Spectrally and Time-Resolved Fluorescence Imaging of 22-NBD-Cholesterol in Human Peripheral Blood Mononuclear Cells in Chronic Kidney Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Reagents
2.3. Analytical Procedures
2.4. Isolation of Human Peripheral Blood Mononuclear Cells (PBMC)
2.5. Fluorescence Staining and Incorporation of Membrane Cholesterol
2.6. Confocal Microscopy
2.7. Fluorescence Lifetime Imaging Microscopy (FLIM)
2.8. Statistical Analysis
3. Results
3.1. Clinical and Biochemical Parameters
3.2. Fluorescence of 22-NBD-Cholesterol in PBMC
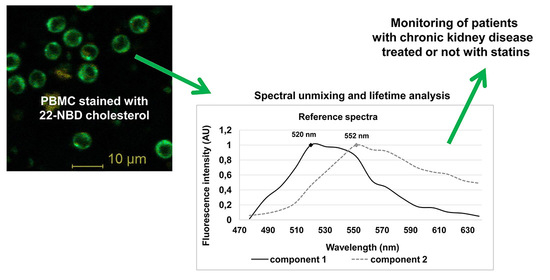
3.3. Spectrally Resolved Analysis of 22-NBD-Cholesterol in PBMC
3.4. Comparison of Groups of Probands by Spectrally Resolved Analysis
3.5. Fluorescence Lifetime Imaging Microscopy (FLIM)
3.6. Comparison of Groups of Probands by Time-Resolved Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chattopadhyay, A. Chemistry and Biology of N-(7-nitrobenz-2-oxa-1, 3-diazol-4-yl)-Labeled Lipids: Fluorescent Probes of Biological and model Membranes. Chem. Phys. Lipids 1990, 53, 1–15. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chattopadhyay, A. Membrane Organization at Low Cholesterol Concentrations: A Study Using 7-Nitrobenz-2-oxa-1,3-diazol-4-yl-Labeled Cholesterol. Biochemistry 1996, 35, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Chattopadhyay, A. Transbilayer Organization of Membrane Cholesterol at Low Concentrations: Implications in Health and Disease. Biochim. Biophys. Acta 2010, 1808, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.A.; Chattopadhyay, A. Cholesterol: An Evergreen Molecule in Biology. Biomed. Spectrosc. Imaging 2016, 5, S55–S66. [Google Scholar] [CrossRef] [Green Version]
- Haldar, S.; Chattopadhyay, A. Application of NBD-Labeled Lipids in Membrane and Cell Biology. In Fluorescent Methods to Study Biological Membranes, 14th ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 37–50. [Google Scholar]
- Pucadyil, T.J.; Mukherjee, S.; Chattopadhyay, A. Organization and Dynamics of NBD-Labeled Lipids in Membranes Analyzed by Fluorescence Recovery after Photobleaching. J. Phys. Chem. B. 2007, 111, 1975–1983. [Google Scholar] [CrossRef]
- De Jong, P.E.; van der Velde, M.; Gansevoort, R.T.; Zoccali, C. Screening for Chronic Kidney Disease: Where Does Europe Go? Clin. J. Am. Soc. Nephrol. 2008, 3, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubina, P.; Lahera, V.; Luno, J. Oxidative Stress and Inflammation, a Link between Chronic Kidney Disease and Cardiovascular Disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [Green Version]
- Recio-Mayoral, A.; Banerjee, D.; Streather, C.H.; Kaski, J.C. Endothelial Dysfunction, Inflammation and Atherosclerosis in Chronic Kidney Disease—A Cross-Sectional Study of Predialysis, Dialysis and Kkidney-Transplantation Patients. Atherosclerosis 2011, 216, 446–451. [Google Scholar] [CrossRef]
- Reiss, A.B.; Voloshyna, I.; De Leon, J.; Miyawaki, N.; Mattana, J. Cholesterol Metabolism in CKD. Am. J. Kidney Dis. 2015, 66, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Bulbul, M.C.; Dagel, T.; Afsar, B.; Ulusu, N.N.; Kuwabara, M.; Covic, A.; Kanbay, M. Disorders of Lipid Metabolism in Chronic Kidney Disease. Blood Purif. 2018, 4, 144–152. [Google Scholar] [CrossRef]
- Vaziri, N.D. HDL Abnormalities in Nephrotic Syndrome and Chronic Kidney Disease. Nat. Rev. Nephrol. 2016, 12, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Wahl, P.; Ducasa, G.M.; Fornoni, A. Systemic and Renal Lipids in Kidney Disease Development and Progression. Am. J. Physiol. Ren. Physiol. 2015, 310, F433–F445. [Google Scholar] [CrossRef] [Green Version]
- Mikolasevic, I.; Žutelija, M.; Mavrinac, V.; Orlic, L. Dyslipidemia in Patients with Chronic Kidney Disease: Etiology and Management. Int. J. Nephrol. Renovasc. Dis. 2017, 10, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Tsimihodimos, V.; Mitrogianni, Z.; Elisaf, M. Dyslipidemia Associated with Chronic Kidney Disease. Open Cardiovasc. Med. J. 2011, 5, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Blaton, V. 8. Dyslipidemia at Chronic Renal Failure. EJIFCC 2009, 20, 59–66. [Google Scholar] [PubMed]
- Becker, W. Advanced Time-Correlated Single Photon Counting Applications; Springer Int. Publishing: New York, NY, USA, 2016; pp. 1–657. [Google Scholar]
- Chorvat, D., Jr.; Chorvatova, A. Multi-Wavelength Fluorescence Lifetime Spectroscopy: A New Approach to the Study of Endogenous Fluorescence in Living Cells and Tissues. Laser Phys. Lett. 2009, 6, 175–193. [Google Scholar] [CrossRef]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B.; Wheeler, D.; Levin, A.; Stevens, P.E.; Bilous, R.W.; Lamb, E.J.; Coresh, J.; et al. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013, 3, S1–S150. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Lajdova, I.; Chorvat, D., Jr.; Spustova, V.; Chorvatova, A. 4-Aminopyridine Activates Calcium Influx through Modulation of the Pore-Forming Purinergic Receptor in Human Peripheral Blood Mononuclear Cells. Can. J. Physiol. Pharm. 2004, 82, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Martináková, Z.; Horilová, J.; Lajdová, I.; Marček Chorvátová, A. Time Resolved Fluorescence Monitoring of Cholesterol in Peripheral Blood Mononuclear Cells. In Proceedings of the 19th Polish-Slovak-Czech Optical Conference on Wave and Quantum Aspects of Contemporary Optics, Jelenia Gora, Poland, 5 December 2014; p. 94411. [Google Scholar]
- Dickinson, M.E.; Bearman, G.; Tilie, S.; Lansford, R.; Fraser, S.E. Multi-spectral imaging and linear unmixing add a whole new dimension to laser scanning fluorescence microscopy. Biotechniques 2001, 31, 1274–1276. [Google Scholar] [CrossRef]
- NLopt. GipHub. Overwiew. Available online: https://nlopt.readthedocs.io/en/latest/ (accessed on 12 April 2019).
- Fan, H.Y.; Heerklotz, H. Digitonin Does Not Flip across Cholesterol-Poor Membranes. J. Colloid Interface Sci. 2017, 504, 283–293. [Google Scholar] [CrossRef]
- Robalo, J.R.; Prates Ramalho, J.P.; Loura, L.M.S. NBD-Labeled Cholesterol Analogues in Phospholipid Bilayers: Insights from Molecular Dynamics. J. Physic. Chem. B 2013, 117, 13731–13742. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W. Probes and Tags to Study Biomolecular Function: For Proteins, RNA and Membranes, 1st ed.; WILEY-VCH: Weinheim, Germany, 2008; pp. 1–193. [Google Scholar]
- Song, W.; Wang, W.; Wang, Y.; Dou., L.; Chen, L.; Yan, X. Characterization of Fluorescent NBD-Cholesterol Efflux in THP-1-Derived Macrophages. Mol. Med. Rep. 2015, 12, 5989–5996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostašov, P.; Sýkora, J.; Brejchová, J.; Olżyńska, A.; Hof, M.; Svoboda, P. FLIM Studies of 22- and 25-NBD-Cholesterol in Living HEK293 Cells: Plasma Membrane Change Induced by Cholesterol Depletion. Chem. Phys. Lipids 2013, 167–168, 62–69. [Google Scholar] [CrossRef]
- Sudji, I.R.; Subburaj, Y.; Frenkel, N.; García-Sáez, A.J.; Wink, M. Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol. Molecules 2015, 20, 20146–20160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D. Causes of Dysregulation of Lipid Metabolism in Chronic Renal Failure. Semin. Dial. 2009, 22, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Ramkumr, N.; Cheung, A.K.; Pappas, L.M.; Roberts, W.L.; Beddhu, S. Association of obesity with inflammation in chronic kidney disease: A cross-sectional study. J. Ren. Nutr. 2004, 14, 201–207. [Google Scholar] [CrossRef]
- Jacob, R.F.; Mason, R.P. Lipid Peroxidation Induces Cholesterol Domain Formation in Model Membranes. J. Biol. Chem. 2005, 280, 39380–39387. [Google Scholar] [CrossRef] [Green Version]
- Galiullina, L.F.; Scheidt, H.A.; Huster, D.; Aganov, A.; Klochkov, V. Interaction of statins with phospholipid bilayers studied by solid-state NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 2019, 1861, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Penkauskas, T.; Zentelyte, A.; Ganpule, S.; Valincius, G.; Preta, G. Pleiotropic effects of statins via interaction with the lipid bilayer: A combined approach. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183306. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Sanford, R.L.; Andersen, O.S.; Scheuring, S. Effect of statins on the nano-mechanical properties of supported lipid bilayers. Biophys. J. 2016, 111, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| HV | CKD without Statin Treatment | CKD with Statin Treatment | |
|---|---|---|---|
| BMI [kg/m2] | 22.5 ± 3.4 | 28.7 ± 6.5 * | 28.8 ± 5.1 * |
| eGFR [mL/s] | 1.6 ± 0.2 | 0.71 ± 0.23 *** | 0.58 ± 0.33 *** |
| TC [mmol/L] | 4.3 ± 0.59 | 5.43 ± 1.47 | 3.73 ± 0.88 # |
| HDL [mmol/L] | 1.55 ± 0.26 | 0.92 ± 0.22 ** | 0.86 ± 0.05 *** |
| LDL [mmol/L] | 2.16 ± 0.6 | 3.39 ± 0.92 | 1.58 ± 0.78 ## |
| VLDL [mmol/L] | 0.52 ± 0.23 | 1.11 ± 0.43 | 1.17 ± 0.23 *** |
| TG [mmol/L] | 1.3 ± 0.32 | 2.44 ± 0.94 * | 2.94 ± 1.08 ** |
| CRP [mg/L] | 0.5 ± 0.2 | 4 ± 4 + | 8 ± 7 + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajdova, I.; Ovsonkova, L.; Spustova, V.; Oksa, A.; Chorvat, D.; Mateasik, A.; Marcek Chorvatova, A. Spectrally and Time-Resolved Fluorescence Imaging of 22-NBD-Cholesterol in Human Peripheral Blood Mononuclear Cells in Chronic Kidney Disease Patients. Molecules 2021, 26, 6800. https://doi.org/10.3390/molecules26226800
Lajdova I, Ovsonkova L, Spustova V, Oksa A, Chorvat D, Mateasik A, Marcek Chorvatova A. Spectrally and Time-Resolved Fluorescence Imaging of 22-NBD-Cholesterol in Human Peripheral Blood Mononuclear Cells in Chronic Kidney Disease Patients. Molecules. 2021; 26(22):6800. https://doi.org/10.3390/molecules26226800
Chicago/Turabian StyleLajdova, Ingrid, Livia Ovsonkova, Viera Spustova, Adrian Oksa, Dusan Chorvat, Anton Mateasik, and Alzbeta Marcek Chorvatova. 2021. "Spectrally and Time-Resolved Fluorescence Imaging of 22-NBD-Cholesterol in Human Peripheral Blood Mononuclear Cells in Chronic Kidney Disease Patients" Molecules 26, no. 22: 6800. https://doi.org/10.3390/molecules26226800
APA StyleLajdova, I., Ovsonkova, L., Spustova, V., Oksa, A., Chorvat, D., Mateasik, A., & Marcek Chorvatova, A. (2021). Spectrally and Time-Resolved Fluorescence Imaging of 22-NBD-Cholesterol in Human Peripheral Blood Mononuclear Cells in Chronic Kidney Disease Patients. Molecules, 26(22), 6800. https://doi.org/10.3390/molecules26226800