In Vitro 31P MR Chemical Shifts of In Vivo-Detectable Metabolites at 3T as a Basis Set for a Pilot Evaluation of Skeletal Muscle and Liver 31P Spectra with LCModel Software
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Energy Metabolites—Phosphocreatine (PCr), Inorganic Phosphate (Pi), Adenosinetriphosphate (ATP), and Adenosinediphosphate (ADP)
3.2. Phosphomonoesters (PME)
3.2.1. Phosphocholine (PC) and Phosphoethanolamine (PE)
3.2.2. Phosphoenolpyruvate (PEP) and Phosphatidylcholine (PtdC)
3.2.3. Glucose-1-Phosphate (G1P) and Glucose-6-Phosphate (G6P)
3.3. Phosphodiesters (PDE)
3.3.1. Glycerophosphocholine (GPC)
3.3.2. 2,3-Diphosphoglycerate (2,3-DPG)
3.4. Nicotinamide Adenine Dinucleotide Metabolites (NAD+, NADH)
3.5. Uridine Diphosphoglucose (UDPG)
3.6. 31P Basis Set for LCModel Calculations
4. Materials and Methods
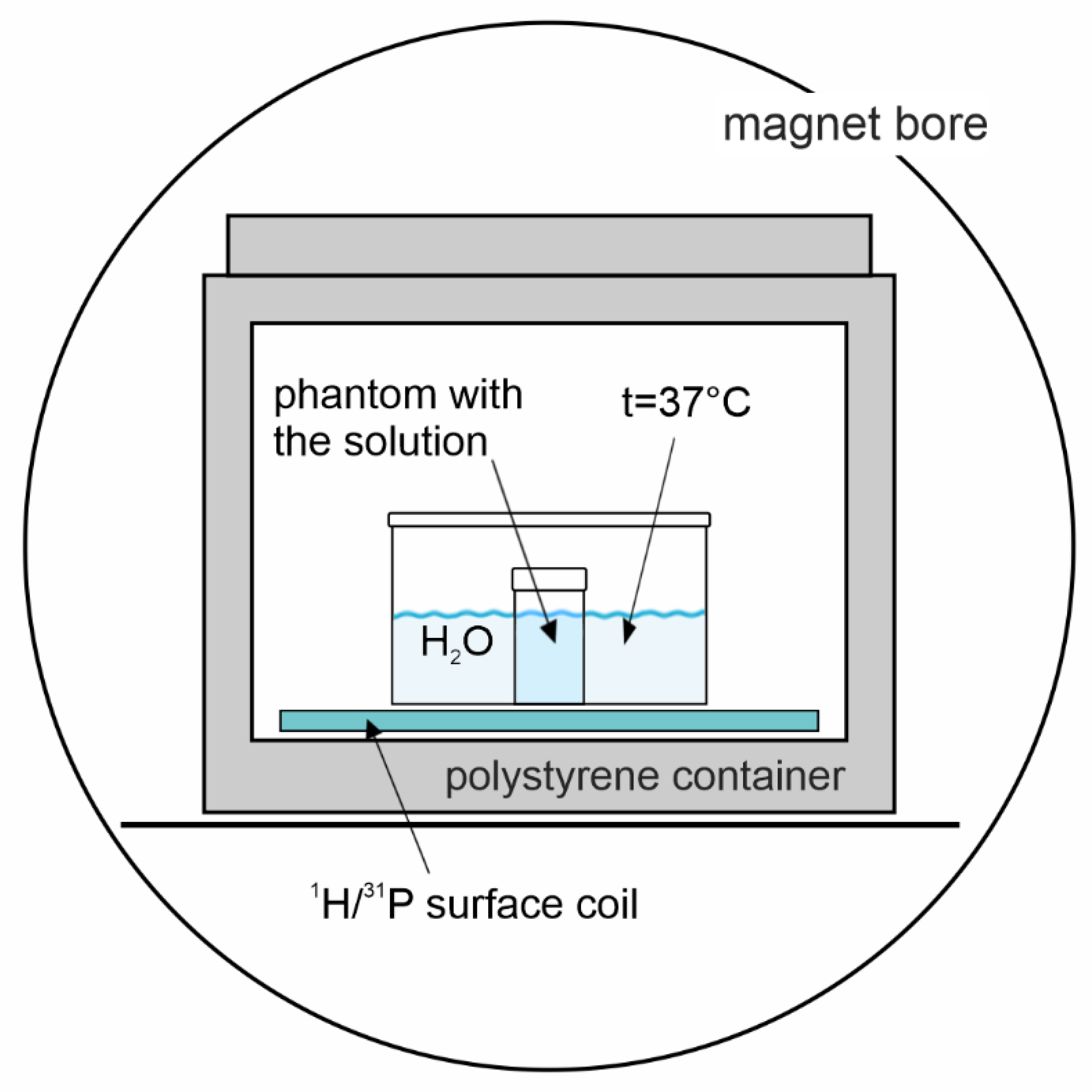
4.1. Phantom Preparation
4.2. 31P MR Spectroscopy
4.3. Spectra Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bottomley, P.A.; Hart, H.R.; Edelstein, W.A.; Schenck, J.F.; Smith, L.S.; Leue, W.M.; Mueller, O.M.; Redington, R.W. Nmr Imaging Spectroscopy System to Study Both Anatomy and Metabolism. Lancet 1983, 2, 273–274. [Google Scholar] [CrossRef]
- Šedivý, P.; Kipfelsberger, M.C.; Dezortová, M.; Krššák, M.; Drobný, M.; Chmelík, M.; Rydlo, J.; Trattnig, S.; Hájek, M.; Valkovič, L. Dynamic 31P MR spectroscopy of plantar flexion: Influence of ergometer design, magnetic field strength (3 and 7 T), and RF-coil design. Med. Phys. 2015, 42, 1678–1689. [Google Scholar] [CrossRef]
- Valkovič, L.; Chmelík, M.; Krššák, M. In-vivo (31)P-MRS of skeletal muscle and liver: A way for non-invasive assessment of their metabolism. Anal. Biochem. 2017, 529, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Chmelík, M.; Valkovič, L.; Wolf, P.; Bogner, W.; Gajdošík, M.; Halilbasic, E.; Gruber, S.; Trauner, M.; Krebs, M.; Trattnig, S.; et al. Phosphatidylcholine contributes to in vivo (31)P MRS signal from the human liver. Eur. Radiol. 2015, 25, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Bierwagen, A.; Begovatz, P.; Nowotny, P.; Markgraf, D.; Nowotny, B.; Koliaki, C.; Giani, G.; Klüppelholz, B.; Lundbom, J.; Roden, M. Characterization of the peak at 2.06 ppm in (31) P magnetic resonance spectroscopy of human liver: Phosphoenolpyruvate or phosphatidylcholine? NMR Biomed. 2015, 28, 898–905. [Google Scholar] [CrossRef]
- Sevastianova, K.; Hakkarainen, A.; Kotronen, A.; Cornér, A.; Arkkila, P.; Arola, J.; Westerbacka, J.; Bergholm, R.; Lundbom, J.; Lundbom, N.; et al. Nonalcoholic fatty liver disease: Detection of elevated nicotinamide adenine dinucleotide phosphate with in vivo 3.0-T 31P MR spectroscopy with proton decoupling. Radiology 2010, 256, 466–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szendroedi, J.; Schmid, A.I.; Chmelik, M.; Krssak, M.; Nowotny, P.; Prikoszovich, T.; Kautzky-Willer, A.; Wolzt, M.; Waldhäusl, W.; Roden, M. Skeletal Muscle Phosphodiester Content Relates to Body Mass and Glycemic Control. PLoS ONE 2011, 6, e21846. [Google Scholar] [CrossRef]
- Sedivy, P.; Dezortova, M.; Drobny, M.; Dubsky, M.; Dusilova, T.; Kovar, J.; Hajek, M. Origin of the (31) P MR signal at 5.3 ppm in patients with critical limb ischemia. NMR Biomed. 2020, 33, e4295. [Google Scholar] [CrossRef]
- Dezortova, M.; Taimr, P.; Skoch, A.; Spicak, J.; Hajek, M. Etiology and functional status of liver cirrhosis by 31P MR spectroscopy. World J. Gastroenterol. 2005, 11, 6926–6931. [Google Scholar] [CrossRef] [PubMed]
- Beiglbock, H.; Wolf, P.; Pfleger, L.; Caliskan, B.; Fellinger, P.; Zettinig, G.; Anderwald, C.H.; Kenner, L.; Trattnig, S.; Kautzky-Willer, A.; et al. Effects of Thyroid Function on Phosphodiester Concentrations in Skeletal Muscle and Liver: An In Vivo NMRS Study. J. Clin. Endocrinol. Metab. 2020, 105, e4866–e4874. [Google Scholar] [CrossRef]
- Valkovič, L.; Chmelík, M.; Ukropcová, B.; Heckmann, T.; Bogner, W.; Frollo, I.; Tschan, H.; Krebs, M.; Bachl, N.; Ukropec, J.; et al. Skeletal muscle alkaline Pi pool is decreased in overweight-to-obese sedentary subjects and relates to mitochondrial capacity and phosphodiester content. Sci. Rep. 2016, 6, 20087. [Google Scholar] [CrossRef] [Green Version]
- Kan, H.E.; Klomp, D.W.J.; Wong, C.S.; Boer, V.O.; Webb, A.G.; Luijten, P.R.; Jeneson, J.A. In vivo 31P MRS detection of an alkaline inorganic phosphate pool with short T1 in human resting skeletal muscle. NMR Biomed. 2010, 23, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Van Oorschot, J.W.M.; Schmitz, J.P.J.; Webb, A.; Nicolay, K.; Jeneson, J.A.L.; Kan, H.E. 31P MR Spectroscopy and Computational Modeling Identify a Direct Relation between Pi Content of an Alkaline Compartment in Resting Muscle and Phosphocreatine Resynthesis Kinetics in Active Muscle in Humans. PLoS ONE 2013, 8, e76628. [Google Scholar] [CrossRef] [Green Version]
- Provencher, S.W. Estimation of Metabolite Concentrations from Localized in-Vivo Proton Nmr-Spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Deelchand, D.K.; Nguyen, T.M.; Zhu, X.H.; Mochel, F.; Henry, P.G. Quantification of in vivo (3)(1)P NMR brain spectra using LCModel. NMR Biomed. 2015, 28, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; van der Veen, J.W.; An, L.; Stolinski, J.; Johnson, C.; Ferraris-Araneta, M.; Victorino, M.; Tomar, J.S.; Shen, J. Cerebral phosphoester signals measured by 31P magnetic resonance spectroscopy at 3 and 7 Tesla. PLoS ONE 2021, 16, e0248632. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.E.; Ali, A.S.; Flores, B.; Jubrias, S.A.; Shankland, E.G. Mitochondrial NAD(P)H In vivo: Identifying Natural Indicators of Oxidative Phosphorylation in the (31)P Magnetic Resonance Spectrum. Front. Physiol. 2016, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Meyerspeer, M.; Boesch, C.; Cameron, D.; Dezortová, M.; Forbes, S.C.; Heerschap, A.; Jeneson, J.A.L.; Kan, H.E.; Kent, J.; Layec, G.; et al. 31P magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purvis, L.A.B.; Clarke, W.T.; Valkovič, L.; Levick, C.; Pavlides, M.; Barnes, E.; Cobbold, J.F.; Robson, M.D.; Rodgers, C.T. Phosphodiester content measured in human liver by in vivo (31) P MR spectroscopy at 7 tesla. Magn. Reson. Med. 2017, 78, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Graaf, R.A.; De Feyter, H.M.; Brown, P.B.; Nixon, T.W.; Rothman, D.L.; Behar, K.L. Detection of cerebral NAD(+) in humans at 7T. Magn. Reson. Med. 2017, 78, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Peeters, T.H.; van Uden, M.J.; Rijpma, A.; Scheenen, T.W.J.; Heerschap, A. 3D (31) P MR spectroscopic imaging of the human brain at 3 T with a (31) P receive array: An assessment of (1) H decoupling, T(1) relaxation times, (1) H-(31) P nuclear Overhauser effects and NAD(+). NMR Biomed. 2019, 34, e4169. [Google Scholar] [CrossRef] [Green Version]
- Pollock, A.S. Intracellular pH of hepatocytes in primary monolayer culture. Am. J. Physiol. 1984, 246, F738–F744. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Poci, C.; Spirlì, C.; Zsembery, A.; Granato, A.; Massimino, M.L.; Crepaldi, G. Intracellular pH regulation in Hep G2 cells: Effects of epidermal growth factor, transforming growth factor-alpha, and insulinlike growth factor-II on Na+/H+ exchange activity. Hepatology 1995, 22, 588–597. [Google Scholar] [PubMed]
- Kim, J.H.; Johannes, L.; Goud, B.; Antony, C.; Lingwood, C.A.; Daneman, R.; Grinstein, S. Noninvasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc. Natl. Acad. Sci. USA 1998, 95, 2997–3002. [Google Scholar] [CrossRef] [Green Version]
- Nedergaard, M.; Kraig, R.P.; Tanabe, J.; Pulsinelli, W.A. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am. J. Physiol. 1991, 260, R581–R588. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, D.; Braun, M.; Mataki, C.; Park, K.S.; Pozzan, T.; Schoonjans, K.; Rorsman, P.; Wollheim, C.B.; Wiederkehr, A. Mitochondrial matrix pH controls oxidative phosphorylation and metabolism-secretion coupling in INS-1E clonal beta cells. FASEB J. 2010, 24, 4613–4626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santo-Domingo, J.; Demaurex, N. The renaissance of mitochondrial pH. J. Gen. Physiol. 2012, 139, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labotka, R.J.; Kleps, R.A. A phosphate-analogue probe of red cell pH using phosphorus-31 nuclear magnetic resonance. Biochemistry 1983, 22, 6089–6095. [Google Scholar] [CrossRef]
- Labotka, R.J. Measurement of intracellular pH and deoxyhemoglobin concentration in deoxygenated erythrocytes by phosphorus-31 nuclear magnetic resonance. Biochemistry 1984, 23, 5549–5555. [Google Scholar] [CrossRef] [PubMed]
- Kemp, G.J.; Meyerspeer, M.; Moser, E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: A quantitative review. NMR Biomed. 2007, 20, 555–565. [Google Scholar] [CrossRef] [PubMed]
- McDowell, R.W.; Stewart, I. Peak assignments for phosphorus-31 nuclear magnetic resonance spectroscopy in pH range 5-13 and their application in environmental samples. Chem. Ecol. 2005, 21, 211–226. [Google Scholar] [CrossRef]
- Moon, R.B.; Richards, J.H. Determination of intracellular pH by 31P magnetic resonance. J. Biol. Chem. 1973, 248, 7276–7278. [Google Scholar] [CrossRef]
- Sedivy, P.; Drobny, M.; Dezortova, M.; Herynek, V.; Roztocil, K.; Cermakova, H.; Nemcova, A.; Dubsky, M.; Hajek, M. 31P-MR spectroscopy in patients with mild and serious lower limb ischemia. Int. Angiol. 2018, 37, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Rata, M.; Giles, S.L.; deSouza, N.M.; Leach, M.O.; Payne, G.S. Comparison of three reference methods for the measurement of intracellular pH using 31P MRS in healthy volunteers and patients with lymphoma. NMR Biomed. 2014, 27, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.I.; Widmaier, S.; Seeger, U.; Bunse, M.; Staubert, A.; Sieverding, L.; Straubinger, K.; van Erckelens, F.; Schick, F.; Dietze, G.; et al. Phosphorus J coupling constants of ATP in human myocardium and calf muscle. J. Magn. Reson. B 1996, 110, 39–46. [Google Scholar] [CrossRef]
- De Graaf, R.A. In Vivo NMR Spectroscopy, Principles and Techniques, 2nd ed.; John Wiley&Sons Ltd.: Chichester, UK, 2007; pp. 1–570. [Google Scholar]
- Turner, B.L.; Mahieu, N.; Condron, L.M. Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH-EDTA extracts. Soil Sci. Soc. Am. J. 2003, 67, 497–510. [Google Scholar] [CrossRef]
- Cade-Menun, B.J. Improved peak identification in 31P-NMR spectra of environmental samples with a standardized method and peak library. Geoderma 2015, 257–258, 102–114. [Google Scholar] [CrossRef]
- Cohn, M.; Hughes, T.R., Jr. Nuclear magnetic resonance spectra of adenosine di- and triphosphate. II. Effect of complexing with divalent metal ions. J. Biol. Chem. 1962, 237, 176–181. [Google Scholar] [CrossRef]
- Szabo, Z. Multinuclear NMR studies of the interaction of metal ions with adenine-nucleotides. Coord. Chem. Rev. 2008, 252, 2362–2380. [Google Scholar] [CrossRef]
- Hernández, E.; Kahl, S.; Seelig, A.; Begovatz, P.; Irmler, M.; Kupriyanova, Y.; Nowotny, B.; Nowotny, P.; Herder, C.; Barosa, C.; et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017, 127, 695–708. [Google Scholar] [CrossRef]
- Hultman, E.; Nilsson, L.H.; Sahlin, K. Adenine nucleotide content of human liver. Normal values and fructose-induced depletion. Scand. J. Clin. Lab. Investig. 1975, 35, 245–251. [Google Scholar] [CrossRef]
- Traussnigg, S.; Kienbacher, C.; Gajdošík, M.; Valkovič, L.; Halilbasic, E.; Stift, J.; Rechling, C.; Hofer, H.; Steindl-Munda, P.; Ferenci, P.; et al. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017, 37, 1544–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.; Bhujwalla, Z.M.; Glunde, K. Targeting Phospholipid Metabolism in Cancer. Front. Oncol. 2016, 6, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosner, Z.; Dezortova, M.; Tintera, J.; Hajek, M. Application of two-dimensional CSI for absolute quantification of phosphorus metabolites in the human liver. Magma 2001, 13, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, L.; Halilbasic, E.; Gajdošík, M.; Benčíková, D.; Chmelík, M.; Scherer, T.; Trattnig, S.; Krebs, M.; Trauner, M.; Krššák, M. Concentration of Gallbladder Phosphatidylcholine in Cholangiopathies: A Phosphorus-31 Magnetic Resonance Spectroscopy Pilot Study. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- De Haan, J.H.; Klomp, D.W.; Tack, C.J.; Heerschap, A. Optimized detection of changes in glucose-6-phosphate levels in human skeletal muscle by 31P MR spectroscopy. Magn. Reson. Med. 2003, 50, 1302–1306. [Google Scholar] [CrossRef]
- Roden, M.; Krssak, M.; Stingl, H.; Gruber, S.; Hofer, A.; Furnsinn, C.; Moser, E.; Waldhausl, W. Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 1999, 48, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Rothman, D.L.; Shulman, R.G.; Shulman, G.I. P-31 Nuclear-Magnetic-Resonance Measurements of Muscle Glucose-6-Phosphate—Evidence for Reduced Insulin-Dependent Muscle Glucose-Transport or Phosphorylation Activity in Non-Insulin-Dependent Diabetes-Mellitus. J. Clin. Investig. 1992, 89, 1069. [Google Scholar] [CrossRef] [PubMed]
- Cline, G.W.; Petersen, K.F.; Krssak, M.; Shen, J.; Hundal, R.S.; Trajanoski, Z.; Inzucchi, S.; Dresner, A.; Rothman, D.L.; Shulman, G.I. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 1999, 341, 240. [Google Scholar] [CrossRef]
- Noren, B.; Dahlqvist, O.; Lundberg, P.; Almer, S.; Kechagias, S.; Ekstedt, M.; Franzén, L.; Wirell, S.; Smedby, O. Separation of advanced from mild fibrosis in diffuse liver disease using 31P magnetic resonance spectroscopy. Eur. J. Radiol. 2008, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Buchli, R.; Meier, D.; Martin, E.; Boesiger, P. Assessment of absolute metabolite concentrations in human tissue by 31P MRS in vivo. Part II: Muscle, liver, kidney. Magn. Reson. Med. 1994, 32, 453–458. [Google Scholar] [CrossRef]
- Li, C.W.; Negendank, W.G.; Murphy-Boesch, J.; Padavic-Shaller, K.; Brown, T.R. Molar quantitation of hepatic metabolites in vivo in proton-decoupled, nuclear Overhauser effect enhanced 31P NMR spectra localized by three-dimensional chemical shift imaging. NMR Biomed. 1996, 9, 141–155. [Google Scholar] [CrossRef]
- Chmelík, M.; Schmid, A.I.; Gruber, S.; Szendroedi, J.; Krššák, M.; Trattnig, S.; Moser, E.; Roden, M. Three-dimensional high-resolution magnetic resonance spectroscopic imaging for absolute quantification of 31P metabolites in human liver. Magn. Reson. Med. 2008, 60, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Laufs, A.; Livingstone, R.; Nowotny, B.; Nowotny, P.; Wickrath, F.; Giani, G.; Bunke, J.; Roden, M.; Hwang, J.-H. Quantitative liver 31 P magnetic resonance spectroscopy at 3T on a clinical scanner. Magn. Reson. Med. 2014, 71, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, L.; Gajdošík, M.; Wolf, P.; Smajis, S.; Fellinger, P.; Kuehne, A.; Krumpolec, P.; Trattnig, S.; Winhofer, Y.; Krebs, M.; et al. Absolute Quantification of Phosphor-Containing Metabolites in the Liver Using (31)P MRSI and Hepatic Lipid Volume Correction at 7T Suggests No Dependence on Body Mass Index or Age. J. Magn. Reson. Imaging 2019, 49, 597–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooijmans, M.T.; Doorenweerd, N.; Baligand, C.; Verschuuren, J.; Ronen, I.; Niks, E.H.; Webb, A.G.; Kan, H.E. Spatially localized phosphorous metabolism of skeletal muscle in Duchenne muscular dystrophy patients: 24-month follow-up. PLoS ONE 2017, 12, e0182086. [Google Scholar] [CrossRef] [PubMed]
- Krumpolec, P.; Klepochova, R.; Just, I.; Tusek Jelenc, M.; Frollo, I.; Ukropec, J.; Ukropcova, B.; Trattnig, S.; Krssak, M.; Valkovic, L. Multinuclear MRS at 7T Uncovers Exercise Driven Differences in Skeletal Muscle Energy Metabolism Between Young and Seniors. Front. Physiol. 2020, 11, 644. [Google Scholar] [CrossRef]
- Klepochova, R.; Valkovic, L.; Hochwartner, T.; Triska, C.; Bachl, N.; Tschan, H.; Trattnig, S.; Krebs, M.; Krssak, M. Differences in Muscle Metabolism Between Triathletes and Normally Active Volunteers Investigated Using Multinuclear Magnetic Resonance Spectroscopy at 7T. Front Physiol. 2018, 9, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, D.; Layton, M. Investigation of the hereditary haemolytic anaemias: Membrane and enzyme abnormalities. In Dacie and Lewis Practical Haematology, 10th ed.; Lewis, S., Bain, B., Bates, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, X.H.; Zhang, Y.; Chen, W. Intracellular Redox State Revealed by In Vivo P-31 MRS Measurement of NAD(+) and NADH Contents in Brains. Magn. Reson. Med. 2014, 71, 1959–1972. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhu, X.H.; Chen, W. In vivo P-31 MRS assessment of intracellular NAD metabolites and NAD(+)/NADH redox state in human brain at 4 T. NMR Biomed. 2016, 29, 1010–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouinard, V.A.; Kim, S.Y.; Valeri, L.; Yuksel, C.; Ryan, K.P.; Chouinard, G.; Cohen, B.M.; Du, F.; Öngür, D. Brain bioenergetics and redox state measured by (31)P magnetic resonance spectroscopy in unaffected siblings of patients with psychotic disorders. Schizoph. Res. 2017, 187, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, J.; Katz, A.; Sahlin, K. Redox state changes in human skeletal muscle after isometric contraction. J. Physiol. 1986, 380, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Sarma, R.H.; Lee, C.H.; Hruska, F.E.; Wood, D.J. H-1, H-1-[P-31], P-31, and P-31-[H-1] fast Fourier transform NMR study of solution conformation of cofactors involved in glycogen synthesis: Adenosinediphosphoglucose and uridinediphosphoglucose. FEBS Lett. 1973, 36, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Stefan, D.; Cesare, F.D.; Andrasescu, A.; Popa, E.; Lazariev, A.; Vescovo, E.; Strbak, O.; Williams, S.; Starcuk, Z.; Cabanas, M.; et al. Quantitation of magnetic resonance spectroscopy signals: The jMRUI software package. Meas. Sci. Technol. 2009, 20, 104035. [Google Scholar] [CrossRef]
- Gajdosik, M.; Landheer, K.; Swanberg, K.M.; Juchem, C. INSPECTOR: Free software for magnetic resonance spectroscopy data inspection, processing, simulation and analysis. Sci. Rep. 2021, 11, 2094. [Google Scholar] [CrossRef]
- Deelchand, D.K.; Ugurbil, K.; Henry, P.G. Investigating brain metabolism at high fields using localized 13C NMR spectroscopy without 1H decoupling. Magn. Res. Med. 2006, 55, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skupienski, R.; Do, K.Q.; Xin, L. In vivo 31P magnetic resonance spectroscopy study of mouse cerebral NAD content and redox state during neurodevelopment. Sci. Rep. 2020, 10, 15623. [Google Scholar] [CrossRef] [PubMed]
- Hájek, M.; Palyzová, D.; Korínek, M.; Kurková, D. Concentrations of free mg2+, pH and 31P MR metabolite ratios in calf muscles of healthy controls and patients with primary juvenile hypertension. Physiol. Res. 2002, 51, 159–167. [Google Scholar] [PubMed]
- Henry, P.G.; Oz, G.; Provencher, S.; Gruetter, R. Toward dynamic isotopomer analysis in the rat brain in vivo: Automatic quantitation of 13C NMR spectra using LCModel. NMR Biomed. 2003, 16, 400–412. [Google Scholar] [CrossRef]
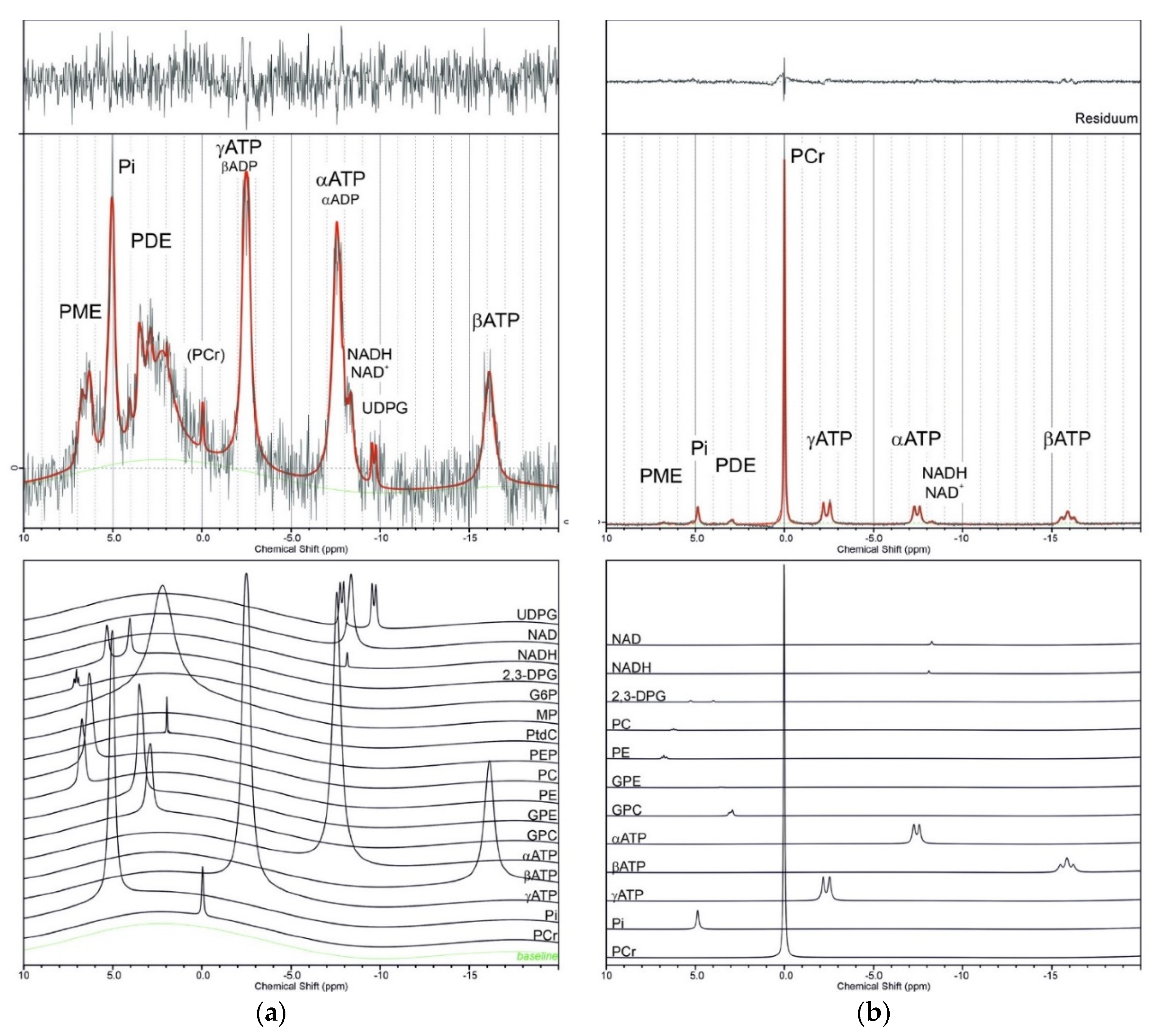
| Metabolite | Product Number | Concentration in Phantoms (mM) | Multiplicity | Chemical Shift at pH = 7.0 | Chemical Shift at pH = 7.5 | Chemical Shift In Vivo | J-Coupling (Hz) | Basis Set Deelchand | BASISp Set Present Study | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phosphocreatine | P7936-5G | 1 PCr | 5 | s | 0 | 0 | 0 | - | 0 | 0 |
| Inorganic phosphate | Pi | 40 | s | 4.78 | 5.27 | ~5 | - | 4.84 | 4.78 | |
| Adenosine triphosphate | A2383-1G | αATP | 10 | d | −7.98 | −7.94 | ~7–8 | 19.5 | −7.56 | −7.53 |
| βATP | 10 | t | −18.80 | −18.58 | ~16 | 20.0 | −16.18 | −16.18 | ||
| γATP | 10 | d | −4.18 | −3.37 | ~3–4 | 19.0 | −2.53 | −2.7 | ||
| Adenosine diphosphate | 29349990900 | αADP | 10 | d | −7.28 | −7.32 | - | 19.0 | - | - |
| βADP | 10 | d | −3.50 | −3.20 | - | 19.5 | - | - | ||
| Phosphoethanolamine | P0503-1G | PE (PME) | 10 | t | 6.74 | 6.85 | 6.78 | 7.0 | 6.77 | 6.77 |
| Phospholcholine | P0378G | PC (PME) | 10 | t | 6.19 | 6.35 | 5.9 | 6.0 | 6.23 | 6.23 |
| Glucose 1-phosphate | G1259-1G | G1P | 10 | d | 4.99 | overlay with Pi (5.20) | overlay with PME | 7.5 | - | 4.99 |
| Glucose 6-phosphate | G7879-1G | G6P | 10 | t | 7.03 | 7.30 | 7.1–7.2 | 6.1 | - | 7.03 |
| Phosphoenolpyruvate | P7127-500MG | PEP (PME) | 10 | s | 2.00 | 2.27 | 2.06 | - | 2 | |
| Phosphatidylcholine | PtdC | 3 10 | t | 2.14 | 2.13 | 2.06 | - | 2.14 | ||
| Glycerol-3-phosphoryl choline | G5291-100MG | GPC (PDE) | 10 | t | 2.97 | 2.96 | 2.76 | 5.5 | 2.94 | 2.97 |
| 2.3-diphosphoglycerate | D9134-100MG | 2,3-DPG(PDE) | 2 2 | 2- d | 4.09 | 4.6 | 5.5 | 6.0 | 5.23 | 4.05 |
| 3- t | 5.338 | 5.84 | 6.3 | 9.5 | 5.71 | 5.32 | ||||
| Nicotinamide adenine dinucleotide (reduced form) | N8129-1G | NADH | 10 | s | −8.16 | −8.16 | ~8.1 | −8.13 | −8.13 | |
| Nicotinamide adenine dinucleotide (oxygenated form) | 10127981001 | NAD+ | 10 | s | −8.32 | −8.32 | ~8.3 | 6.0 | −8.31 | −8.31 |
| Uridine diphosphoglucose | U4625-500MG | UDPG | 2 2 | d | −8.11 | −8.11 | −8.1 | 10.0 | - | −7.98 |
| UDPG | 2 2 | d | −9.78 | −9.78 | −9.8 | 10.0 | - | −9.78 | ||
| 4Membrane phospholipids | MP | 2.3 | 2.3 | |||||||
| 4Glycerophosphoethanolamine | GPE | 3.49 | 3.49 |
| Liver | γ-ATP | α-ATP | β-ATP | GPC | PCr | Pi | |
| jMRUI | Relative signal intensity | 23.3 | 26.9 | 13.4 | 21.6 | 2.5 | 12.3 |
| Relative CRLB | 2.0 | 1.9 | 4.0 | 3.9 | 19.6 | 3.9 | |
| LCModel | Relative signal intensity | 26.0 | 34.1 | 11.3 | 15.6 | 3.3 | 9.7 |
| Relative CRLB | 3.3 | 2.7 | 4.7 | 6.0 | 11.0 | 5.0 | |
| mean CV | 11 | 23 | 16 | 37 | 33 | 23 | |
| Muscle | γ-ATP | α-ATP | β-ATP | GPC | PCr | Pi | |
| jMRUI | Relative signal intensity | 12.4 | 10.3 | 10.0 | 2.6 | 60.2 | 4.4 |
| Relative CRLB | 1.2 | 1.5 | 2.0 | 8.8 | <1 | 1.9 | |
| LCModel | Relative signal intensity | 14.6 | 12.2 | 10.5 | 3.4 | 53.6 | 5.7 |
| Relative CRLB | 1.7 | 2.0 | 2.3 | 8.7 | <1 | 2.0 | |
| mean CV | 16 | 17 | 5 | 24 | 12 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedivy, P.; Dusilova, T.; Hajek, M.; Burian, M.; Krššák, M.; Dezortova, M. In Vitro 31P MR Chemical Shifts of In Vivo-Detectable Metabolites at 3T as a Basis Set for a Pilot Evaluation of Skeletal Muscle and Liver 31P Spectra with LCModel Software. Molecules 2021, 26, 7571. https://doi.org/10.3390/molecules26247571
Sedivy P, Dusilova T, Hajek M, Burian M, Krššák M, Dezortova M. In Vitro 31P MR Chemical Shifts of In Vivo-Detectable Metabolites at 3T as a Basis Set for a Pilot Evaluation of Skeletal Muscle and Liver 31P Spectra with LCModel Software. Molecules. 2021; 26(24):7571. https://doi.org/10.3390/molecules26247571
Chicago/Turabian StyleSedivy, Petr, Tereza Dusilova, Milan Hajek, Martin Burian, Martin Krššák, and Monika Dezortova. 2021. "In Vitro 31P MR Chemical Shifts of In Vivo-Detectable Metabolites at 3T as a Basis Set for a Pilot Evaluation of Skeletal Muscle and Liver 31P Spectra with LCModel Software" Molecules 26, no. 24: 7571. https://doi.org/10.3390/molecules26247571
APA StyleSedivy, P., Dusilova, T., Hajek, M., Burian, M., Krššák, M., & Dezortova, M. (2021). In Vitro 31P MR Chemical Shifts of In Vivo-Detectable Metabolites at 3T as a Basis Set for a Pilot Evaluation of Skeletal Muscle and Liver 31P Spectra with LCModel Software. Molecules, 26(24), 7571. https://doi.org/10.3390/molecules26247571







