Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice
Abstract
:1. Introduction
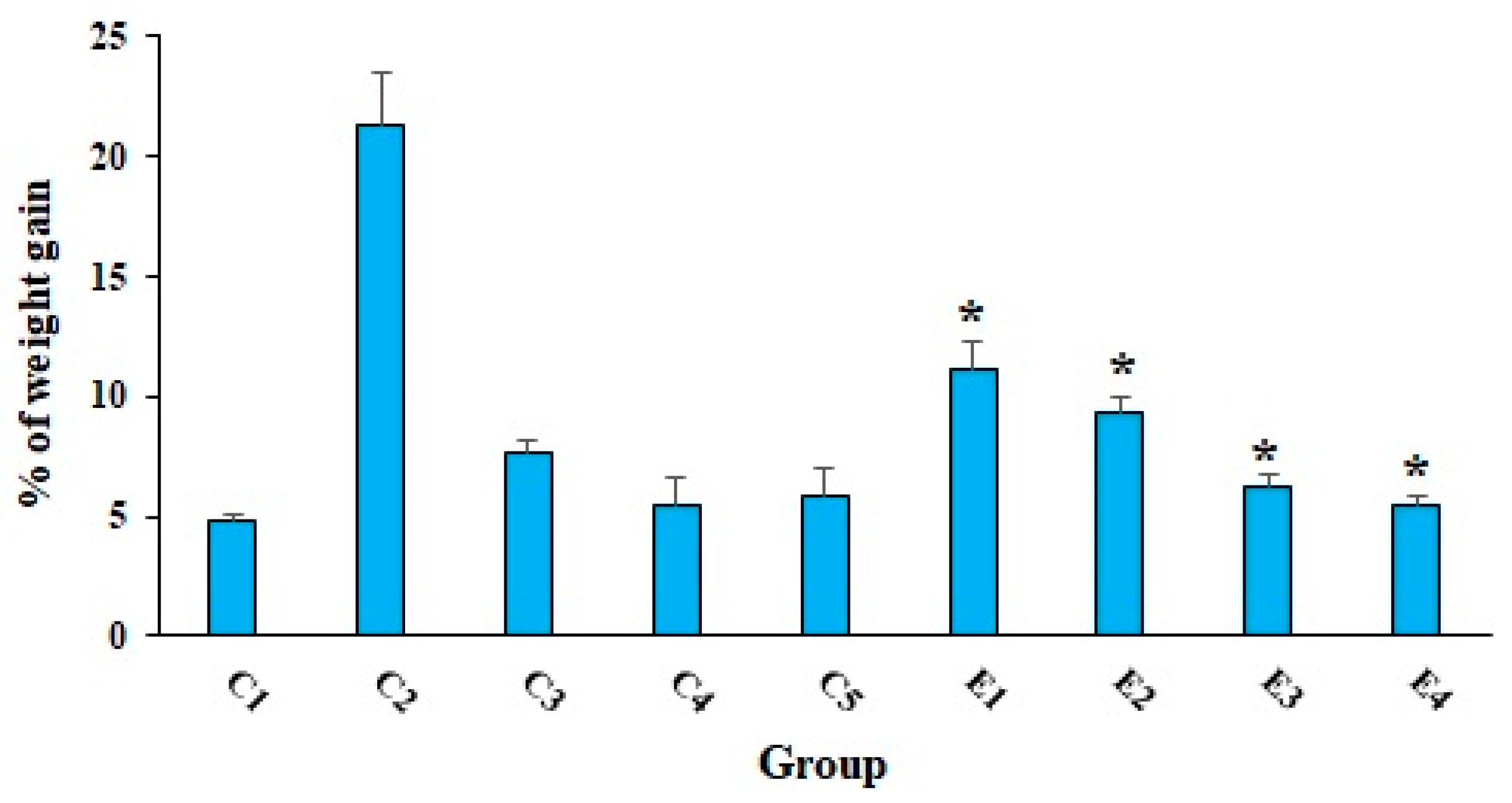
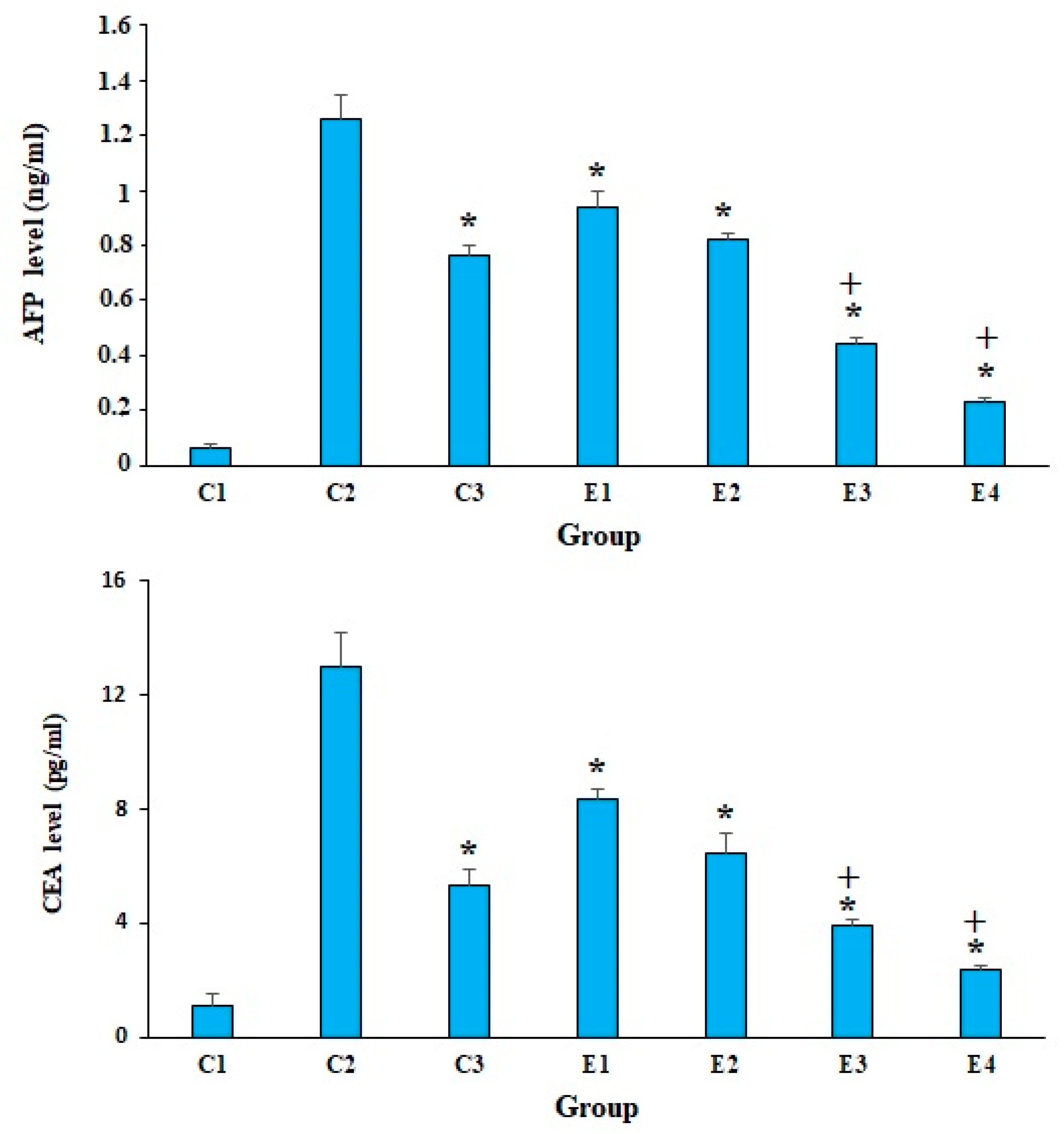
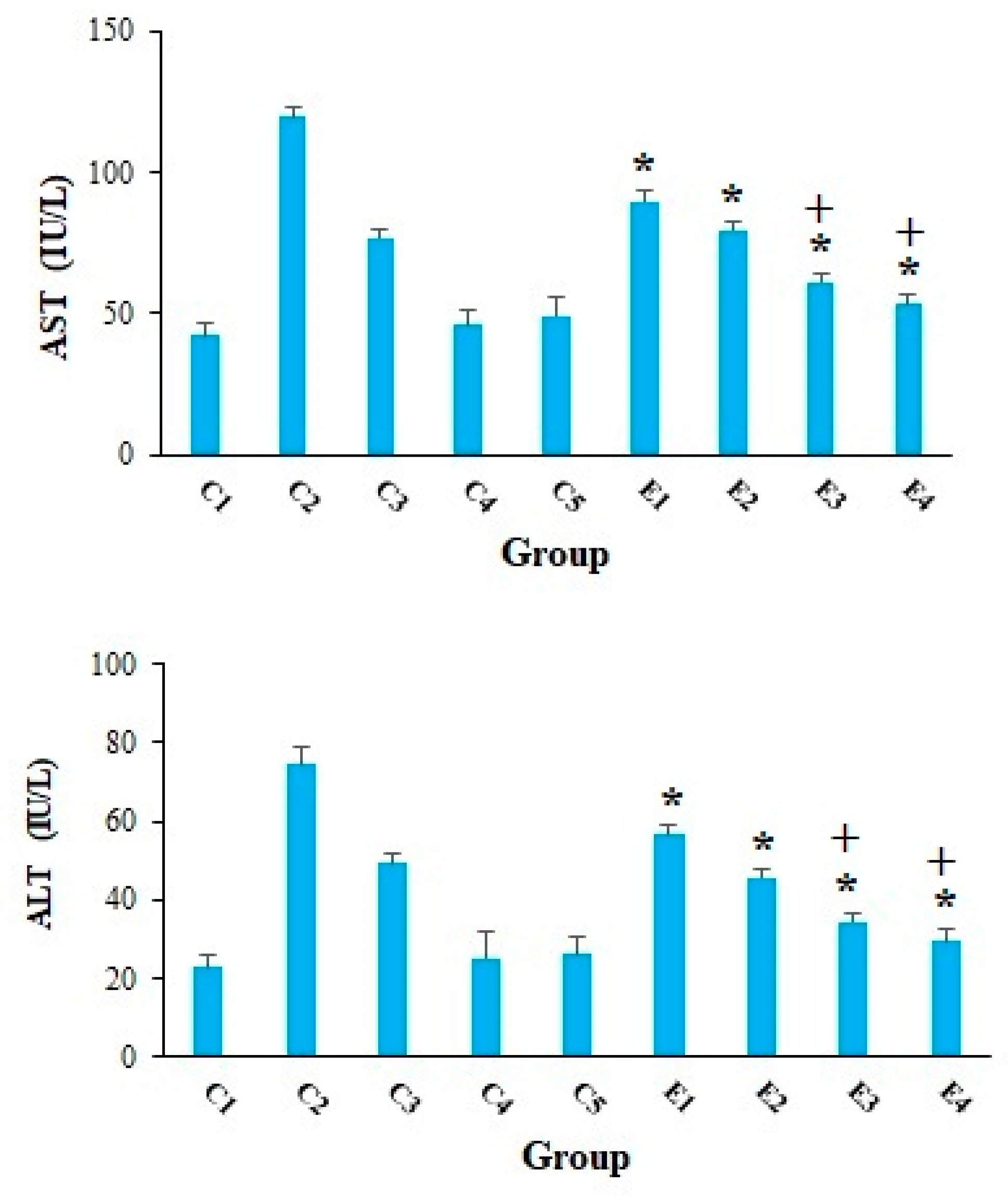
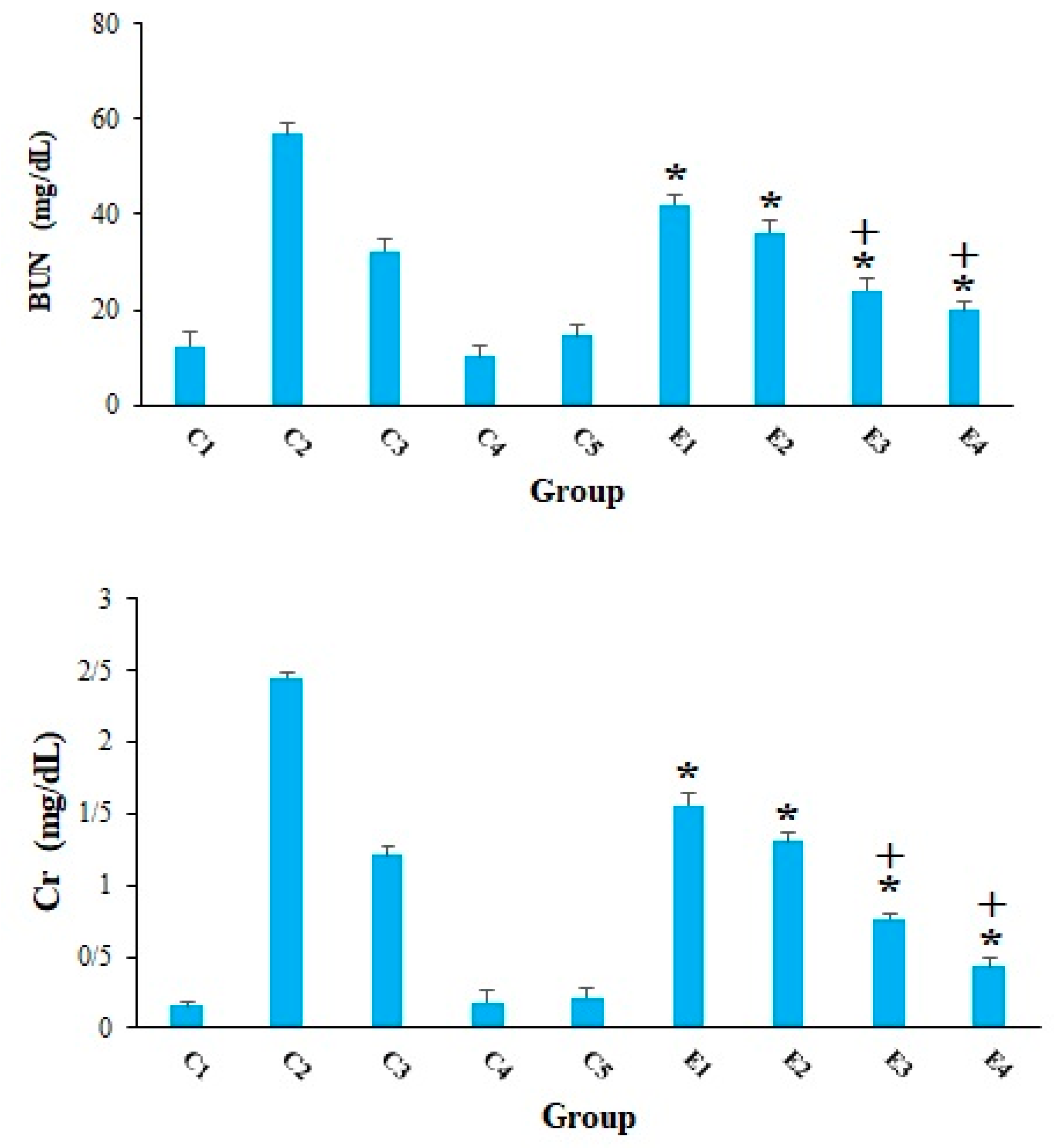
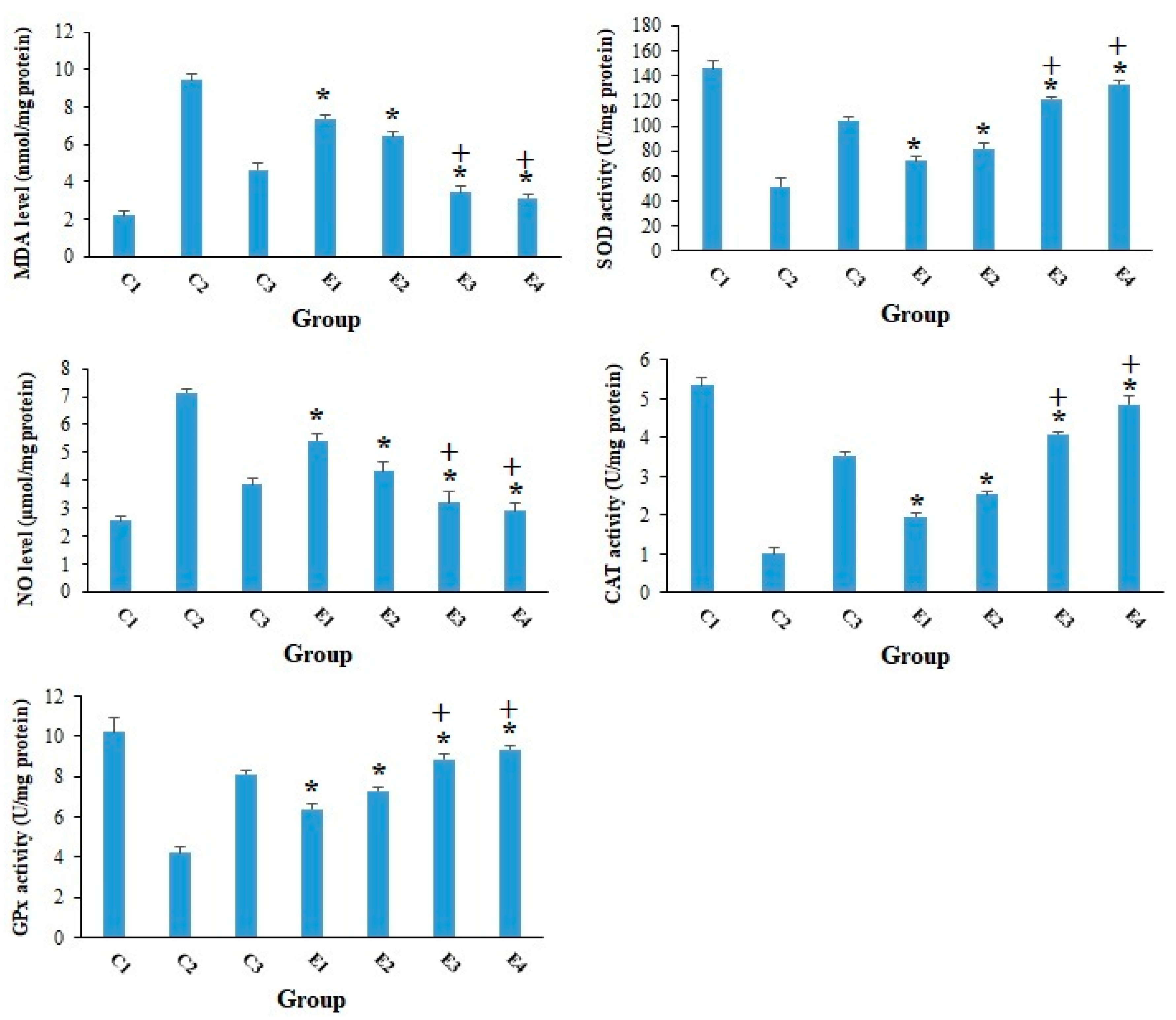
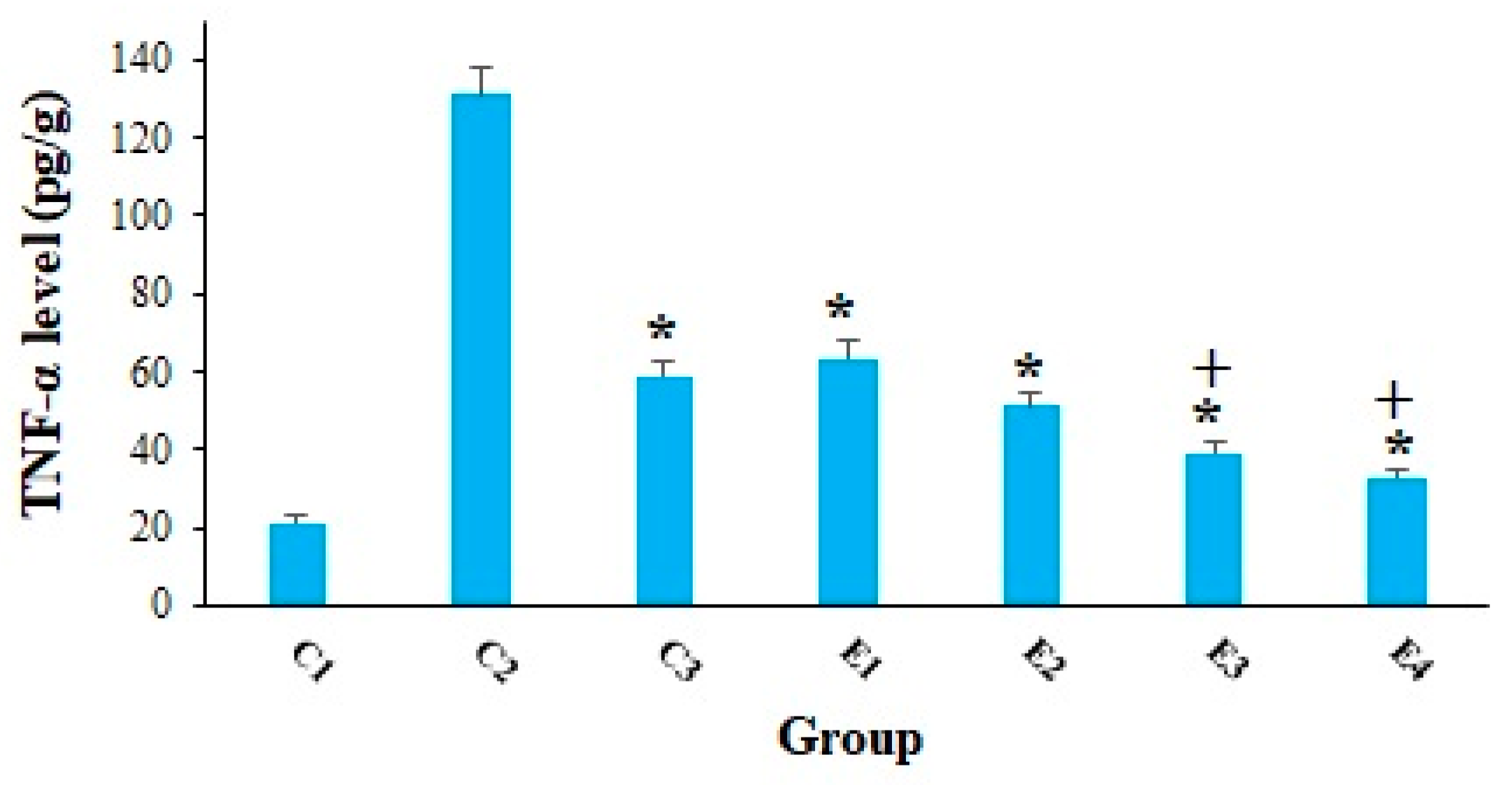
2. Results and Discussion
3. Materials and Methods
3.1. 10-HAD
3.2. Animals
3.3. The Ehrlich Ascites Tumor (EAT) Cell Line and Induction of EST in Mice
3.4. Study Design
3.5. Blood and Tumor Sampling
3.6. Tumor Growth Inhibition
3.7. Body Weight (BW) Changes
3.8. Evaluating the Tumor Markers
3.9. Evaluation of Serum Levels of Liver Enzymes
3.10. Evaluation of Serum Levels of Kidney Enzymes
3.11. Evaluation of the Oxidative Stress Markers
3.12. Evaluation of the Antioxidant Enzymes
3.13. Measuring the Tumor Necrosis Factor Alpha Level (TNF-α)
3.14. Evaluating the Apoptosis-Regulatory Genes Expression
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Golemis, E.A.; Scheet, P.; Beck, T.N.; Scolnick, E.M.; Hunter, D.J.; Hawk, E.; Hopkins, N. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018, 32, 868–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaguchi, T.; Sumimoto, H.; Kudo-Saito, C.; Tsukamoto, N.; Ueda, R.; Iwata-Kajihara, T.; Nishio, H.; Kawamura, N.; Kawakami, Y. The mechanisms of cancer immunoescape and development of overcoming strategies. Int. J. Hematol. 2011, 93, 294–300. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Diaby, V.; Tawk, R.; Sanogo, V.; Xiao, H.; Montero, A.J. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res. Treat. 2015, 151, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, R.B. Chemotherapy education for patients with cancer: A literature review. Clin. J. Oncol. Nurs. 2014, 18, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [Green Version]
- Pavel, C.I.; Mărghitaş, L.A.; Bobiş, O.; Dezmirean, D.S.; Şapcaliu, A.; Radoi, I.; Mădaş, M.N. Biological activities of royal jelly-review. Sci. Pap. Anim. Sci. Biotech. 2011, 44, 108–118. [Google Scholar]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods. 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isidorov, V.A.; Bakier, S.; Grzech, I. Gas chromatographic–mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B 2012, 885, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Peng, C.C.; Sun, H.T.; Lin, I.P.; Kuo, P.C.; Li, J.C. The functional property of royal jelly 10-hydroxy-2-decenoic acid as a melanogenesis inhibitor. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vucevic, D.; Melliou, E.; Vasilijic, S.; Gasic, S.; Ivanovski, P.; Chinou, I.; Colic, M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. [Google Scholar] [CrossRef]
- Weaver, N.; Law, J.H. Heterogeneity of fatty acids from royal jelly. Nature 1960, 188, 938–939. [Google Scholar] [CrossRef]
- Antinelli, J.F.; Zeggane, S.; Davico, R.; Rognone, C.; Faucon, J.P.; Lizzani, L. Evaluation of (E)-10-hydroxydec-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Townsend, G.F.; Morgan, J.F.; Hazlett, B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukaemia and ascitic tumours. Nature 1959, 183, 1270–1271. [Google Scholar] [CrossRef]
- Townsend, G.F.; Morgan, J.F.; Tolnai, S.; Hazlett, B.; Morton, H.J.; Shuel, R.W. Studies on the in vitro antitumor activity of fatty acids. I. 10-Hydroxy-2-decenoic acid from royal jelly. Cancer Res. 1960, 20, 503–510. [Google Scholar]
- Filipič, B.; Gradišnik, L.; Rihar, K.; Šooš, E.; Pereyra, A.; Potokar, J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arh Hig Rada Toksikol. 2015, 66, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.M.; Liu, S.B.; Luo, Y.H.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Lu, B.X.; et al. 10-HDA Induces ROS-Mediated Apoptosis in A549 Human Lung Cancer Cells by Regulating the MAPK, STAT3, NF-κB, and TGF-β1 Signaling Pathways. Biomed Res. Int. 2020, 8, 2020. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Huang, Q.; Huang, Z.; Li, H.; Qi, X.; Wang, Y.; Liu, Z.; Liu, X.; Lu, L. Optimized animal model of cyclophosphamide-induced bone marrow suppression. Basic Clin. Pharmacol. Toxicol. 2016, 119, 428–435. [Google Scholar] [CrossRef]
- Khazaei, F.; Ghanbari, E.; Khazaei, M. Protective Effect of Royal Jelly against Cyclophosphamide-Induced Thrombocytopenia and Spleen and Bone Marrow Damages in Rats. Cell J. 2020, 22, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.A.; Hassan, N.H.; El-Fiky, S.A.; Elalfy, H.G. A mixture of honey bee products ameliorates the genotoxic side effects of cyclophosphamide. Asian Pac. J. Trop. Dis. 2015, 5, 638–644. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020, 871, 172937. [Google Scholar] [CrossRef]
- Liang, C.H.; Ye, W.L.; Zhu, C.L.; Na, R.; Cheng, Y.; Cui, H.; Liu, D.Z.; Yang, Z.F.; Zhou, S.Y. Synthesis of doxorubicin α-linolenic acid conjugate and evaluation of its antitumor activity. Mol. Pharmaceutics. 2014, 11, 1378–1390. [Google Scholar] [CrossRef]
- Mielczarek-Puta, M.; Struga, M.; Roszkowski, P. Synthesis and anticancer effects of conjugates of doxorubicin and unsaturated fatty acids (LNA and DHA). Med. Chem. Res. 2019, 28, 2153–2164. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, G.; El Sadda, R.; Botchkina, G.; Ojima, I.; Egan, J.; Amiji, M. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017, 406, 71–80. [Google Scholar] [CrossRef]
- Edoo, M.I.A.; Chutturghoon, V.K.; Wusu-Ansah, G.K.; Zhu, H.; Zhen, T.Y.; Xie, H.Y.; Zheng, S.S. Serum Biomarkers AFP, CEA and CA19-9 Combined Detection for Early Diagnosis of Hepatocellular Carcinoma. Iran J. Public Health 2019, 48, 314–322. [Google Scholar] [PubMed]
- Kim, S.K.; Karadeniz, F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Viswanathan, P.; Pugalendi, K.V. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006, 78, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Sivakumar, T.; Vamsi, M.L. Antitumor activity and antioxidant status of Caesalpiniabonducella against Ehrlich ascites carcinoma in Swiss albino mice. J. Pharmacol. Sci. 2004, 94, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, S.A.; Azab, M.E. Effect of insulin treatment on some metabolic changes on experimentally induced tumor in female mice, Egypt. J. Biochem. Mol. Biol. 1997, 15, 61–80. [Google Scholar]
- Abou, Z.O.A.; Hassanein, M.R.; EL-Senosi, Y.A.; EL-Shiekha, M.F. Biochemical effect of some antioxidant on metabolic changes in experimentally induced tumor in female mice. Benha Vet. Med J. 2011, 1, 52–60. [Google Scholar]
- Weiser, M.J.; Grimshaw, V.; Wynalda, K.M.; Mohajeri, M.H.; Butt, C.M. Long-Term Administration of Queen Bee Acid (QBA) to Rodents Reduces Anxiety-Like Behavior, Promotes Neuronal Health and Improves Body Composition. Nutrients 2017, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Visconti, R.; Grieco, D. New insights on oxidative stress in cancer. Curr. Opin. Drug Discov. Dev. 2009, 12, 240–245. [Google Scholar]
- Chen, W.; Jia, Z.; Pan, M.H.; AnandhBabu, P.V. Natural products for the prevention of oxidative stress-related diseases: Mechanisms and strategies. Oxid. Med. Cell. Longevity. 2016, 26, 2016. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Takahashi, K.; Kuzumaki, A.; Tokoro, S.; Neri, P.; Mori, H. Inhibitory mechanism of 10-hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide-and interferon-β-induced nitric oxide production. Inflammation 2013, 36, 372–378. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Ling, I.P.; Peng, C.C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef]
- Sankari, S.L.; Masthan, K.M.; Babu, N.A.; Bhattacharjee, T.; Elumalai, M. Apoptosis in cancer-an update. Asian Pac. J. Cancer Prev. 2012, 13, 4873–4878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharawi, Z.W. Therapeutic effect of Arthrocnemummachrostachyummethanolic extract on Ehrlich solid tumor in mice. BMC Complement. Med. Ther. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013, 51, 58–63. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Luck, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1965; pp. 855–888. [Google Scholar]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Selim, N.M.; Elgazar, A.A.; Abdel-Hamid, N.M.; El-Magd, M.R.A.; Yasri, A.; Hefnawy, H.M.E.; Sobeh, M. Chrysophanol, physcion, hesperidin and curcumin modulate the gene expression of pro-inflammatory mediators induced by lps in hepg2: In silico and molecular studies. Antioxidants 2019, 8, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]

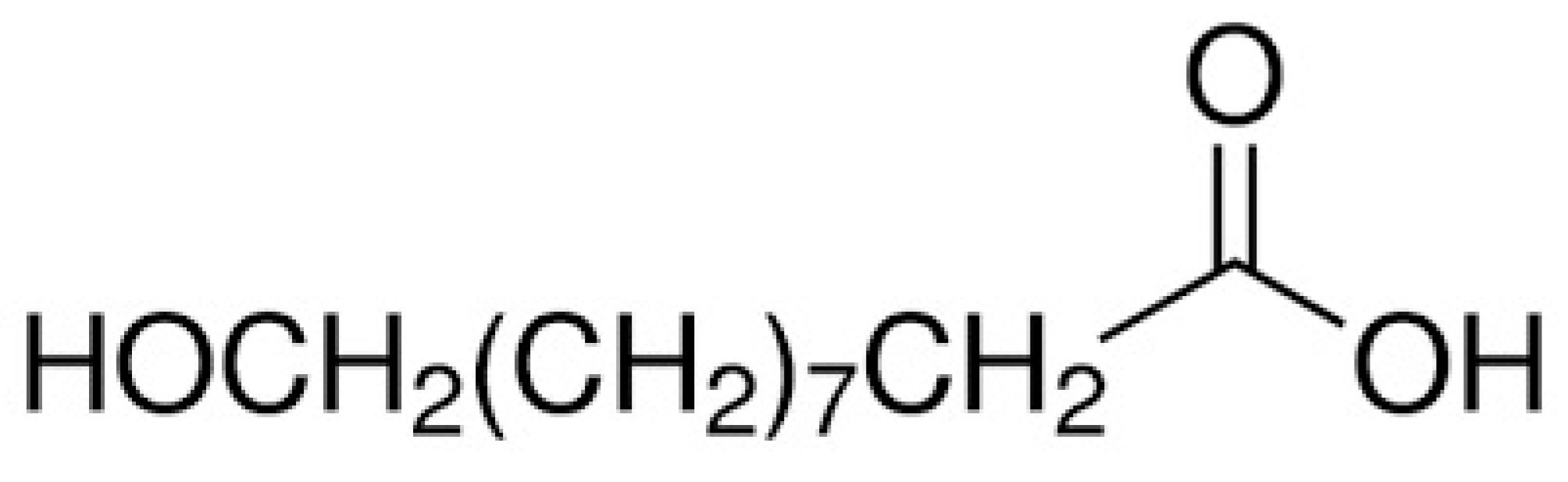
| Group | Tumor Volume (g) | % of Inhibition |
|---|---|---|
| 10-HDA 2.5 mg/kg | 1.96 ± 0.083 | 37.2 |
| 10-HAD 5 mg/kg | 1.32 ± 0.13 | 57.7 |
| 10-HAD 2.5 mg/kg + CP (25 mg/kg) | 0.62 ± 0.031 | 80.1 |
| 10-HAD 5 mg/kg + CP (25 mg/kg) | 0.32 ± 0.024 | 89.7 |
| Control C2 | 3.12 ± 0.12 | - |
| Control C3 | 1.27 ± 0.03 | 59.3 |
| Amplicon | Primers | Sequence (5′–3′) |
|---|---|---|
| Bax | F R | GGCTGGACACTGGACTTCCT GGTGAGGACTCCAGCCACAA |
| Bcl-2 | F R | CATGCCAAGAGGGAAACACCAGAA GTGCTTTGCATTCTTGGA TGAGGG |
| Caspase-3 | F R | TTCATTATTCAGGCCTGCCGAGG TTCTGACAGGCCATGTCATCCTCA |
| β-actin | F R | GTGACGTTGACATCCGTAAAGA GCCGGACTCATCGTACTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalawi, A.E.; Althobaiti, N.A.; Alrdahe, S.S.; Alhasani, R.H.; Alaryani, F.S.; BinMowyna, M.N. Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. Molecules 2021, 26, 7021. https://doi.org/10.3390/molecules26227021
Albalawi AE, Althobaiti NA, Alrdahe SS, Alhasani RH, Alaryani FS, BinMowyna MN. Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. Molecules. 2021; 26(22):7021. https://doi.org/10.3390/molecules26227021
Chicago/Turabian StyleAlbalawi, Aishah E., Norah A. Althobaiti, Salma Saleh Alrdahe, Reem Hasaballah Alhasani, Fatima S. Alaryani, and Mona Nasser BinMowyna. 2021. "Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice" Molecules 26, no. 22: 7021. https://doi.org/10.3390/molecules26227021
APA StyleAlbalawi, A. E., Althobaiti, N. A., Alrdahe, S. S., Alhasani, R. H., Alaryani, F. S., & BinMowyna, M. N. (2021). Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. Molecules, 26(22), 7021. https://doi.org/10.3390/molecules26227021







