from Rat Heart. Circ. Res. 1991, 69, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Kimes, B.W.; Brandt, B.L. Properties of a Clonal Muscle Cell Line from Rat Heart. Exp. Cell Res. 1976, 98, 367–381. [Google Scholar] [CrossRef]
- Watkins, S.J.; Borthwick, G.M.; Arthur, H.M. The H9C2 Cell Line and Primary Neonatal Cardiomyocyte Cells Show Similar Hypertrophic Responses in Vitro. Vitr. Cell. Dev. Biol. Animal 2011, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Frey, W.; White, J.A.; Price, R.O.; Blackmore, P.F.; Joshi, R.P.; Nuccitelli, R.; Beebe, S.J.; Schoenbach, K.H.; Kolb, J.F. Plasma Membrane Voltage Changes during Nanosecond Pulsed Electric Field Exposure. Biophys. J. 2006, 90, 3608–3615. [Google Scholar] [CrossRef] [Green Version]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Craft, C.M.; Gundersen, M.A. Nanosecond Pulsed Electric Fields Perturb Membrane Phospholipids in T Lymphoblasts. FEBS Lett. 2004, 572, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rems, L.; Kasimova, M.A.; Testa, I.; Delemotte, L. Pulsed Electric Fields Can Create Pores in the Voltage Sensors of Voltage-Gated Ion Channels. Biophys. J. 2020, 119, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Diederich, M.; Ghibelli, L. The Dual Role of Calcium as Messenger and Stressor in Cell Damage, Death, and Survival. Available online: https://www.hindawi.com/journals/ijcb/2010/546163/ (accessed on 9 November 2020).
- Ciobanu, F.; Golzio, M.; Kovacs, E.; Teissié, J. Control by Low Levels of Calcium of Mammalian Cell Membrane Electropermeabilization. J. Membr. Biol. 2018, 251, 221–228. [Google Scholar] [CrossRef]
- Navickaite, D.; Ruzgys, P.; Novickij, V.; Jakutaviciute, M.; Maciulevicius, M.; Sinceviciute, R.; Satkauskas, S. Extracellular-Ca2+-Induced Decrease in Small Molecule Electrotransfer Efficiency: Comparison between Microsecond and Nanosecond Electric Pulses. Pharmaceutics 2020, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Batista Napotnik, T.; Reberšek, M.; Kotnik, T.; Lebrasseur, E.; Cabodevila, G.; Miklavčič, D. Electropermeabilization of Endocytotic Vesicles in B16 F1 Mouse Melanoma Cells. Med. Biol. Eng. Comput. 2010, 48, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Reberšek, M.; Miklavčič, D. Advantages and Disadvantages of Different Concepts of Electroporation Pulse Generation. Automatika 2011, 52, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Dermol-Černe, J.; Miklavčič, D.; Reberšek, M.; Mekuč, P.; Bardet, S.M.; Burke, R.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R. Plasma Membrane Depolarization and Permeabilization Due to Electric Pulses in Cell Lines of Different Excitability. Bioelectrochemistry 2018, 122, 103–114. [Google Scholar] [CrossRef] [PubMed]


| Reference | Pulse Duration Range | Pulse Parameters | Detection Molecule/Ion | Preferential Electrode Uptake | Cells |
|---|---|---|---|---|---|
| Beier 2012 [26] | ns | 1 × 600 ns, 50 kV/cm | Ca2+ | C− | NG108-15 rodent neuroblastoma |
| Semenov 2015 [22] | ns, ms | 1 × 10 ns, 270 kV/cm | Ca2+ | Symmetrical | Embryonic rat cardiac myocytes |
| 1 × 4 ms, 1100 V/cm | Ca2+ | A+ | |||
| Bo 2020 [41] | ns | 1 × 600 ns, 10 kV/cm | Ca2+, Ba2+ | A+ | HEK human epithelial kidney cells |
| Sözer 2018 [39] | ns, µs | 1 × 6 ns, 200 kV/cm | YO-PRO-1, PI | A+ | U-937 human histiocytic lymphoma monocyte |
| 1 × 6 ns, 200 kV/cm | Calcein | Symmetrical | |||
| 1 × 220 µs, 2.5 kV/cm | YO-PRO-1, PI | C− | |||
| 1 × 220 µs, 2.5 kV/cm | Calcein | Symmetrical | |||
| Sun 2006 [49] | ns, ms | 1 × 30 ns, 25 kV/cm | Ca2+ | Symmetrical | Jurkat Human T lymphocytes |
| 1 × 5 ms, 1 kV/cm | PI | A+ | |||
| Vernier 2006 [52] | ns | 100 × 4 ns, 80 kV/cm, 1 kHz | YO-PRO-1 | A+ | Jurkat Human T lymphocytes |
| Michel 2020 [48] | ns, µs | 1 × 300 ns, 4.8–8.4 kV/cm 1 × 100 μs, 480–720 V/cm 8 × 100 μs, 240–480 V/cm, 5 kHz | Ca2+, YO-PRO-1 | A+ | CHO Chinese hamster ovary cells |
| Wang 2009 [53] | ns | 4 ns, 10–80 kV/cm | Ca2+ | A+ | Rat ventricular myocytes |
| Djuzenova 1996 [43] | µs | Exponentially decaying pulse, time constant of 40 μs, 2–6 kV/cm | PI | A+ | Sp2/0-Agl4 murine myeloma |
| Gabriel 1997 [45] | µs, ms | 1 × 240 µs, 1.5 kV/cm | PI | Symmetrical | CHO Chinese hamster ovary cells |
| 1 × 0.5–3 ms, 1.5 kV/cm | PI | A+ | |||
| 1 × 20 ms, 500–1200 V/cm | PI | A+, C− | |||
| Guionet 2018 [12] | µs | 6–278 × 10 μs, 270–1800 V/cm, 1 Hz | Ca2+ | A+ | HeLa human cervical cancer |
| Hibino 1991 [36] | µs | 1 × 10 μs, 100 V/cm | Ca2+, envelope | C− | Sea urchin egg |
| Hibino 1993 [35] | µs | 1 × 100 μs, 400 V/cm | Ca2+, envelope | C− | Sea urchin egg |
| 1 × 400 μs, 400 V/cm | Ca2+, envelope | A+ | |||
| Kinosita 1991 [56] | µs | 1 × 400 μs, 400 V/cm | Ca2+ | C− | Sea urchin egg |
| Mehrle 1985 [47] | µs | 1 × 10 µs, 2.3 kV/cm 1 × 20 µs, 1 kV/cm 1 × 40 µs, 1 kV/cm 1 × 60 µs, 500 V/cm | Ethidium bromide, berberine hemisulfate, fluorescein diacetate | A+ | Oat mesophyll protoplasts |
| Sowers 1988 [37] | µs | Exponentially decaying pulse, 600 μs ms decay halftime, 7 kV/cm | FITC dextran | C− | Human erythrocyte ghosts |
| Tekle 1990 [55] | µs | 1 × 400 μs, 5 kV/cm | Ethidium bromide | A+ | NIH 3T3 fibroblasts |
| Tekle 1994 [40] | µs, ms | 1 × 250 μs, 1.2 kV/cm (similar results also for 10 μs and 1 ms pulses) | PI, ethidium homodimer EthD-1 | C− | CHO, HeLa, NIH 3T3 |
| Ethidium bromide, Ca2+ in high salt medium | C− | ||||
| Ethidium bromide, Ca2+ in low salt medium | A+ | ||||
| Sweeney 2016 [50] | µs | 100 × 100 μs, 1250 V/cm, 2 kHz 200 × 100 μs, 750 V/cm, 2 kHz | PI | A+ | CHO Chinese hamster ovary cells |
| Cheek 2004 [42] | ms | 1 × 10 ms, 10 V/cm | PI | A+ | Rat ventricular myocytes |
| Gabriel 1999 [44] | ms | 1 × 1.2–16 ms, 1.2 kV/cm | Ca2+ leak out, PI | A+ | CHO, HeLa |
| Klauke 2010 [46] | ms | 1 × 4 ms, 40–68 V/cm | Ca2+, SNARF-1 dextran | A+ | Primary rabbit ventricular myocytes |
| Reberšek 2007 [61] | ms | 8 × 1 ms, 100–400 V, 1 Hz | DNA plasmid | C− | CHO Chinese hamster ovary cells |
| Sowers 1986 [38] | ms | 1 × 1.2 ms, 7 kV/cm | FITC dextran | C− | Human erythrocyte ghosts |
| Teruel 1997 [51] | ms | 1 × 5–200 ms, 167–340 V/cm | Ca2+ | A+ | 2H3 rat basophilic leukemia cells, neocortical neuroblastoma cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista Napotnik, T.; Miklavčič, D. Pulse Duration Dependent Asymmetry in Molecular Transmembrane Transport Due to Electroporation in H9c2 Rat Cardiac Myoblast Cells In Vitro. Molecules 2021, 26, 6571. https://doi.org/10.3390/molecules26216571
Batista Napotnik T, Miklavčič D. Pulse Duration Dependent Asymmetry in Molecular Transmembrane Transport Due to Electroporation in H9c2 Rat Cardiac Myoblast Cells In Vitro. Molecules. 2021; 26(21):6571. https://doi.org/10.3390/molecules26216571
Chicago/Turabian StyleBatista Napotnik, Tina, and Damijan Miklavčič. 2021. "Pulse Duration Dependent Asymmetry in Molecular Transmembrane Transport Due to Electroporation in H9c2 Rat Cardiac Myoblast Cells In Vitro" Molecules 26, no. 21: 6571. https://doi.org/10.3390/molecules26216571
APA StyleBatista Napotnik, T., & Miklavčič, D. (2021). Pulse Duration Dependent Asymmetry in Molecular Transmembrane Transport Due to Electroporation in H9c2 Rat Cardiac Myoblast Cells In Vitro. Molecules, 26(21), 6571. https://doi.org/10.3390/molecules26216571






