Determination of Trimethylamine N-oxide and Betaine in Serum and Food by Targeted Metabonomics
Abstract
1. Introduction
2. Results
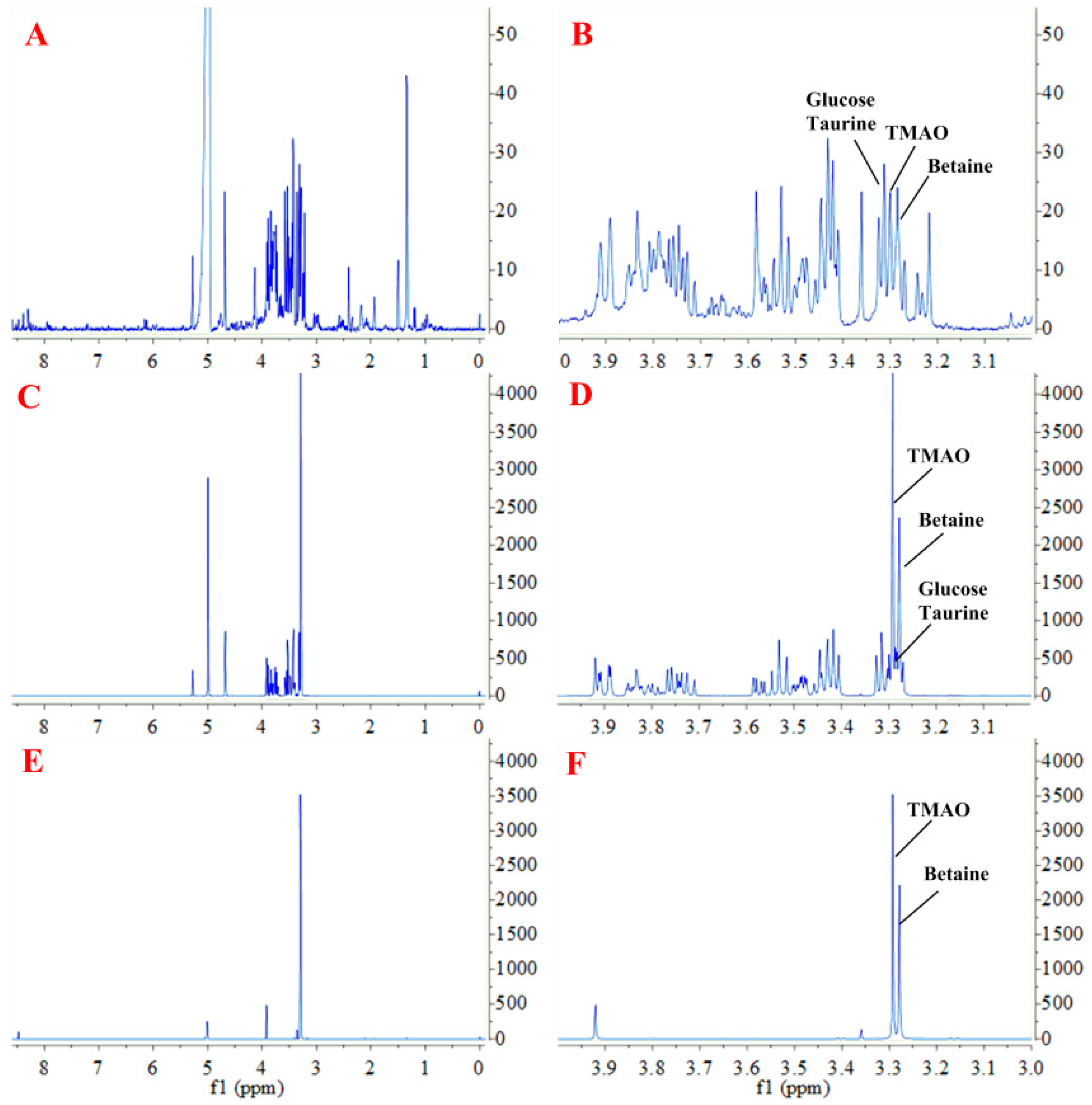
2.1. Sample Preparation for 1H NMR Measurement
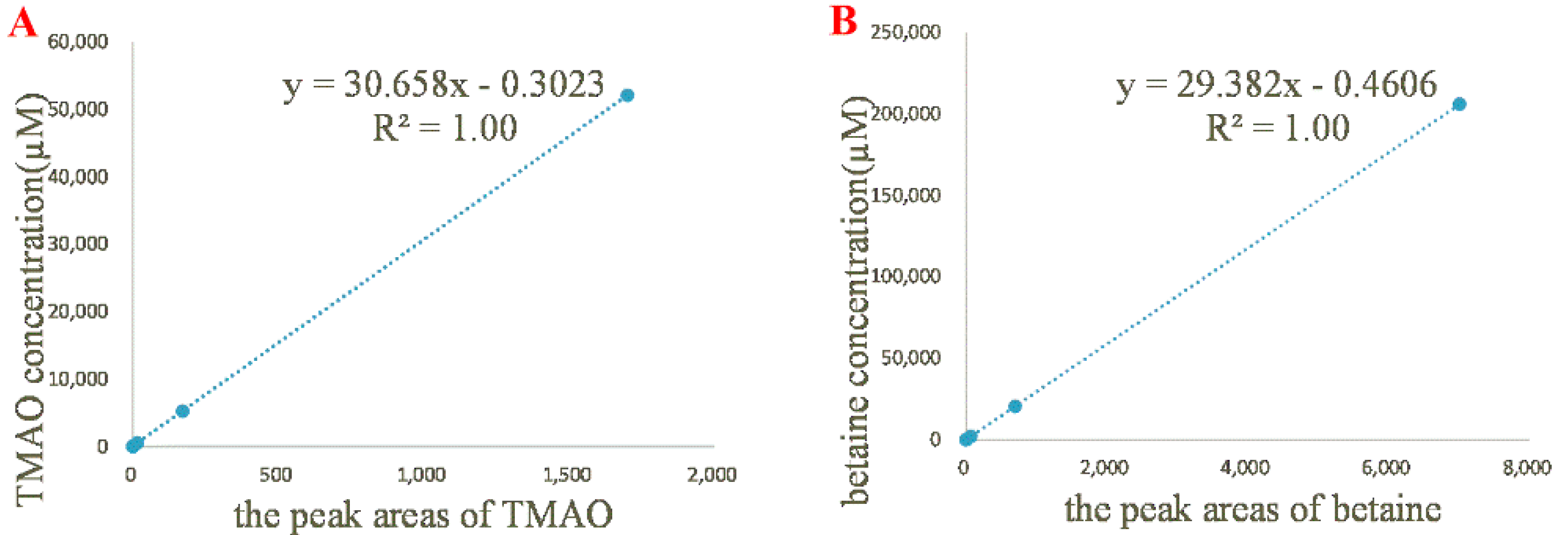
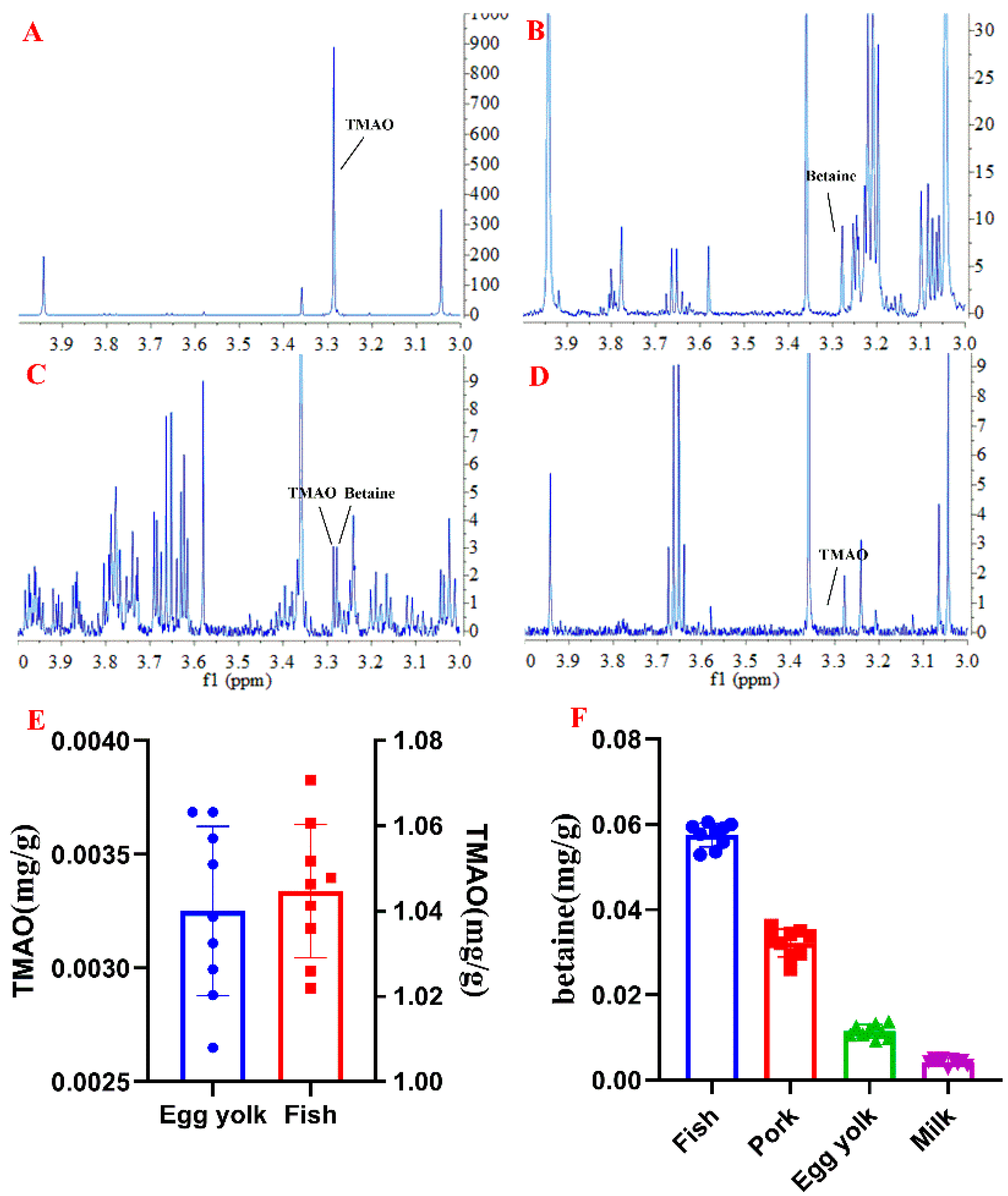
2.2. Quantitative 1H NMR Analysis and Method Validation
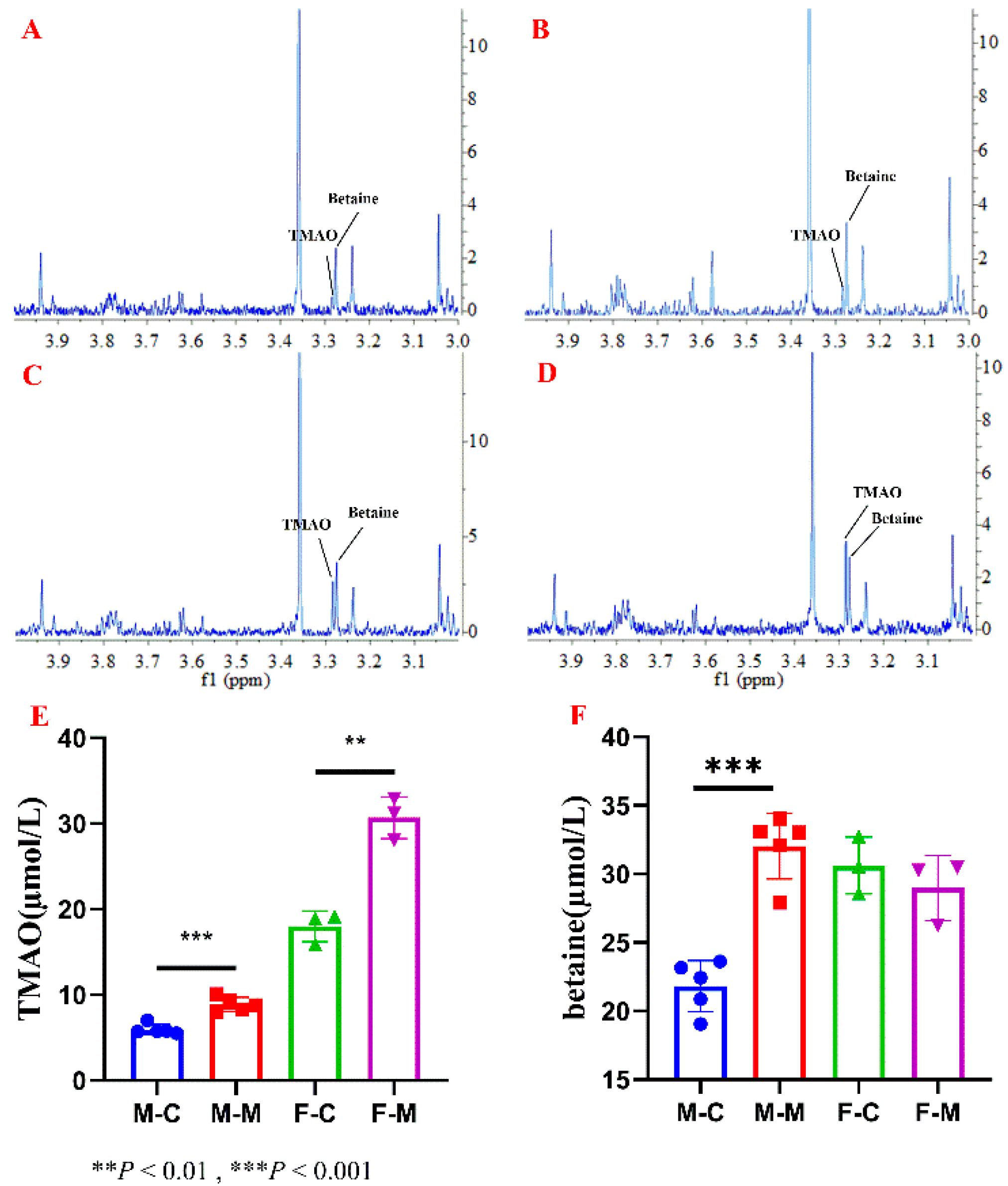
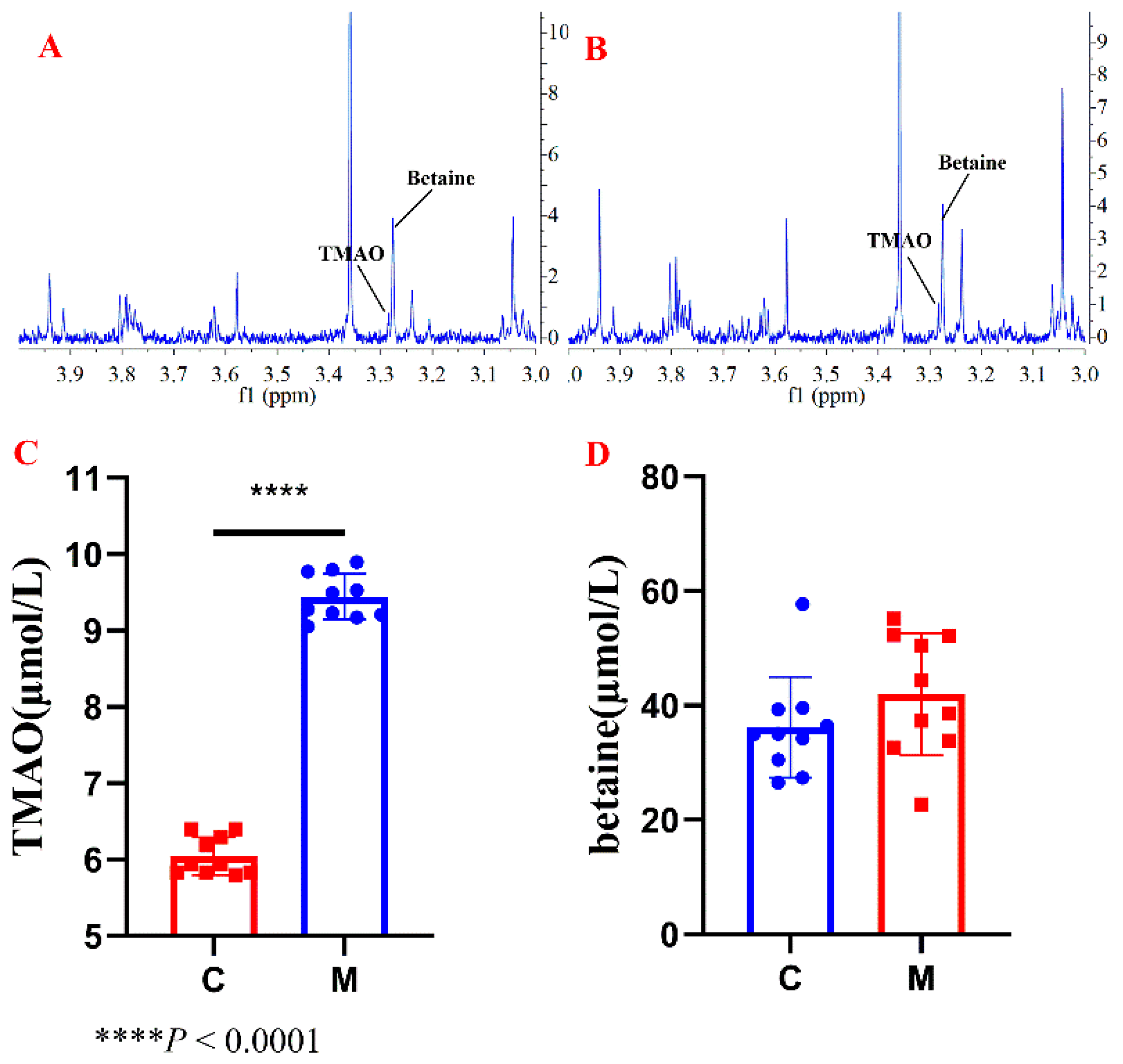
2.3. Applications to Serum and Food Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Animals
3.3. Sample Preparation
3.4. NMR Spectroscopy
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Quan, L.; Yi, J.; Zhao, Y.; Zhang, F.; Shi, X.-T.; Feng, Z.; Miller, H.L. Plasma trimethylamine N-oxide, a gut microbe–generated phosphatidylcholine metabolite, is associated with autism spectrum disorders. NeuroToxicology 2020, 76, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.A.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of Choline-Containing Compounds and Betaine in Common Foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; Von Scholten, B.J. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef] [PubMed]
- Al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metabolism 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Lang, D.; Yeung, C.; Peter, R.; Ibarra, C.; Gasser, R.; Itagaki, K.; Philpot, R.; Rettie, A. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes. Biochem. Pharmacol. 1998, 56, 1005–1012. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; Von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Ming-Liang, C. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Bjørnestad, E.Ø.; Olset, H.; Dhar, I.; Løland, K.; Pedersen, E.K.R.; Svingen, G.F.; Svardal, A.; Berge, R.K.; Ueland, P.M.; Tell, G.S.; et al. Circulating trimethyllysine and risk of acute myocardial infarction in patients with suspected stable coronary heart disease. J. Intern. Med. 2020, 288, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, B.; Księżarczyk, K.; Raćkowska, E.; Szlufik, S.; Koziorowski, D.; Giebułtowicz, J. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin. Chim. Acta 2020, 501, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; You, T.; Li, X.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Millard, H.R.; Musani, S.K.; Dibaba, D.T.; Talegawkar, S.A.; Taylor, H.A.; Tucker, K.L.; Bidulescu, A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: The Jackson Heart Study. Eur. J. Nutr. 2018, 57, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Awwad, H.M.; Kirsch, S.H.; Geisel, J.; Obeid, R. Measurement of concentrations of whole blood levels of choline, betaine, and dimethylglycine and their relations to plasma levels. J. Chromatogr. B 2014, 957, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Awwad, H.M.; Geisel, J.; Obeid, R. Determination of trimethylamine, trimethylamine N-oxide, and taurine in human plasma and urine by UHPLC–MS/MS technique. J. Chromatogr. B 2016, 1038, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Osté, M.C.J.; Bennett, D.W.; Jeyarajah, E.J.; Shalaurova, I.; Gruppen, E.G.; Hazen, S.L.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.; et al. High Betaine, a Trimethylamine N-Oxide Related Metabolite, Is Prospectively Associated with Low Future Risk of Type 2 Diabetes Mellitus in the PREVEND Study. J. Clin. Med. 2019, 8, 1813. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Zhang, M.Y.; Zhou, X.D.; Lai, X.R.; Yue, Q.H.; Tang, C.; Luo, W.Z.; Zhang, Y. Quality evaluation and species differentiation of Rhizoma coptidis by using proton nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2012, 747, 76–83. [Google Scholar] [CrossRef] [PubMed]
| TMAO | Betaine | |
|---|---|---|
| Chemical shift δ (ppm) | 3.29 | 3.28 |
| Linear regression equation | y = 30.66 x − 0.3023 | y = 29.38 x − 0.4606 |
| correlation coefficient | R2 = 1.00 | R2 = 1.00 |
| LOD (µM) | 1.00 | 1.52 |
| LOQ (µM) | 3.02 | 4.60 |
| Precision RSD (%) | 0.85 | 0.93 |
| Repeatability RSD (%) | 3.06 | 2.76 |
| Stability RSD (%) | 2.79 | 2.81 |
| Compound | Original (µmol) | Spiked (µmol) | Found (µmol) | Recovery (%) | Average Recovery (%) ± SD | RSD (%) |
|---|---|---|---|---|---|---|
| TMAO | 12.11 | 6.21 | 18.21 | 98.23 | 98.48 ± 0.23 | 0.23 |
| 12.06 | 12.14 | 24.02 | 98.52 | |||
| 12.08 | 18.17 | 30.01 | 98.68 | |||
| betaine | 48.24 | 24.13 | 71.79 | 97.60 | 98.84 ± 0.35 | 0.36 |
| 48.17 | 48.22 | 95.54 | 98.24 | |||
| 48.21 | 72.09 | 118.62 | 97.67 |
| Compound | Original (µmol) | Spiked (µmol) | Found (µmol) | Recovery (%) | Average Recovery (%) ± SD | RSD (%) |
|---|---|---|---|---|---|---|
| TMAO | 9.53 | 9.87 | 18.21 | 98.12 | 98.47 ± 0.50 | 0.51 |
| 9.52 | 9.87 | 24.02 | 99.04 | |||
| 9.55 | 9.87 | 30.01 | 98.25 | |||
| betaine | 37.33 | 38.79 | 71.79 | 98.18 | 97.95 ± 0.64 | 0.65 |
| 37.36 | 38.79 | 95.54 | 97.23 | |||
| 37.35 | 38.79 | 118.62 | 98.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Yu, H.; Lei, P.; Huang, S.; Ren, J.; Fan, W.; Han, L.; Yu, H.; Wang, Y.; Ren, M.; et al. Determination of Trimethylamine N-oxide and Betaine in Serum and Food by Targeted Metabonomics. Molecules 2021, 26, 1334. https://doi.org/10.3390/molecules26051334
He M, Yu H, Lei P, Huang S, Ren J, Fan W, Han L, Yu H, Wang Y, Ren M, et al. Determination of Trimethylamine N-oxide and Betaine in Serum and Food by Targeted Metabonomics. Molecules. 2021; 26(5):1334. https://doi.org/10.3390/molecules26051334
Chicago/Turabian StyleHe, Mingshuai, Heshui Yu, Peng Lei, Shengjie Huang, Juanning Ren, Wenjing Fan, Lifeng Han, Haiyang Yu, Yuefei Wang, Ming Ren, and et al. 2021. "Determination of Trimethylamine N-oxide and Betaine in Serum and Food by Targeted Metabonomics" Molecules 26, no. 5: 1334. https://doi.org/10.3390/molecules26051334
APA StyleHe, M., Yu, H., Lei, P., Huang, S., Ren, J., Fan, W., Han, L., Yu, H., Wang, Y., Ren, M., & Jiang, M. (2021). Determination of Trimethylamine N-oxide and Betaine in Serum and Food by Targeted Metabonomics. Molecules, 26(5), 1334. https://doi.org/10.3390/molecules26051334







