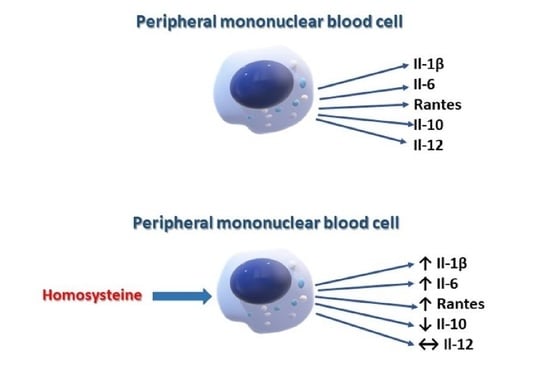
The Effect of Homocysteine on the Secretion of Il-1β, Il-6, Il-10, Il-12 and RANTES by Peripheral Blood Mononuclear Cells—An In Vitro Study
Abstract
:1. Introduction
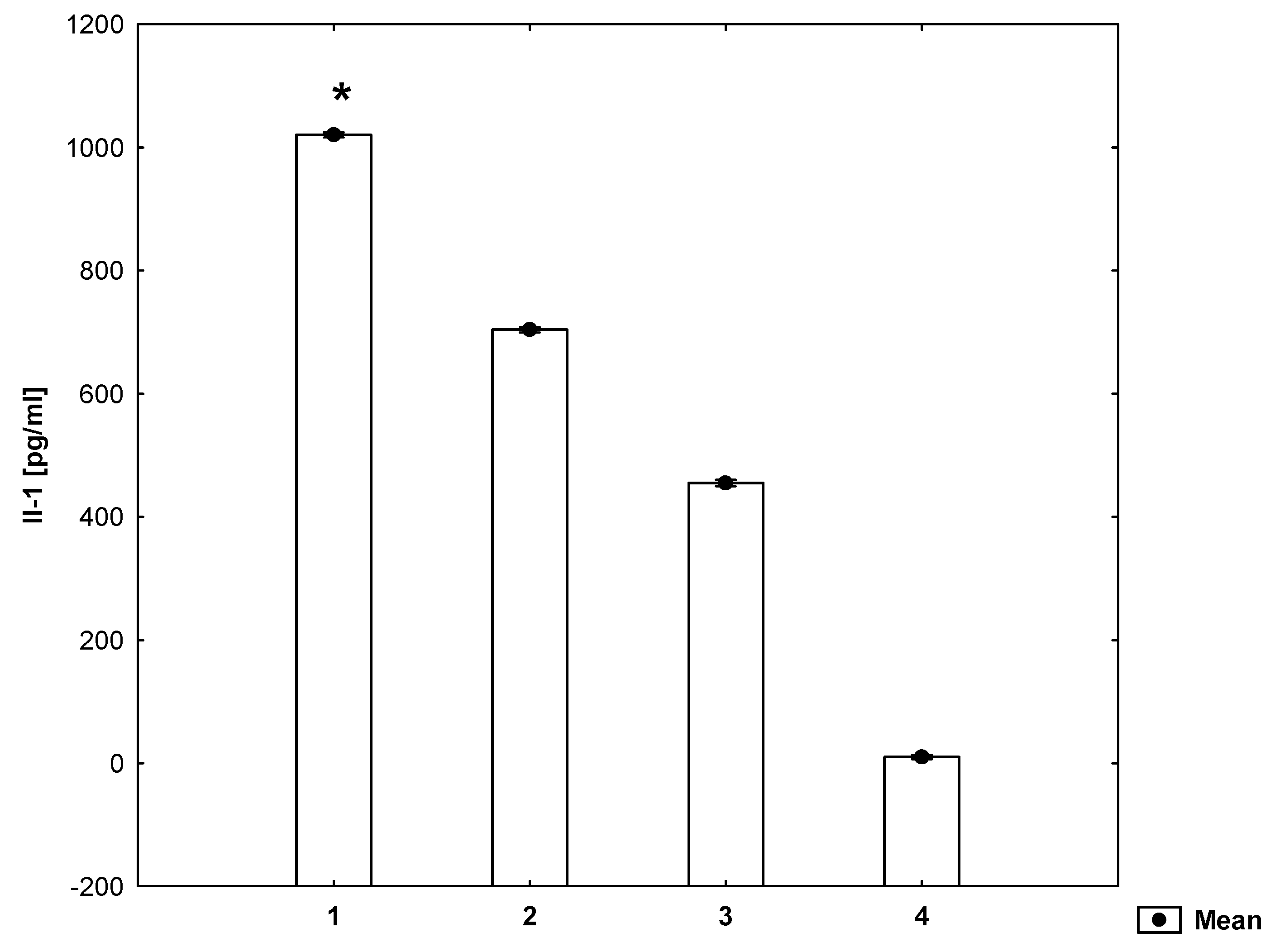
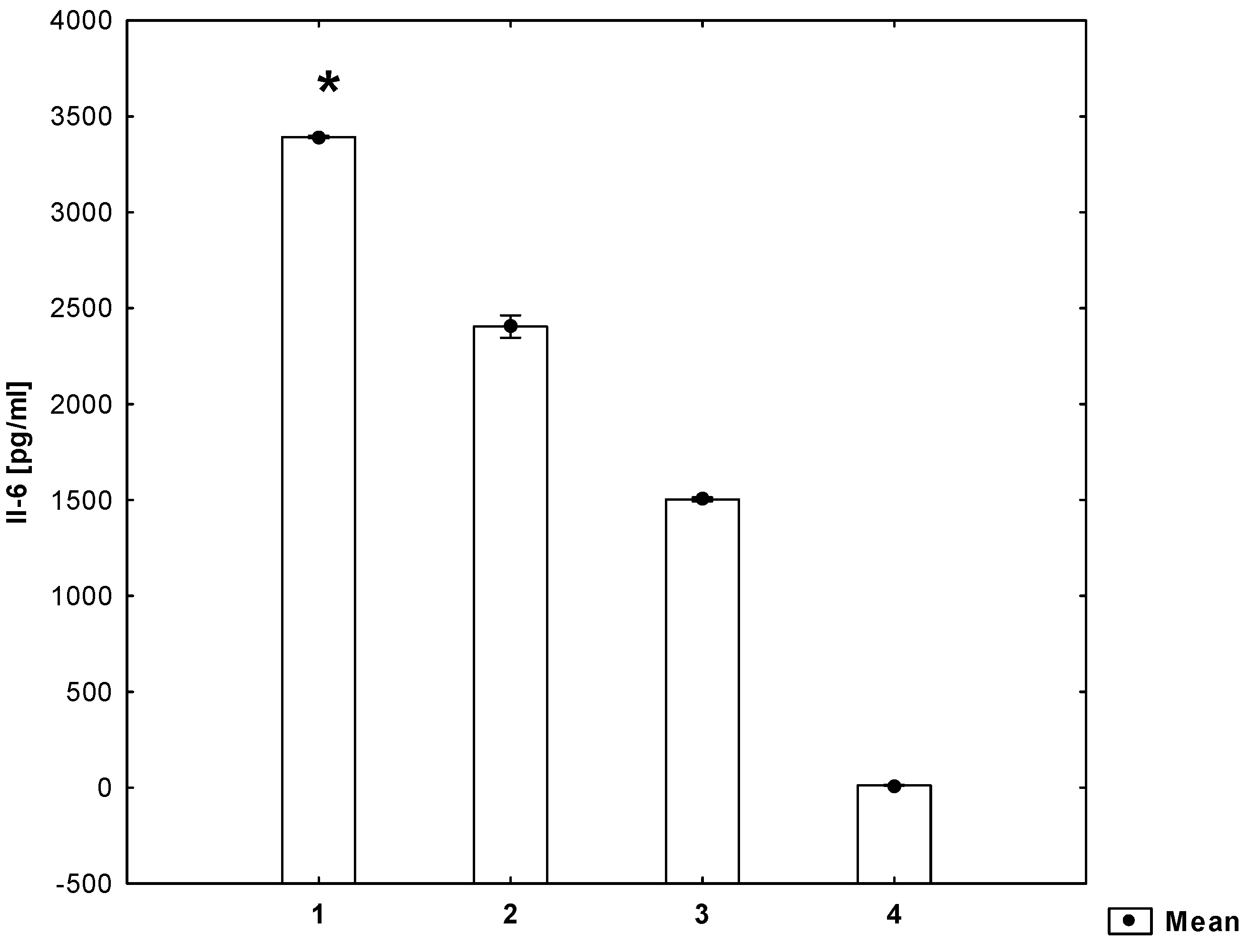
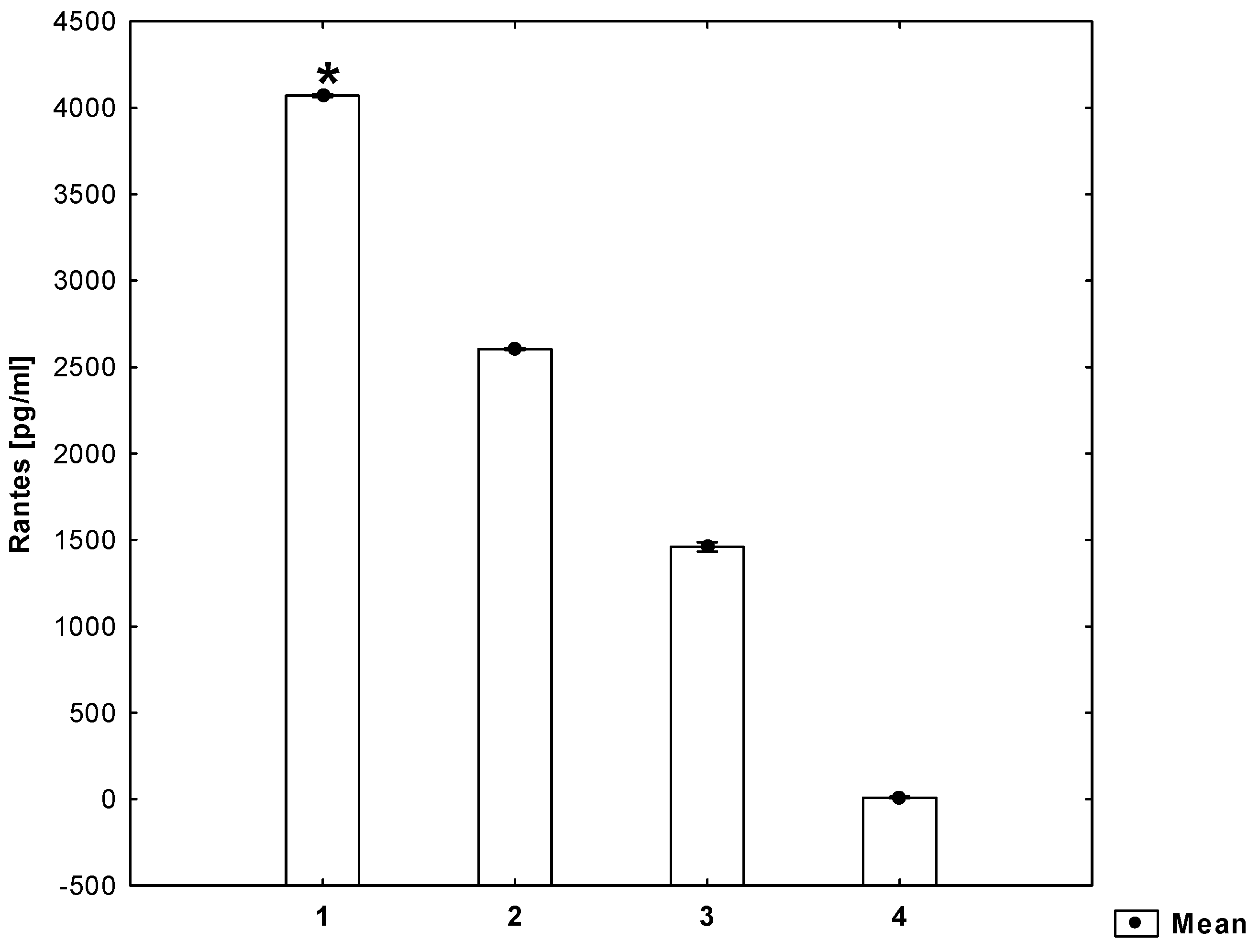
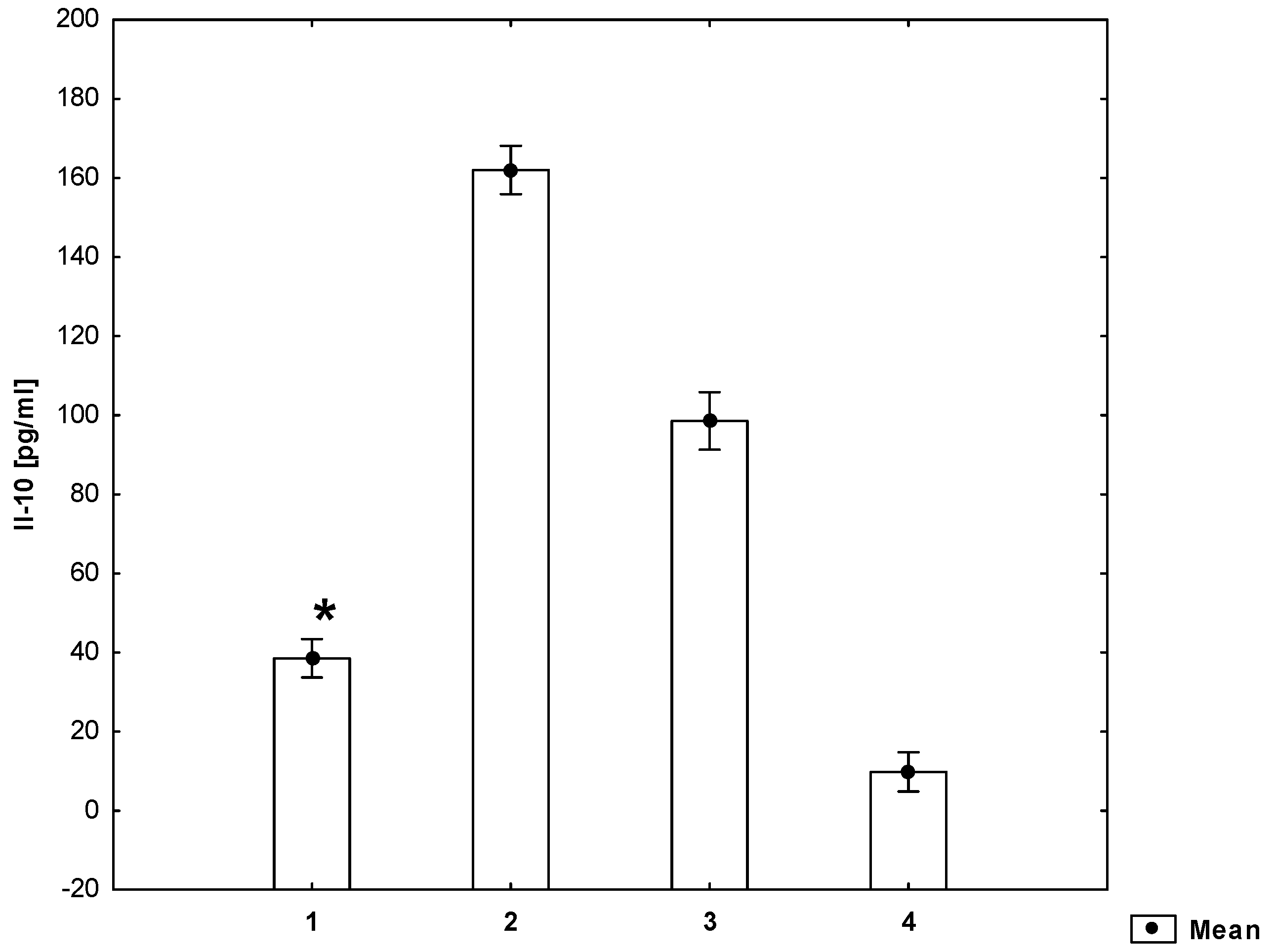
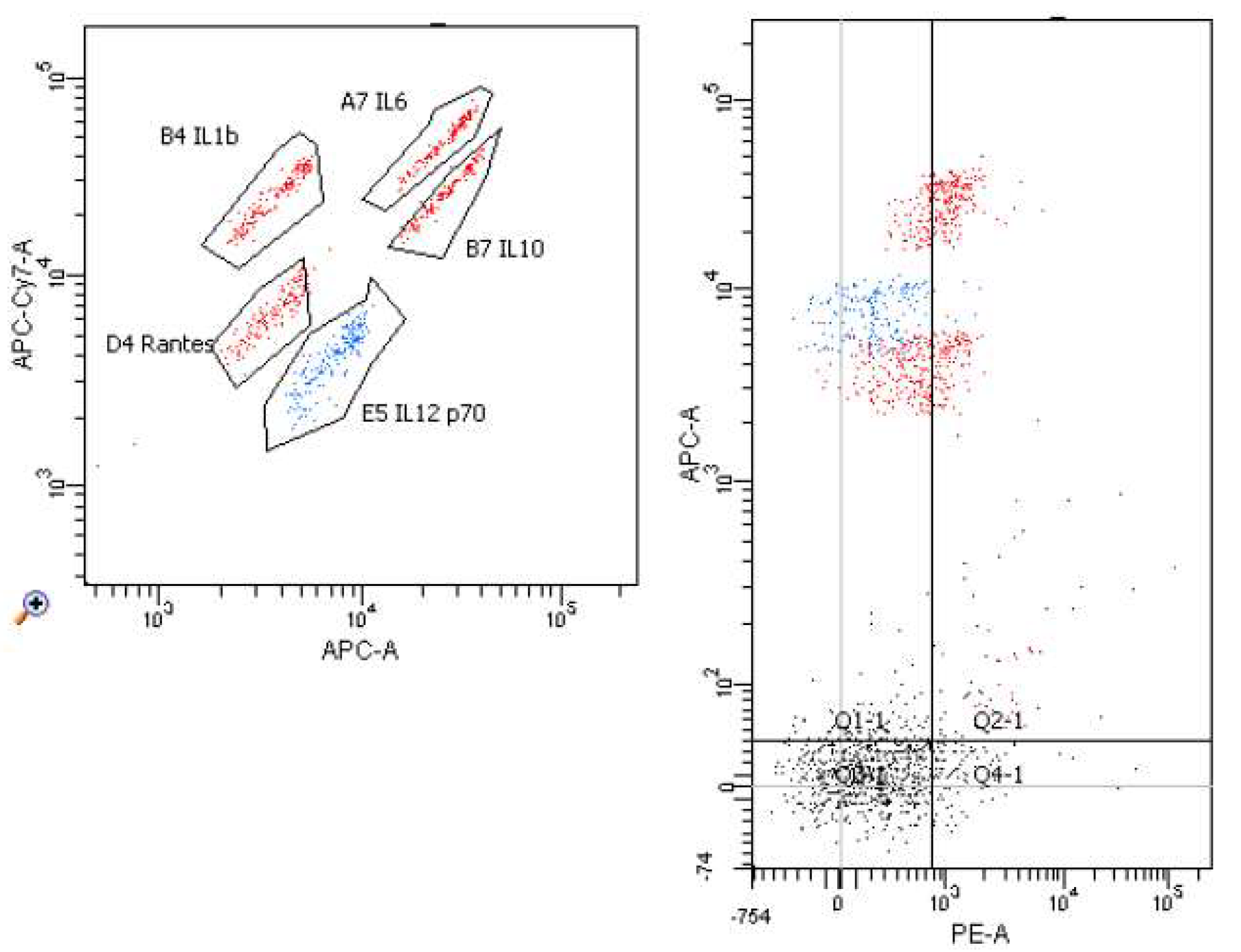
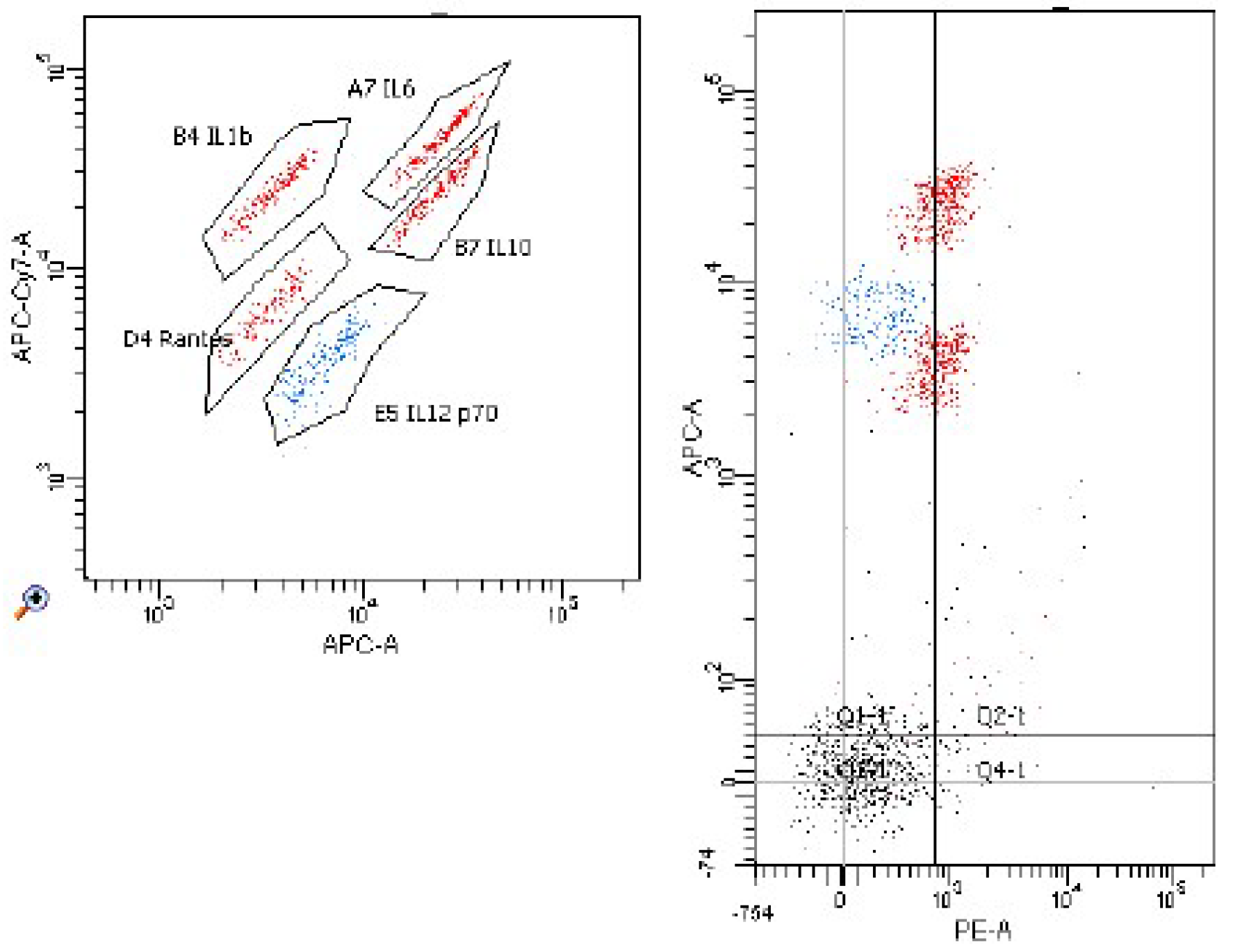
2. Results
3. Discussion
4. Materials and Methods
4.1. Examined Subjects
4.2. The Isolation of Lymphocytes and Peripheral Blood Monocytes
4.3. Cell Cultures
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. 2020. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, 139–596. [Google Scholar] [CrossRef]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Mali, V.; Haddox, S.; AbdelGhany, S.M.; El-deek, S.E.M.; Abulfadl, A.; Khalid Matrougui, K.; Belmadani, S. Essential Role of IL-12 in Angiogenesis in Type 2 Diabetes. Am. J. Clin. Pathol. 2017, 187, 2590–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Herder, C.; Illig, T.; Baumert, J.; Müller, M.; Klopp, N.; Khuseyinova, N.; Meisinger, C.; Poschen, U.; Martin, S.; Koenig, W.; et al. RANTES/CCl5 gene polymorphisms, serum concentrations, and incident type 2 diabetes: Result from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Eur. J. Endocrinol. 2008, 158, R1–R5. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Virani, S.S.; Nambi, V.; Hoogeveen, R.; Wasserman, B.A.; Coresh, J.; Gonzalez, F.; Chambless, L.E.; Mosley, T.H.; Boerwinkle, B.; Ballantyne, C.M. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: The Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur. Heart J. 2011, 32, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Gokkusu, C.; Tulubas, F.; Unlucerci, Y.; Ozkok, E.; Umman, B.; Aydin, M. Homocysteine and pro-inflammatory cytokine concentrations in acute heart disease. Cytokine 2010, 50, 15–18. [Google Scholar] [CrossRef]
- Holven, K.B.; Aukrust, P.; Retterstol, K.; Hagve, T.A.; Mørkrid, L.; Ose, L.; Nenseter, M.S. Increased levels of C-reactive protein and interleukin-6 in hyperhomocysteinemic subjects. Scand. J. Clin. Lab. Investig. 2006, 66, 45–54. [Google Scholar] [CrossRef]
- Su, S.J.; Huang, L.W.; Pai, L.S.; Liu, H.W.; Chang, K.L. Homocysteine at pathophysiologic concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrient 2005, 21, 994–1002. [Google Scholar] [CrossRef]
- Dawson, H.; Collins, G.; Pyle, R.; Deep-Dixit, V.; Taub, D.D. The immunoregulatory effects of homocysteine and its intermediates on T-lymphocyte function. Mech. Ageing Dev. 2004, 125, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Holven, K.B.; Aukrust, P.; Holm, T.; Ose, L.; Nenseter, M.S. Folic acid treatment reduces chemokine release from peripheral blood mononuclear cells in hyperhomocysteinemic subjects. Arterioscler. Thromb. Vasc Biol. 2002, 22, 699–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Wang, G.; Zhang, Z.M.; Zeng, X.K.; Wang, X. Chemokine RANTES is upregulated in monocytes from patients with hyperhomocysteinemia. Acta Pharmacol. Sin. 2005, 26, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation and atherosclerosis. J. Am. Coll. Cardiol. 2009, 1, 2129–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Packard, R.R.; Libby, P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin. Chem. 2008, 54, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Akalin, A.; Alatas, O.; Colak, O. Relation of plasma homocysteine levels to atherosclerotic vascular disease and inflammation markers in type 2 diabetic patients. Eur. J. Endocrinol. 2008, 158, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Kanno, K.; Hirata, Y.; Imai, T.; Iwashina, M.; Marumo, F. Regulation of inducible nitric oxide synthase gene by interleukin-1 beta in rat vascular endothelial cells. Am. J. Physiol. 1994, 267, 2318–2324. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Anti-inflammatory mechanism in vascular wall. Circ. Res. 2001, 88, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Von der Thüsen, J.H.; Kuiper, J.; Van Berkel, T.J.; Biessen, E.A. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol. Rev. 2003, 55, 133–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, C.M. Proinfalmmatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 2009, 48, 111–136. [Google Scholar]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caligiuri, G.; Rudling, M.; Ollivier, V.; Jacob, M.P.; Michel, J.B.; Hansson, O. Interleukin 10 Deficiency increases Atherosclerosis, Thrombosis, and Low-density Lipoproteins in Apolipoprotein E Knockout Mice. Mol. Med. 2003, 9, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernecke, A.; Weber, C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc. Res. 2010, 86, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.; Hackeng, T.M.; Weber, C. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005, 105, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Shanmugham, L.N.; Petrarca, C.; Castellani, M.L.; Frydas, S.; Vecchiet, J.; Conti, P.; Tete, S. Rantes potentiates human macrophage aggregation and activation responses to calcium ionophore (A23187) and activates arachidonic acid pathways. J. Biol. Regul. Homeost. Agents 2006, 20, 15–23. [Google Scholar]
- Barlic, J.; Murphy, M. ; Murphy, M. Chemokine regulation of atherosclerosis. J. Leukocyte Biol. 2009, 82, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Dong, J.; Lobe, C.G.; Gong, P.; Liu, J.; Liao, L. CCR5 facilitates endothelial progenitor cell recruitment and promotes the stabilization of atherosclerotic plaques in ApoE-/- mice. Stem. Cell Res. Ther. 2015, 19, 6–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial dysfunction: The link between homocysteine and hydrogen sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef]
- Signorello, M.G.; Segantin, A.; Passalacqua, M.; Leoncini, G. Homocysteine decreased platelet NO level via protein kinase C activation. Nitric Oxide 2009, 20, 104–113. [Google Scholar] [CrossRef]
- Gurda, D.; Handschuh, L.; Kotkowiak, W.; Jakubowski, H. Homocysteine thiolactone and N-homocysteinylated protein induce pro-atherogenic changes in gene expression in human vascular endothelial cells. Amino Acids 2015, 47, 1319–1339. [Google Scholar] [CrossRef] [Green Version]
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The contribution of homocysteine metabolism disruption to endothelial dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, H.; Oh, H.; Park, H.; Park, M.; Jang, Y.; Lee, M. Contribution of dietary intakes of antioxidants to homocysteine-induced low density lipoprotein (LDL) oxidation in atherosclerotic patients. Yonsei Med. J. 2010, 51, 526–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, P.; Sreyoshi, F.A. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Araki, A.; Hosoi, T.; Orimo, H.; Ito, H. Association of plasma homocysteine with serum interleukin-6 and C-peptide levels in patients with type 2 diabetes. Metabolism 2005, 54, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Ciment, S.; Jan, M.; Tran, T.; Pham, H.; Cueto, R.; Yang, X.F.; Wang, H. Homocysteine induces inflammatory transcriptional signaling in monocytes. Front. Biosci. 2013, 1, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Yideng, J.; Zhihong, L.; Jiantuan, X.; Jun, C.; Guizhong, L.; Shuren, W. Homocysteine-mediated PPAR alpha, gamma DNA methylation and its potential pathogenic mechanism in monocytes. DNA Cell Biol. 2008, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dievart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Maki, M.; Schmitt, M.J.; Hatanaka, M.; Verma, I.M. Tumor necrosis factor alpha-induced phosphorylation of I kappa B alpha is a signal for its degradation but not dissociation from NF-kappa B. Proc. Natl. Acad. Sci. USA 1994, 91, 12740–12744. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowska, M.; Winiarska, H.; Dworacka, M.; Wesołowska, A.; Dworacki, G.; Mikołajczak, P.Ł. The Effect of Homocysteine on the Secretion of Il-1β, Il-6, Il-10, Il-12 and RANTES by Peripheral Blood Mononuclear Cells—An In Vitro Study. Molecules 2021, 26, 6671. https://doi.org/10.3390/molecules26216671
Borowska M, Winiarska H, Dworacka M, Wesołowska A, Dworacki G, Mikołajczak PŁ. The Effect of Homocysteine on the Secretion of Il-1β, Il-6, Il-10, Il-12 and RANTES by Peripheral Blood Mononuclear Cells—An In Vitro Study. Molecules. 2021; 26(21):6671. https://doi.org/10.3390/molecules26216671
Chicago/Turabian StyleBorowska, Magdalena, Hanna Winiarska, Marzena Dworacka, Anna Wesołowska, Grzegorz Dworacki, and Przemysław Łukasz Mikołajczak. 2021. "The Effect of Homocysteine on the Secretion of Il-1β, Il-6, Il-10, Il-12 and RANTES by Peripheral Blood Mononuclear Cells—An In Vitro Study" Molecules 26, no. 21: 6671. https://doi.org/10.3390/molecules26216671
APA StyleBorowska, M., Winiarska, H., Dworacka, M., Wesołowska, A., Dworacki, G., & Mikołajczak, P. Ł. (2021). The Effect of Homocysteine on the Secretion of Il-1β, Il-6, Il-10, Il-12 and RANTES by Peripheral Blood Mononuclear Cells—An In Vitro Study. Molecules, 26(21), 6671. https://doi.org/10.3390/molecules26216671