Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars
Abstract
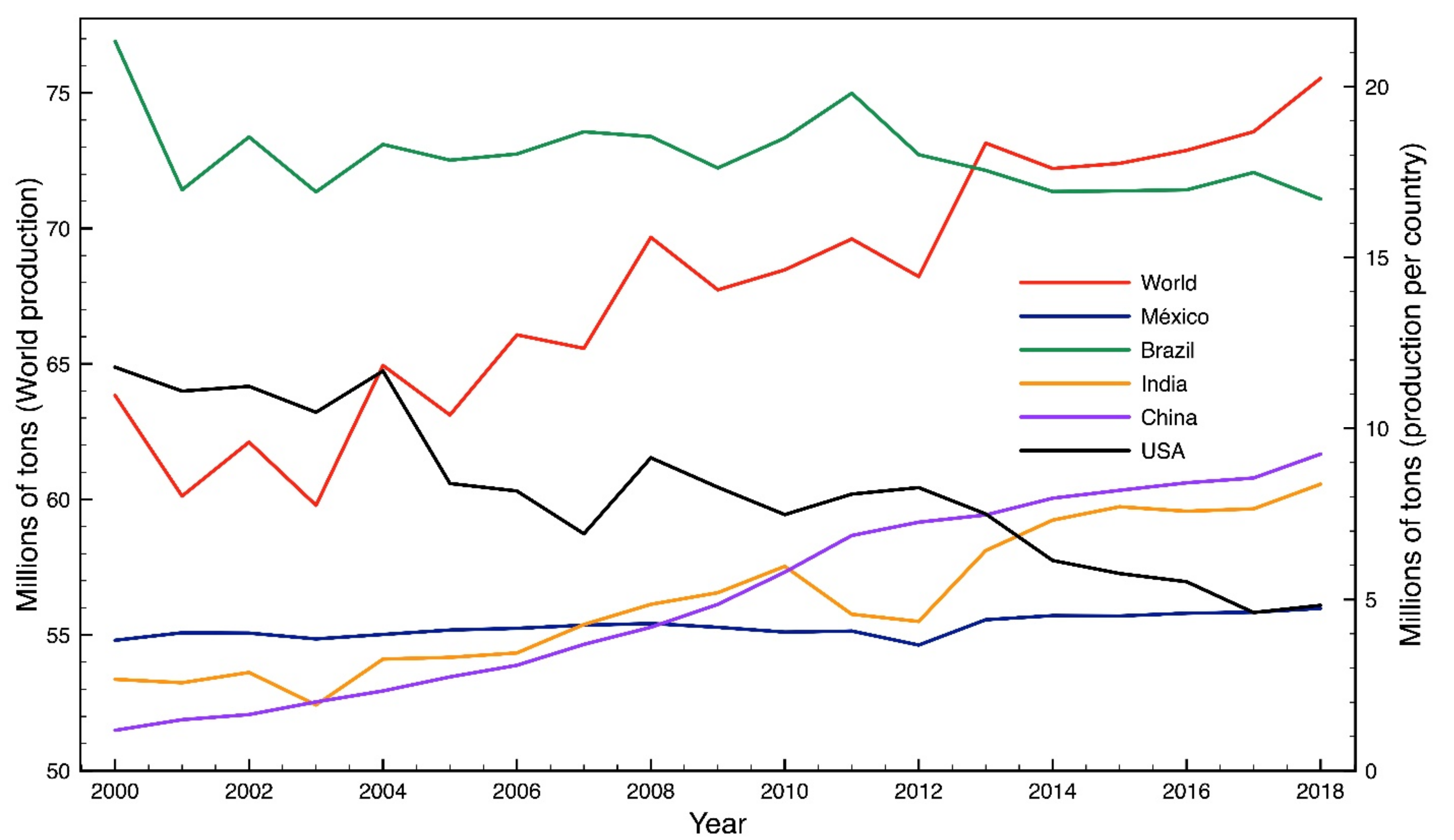
:1. Introduction
2. Results
2.1. Results for the Proximate and Chemical Analysis of Orange Peel
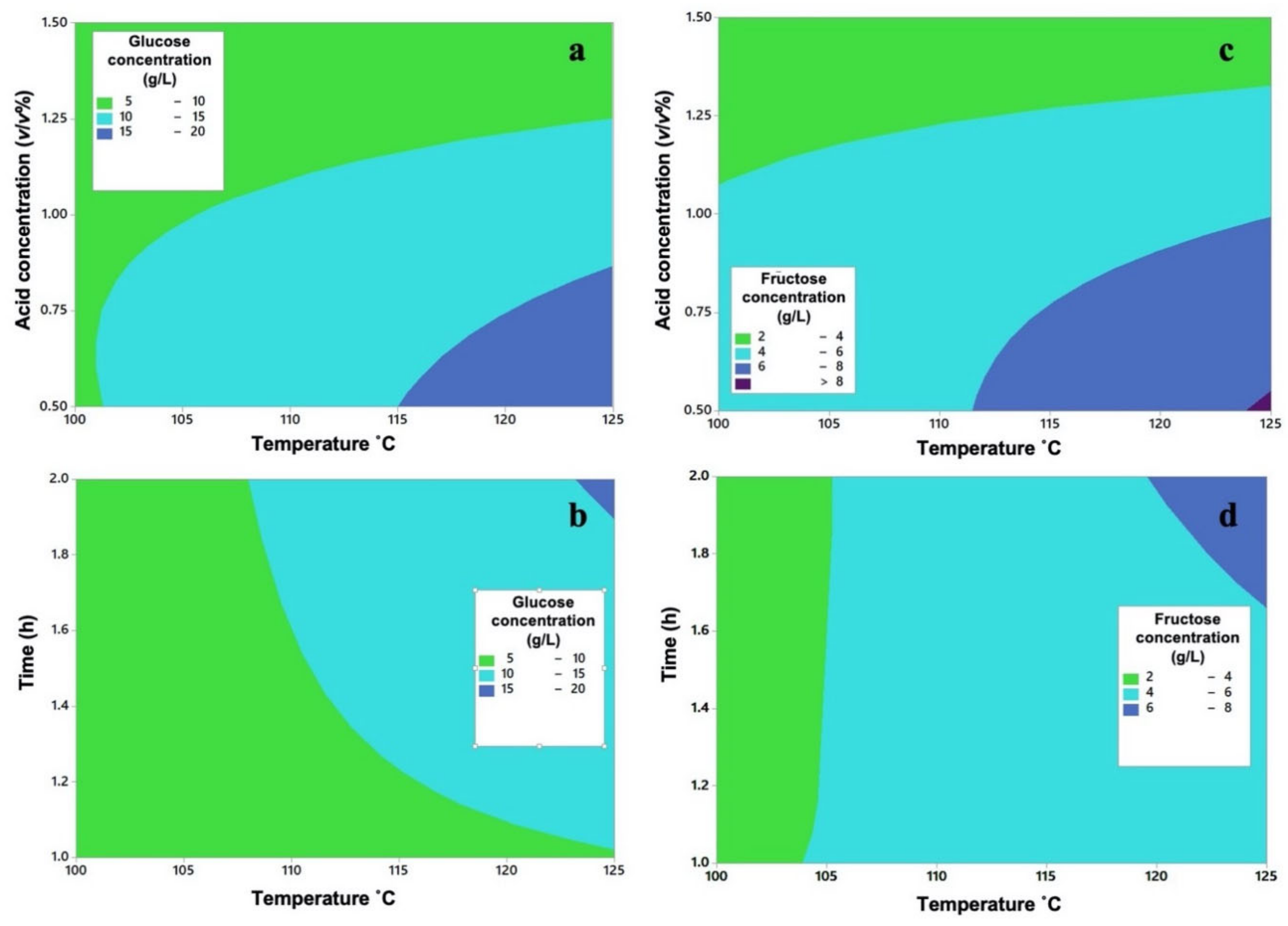
2.2. Design of Experiments of the Dilute Acid Hydrolysis
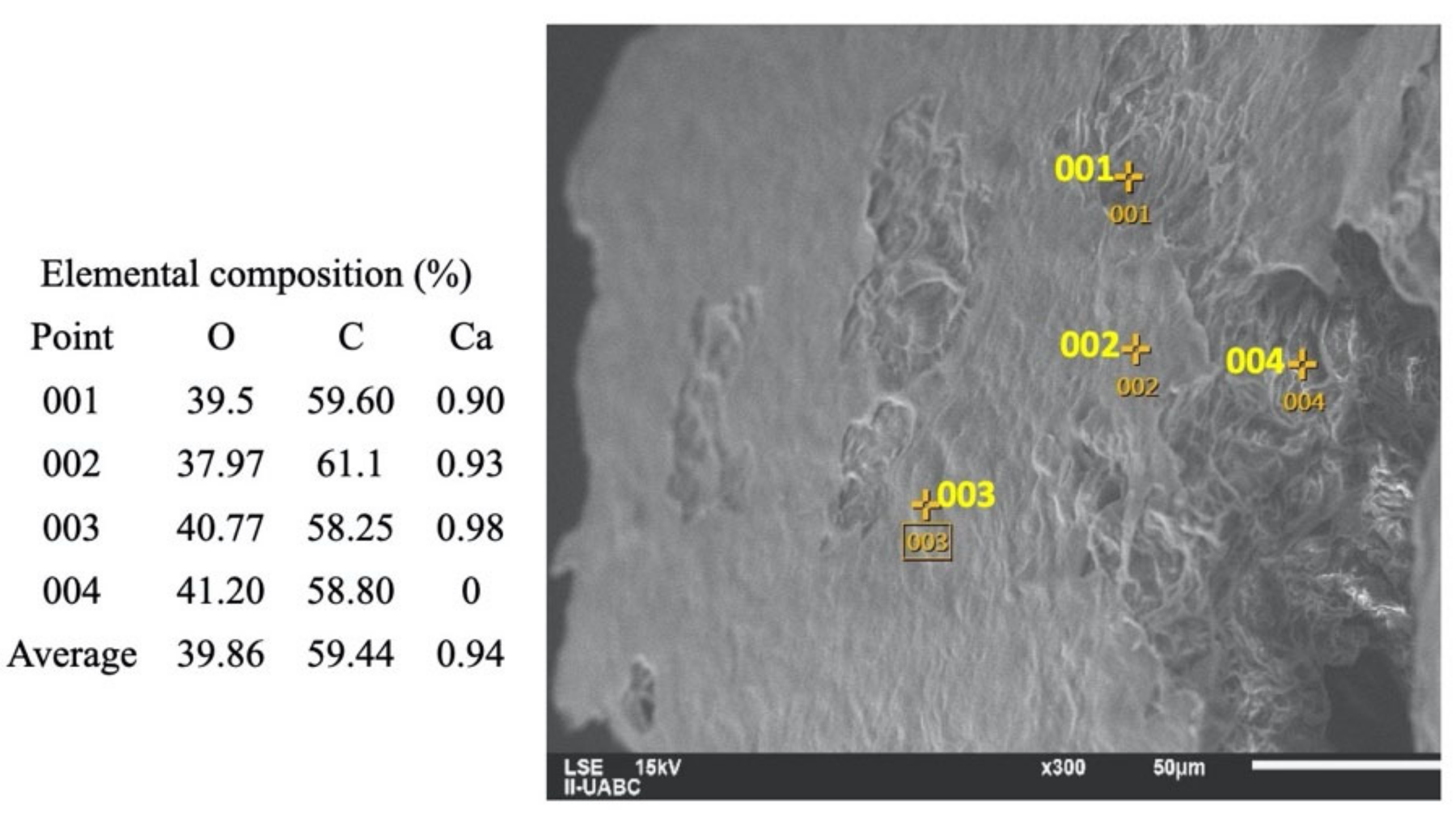
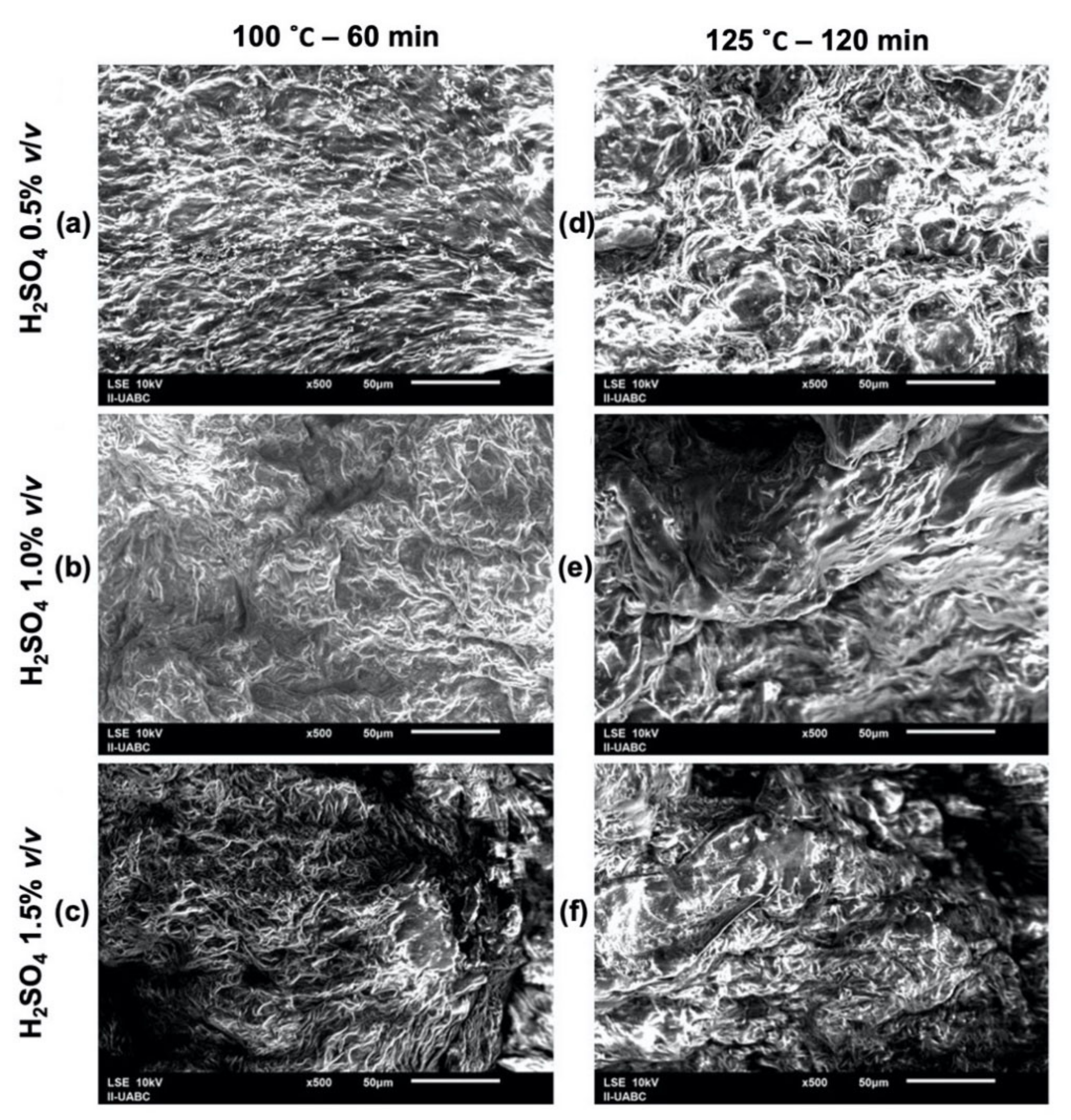
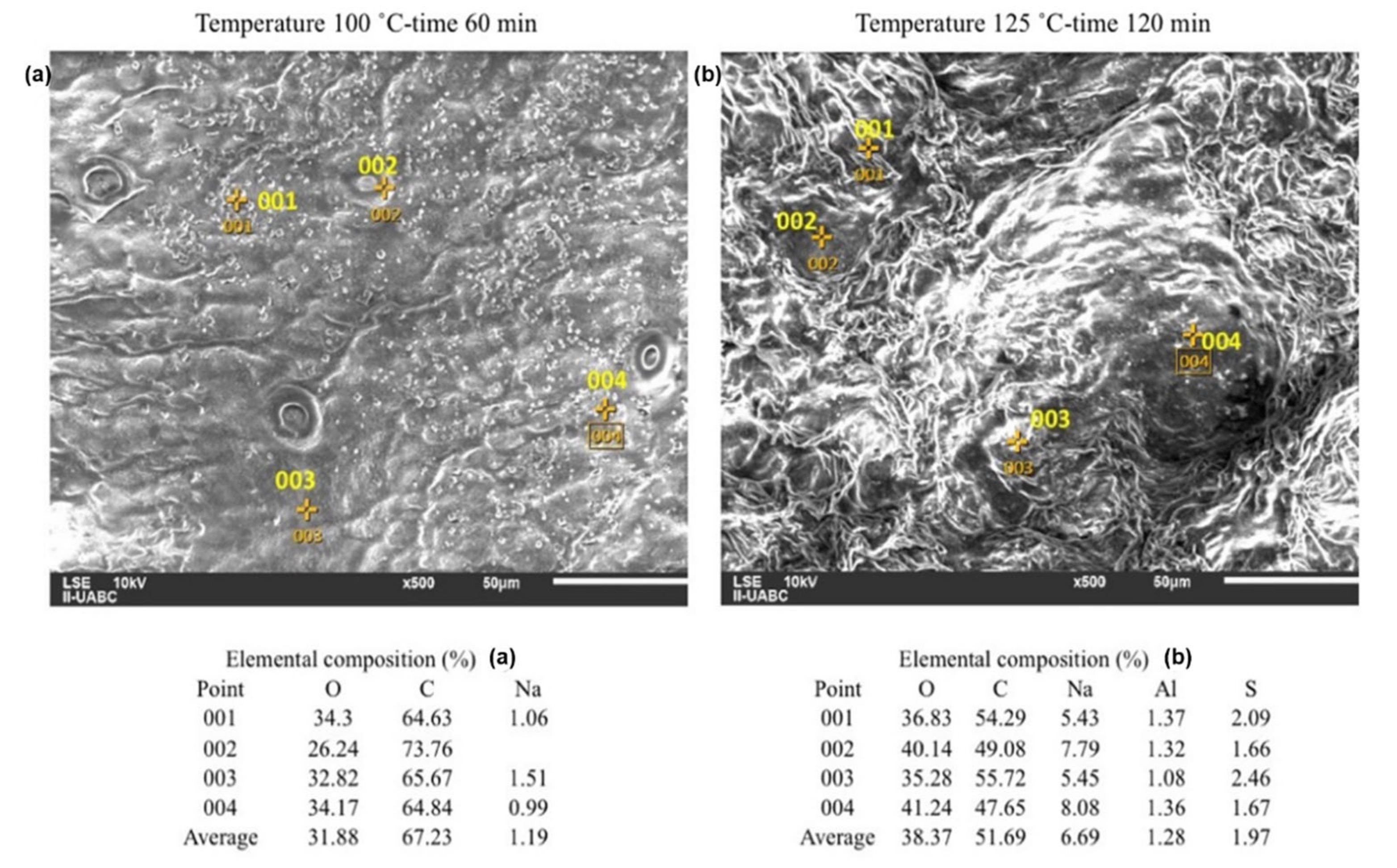
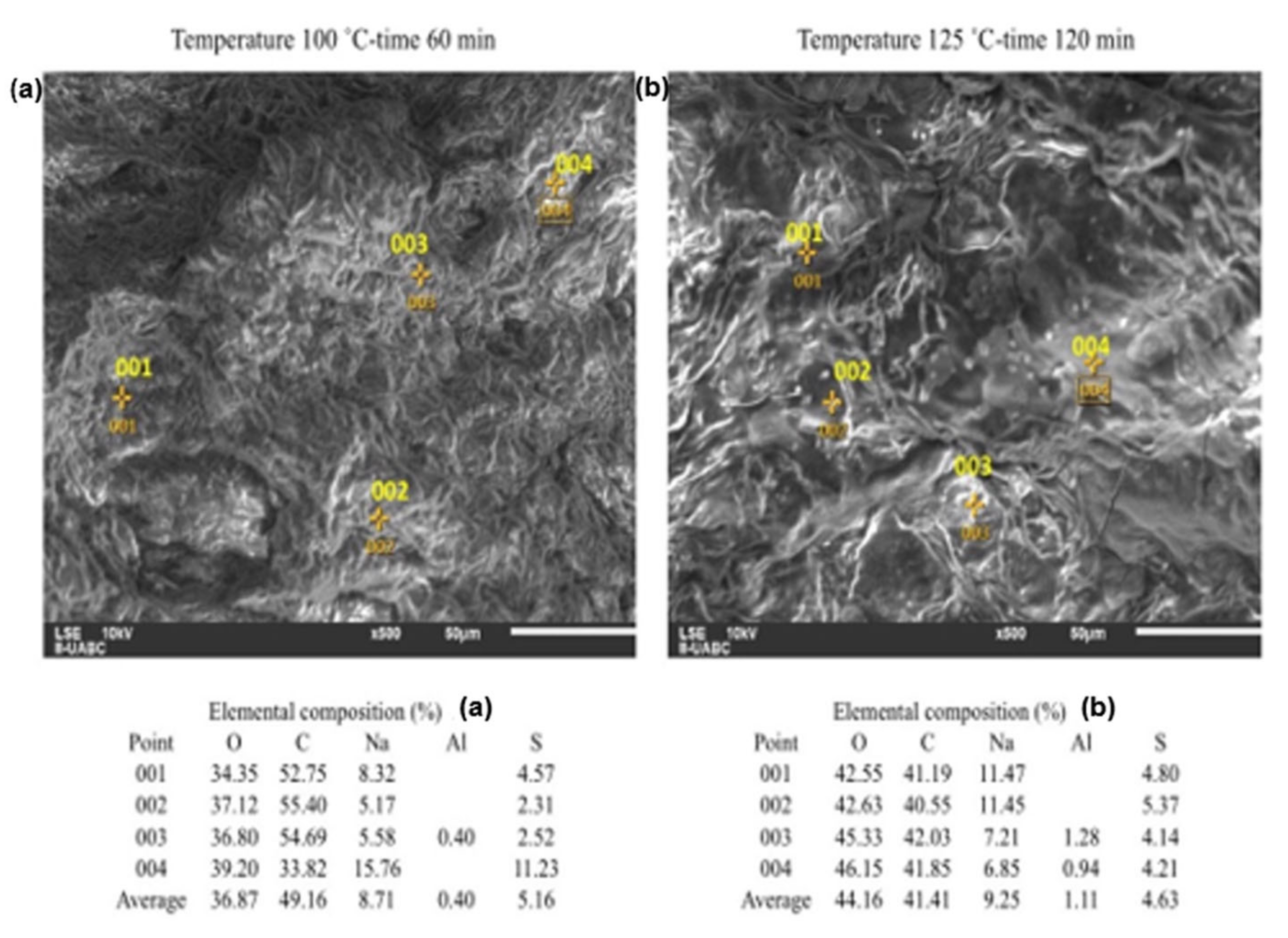
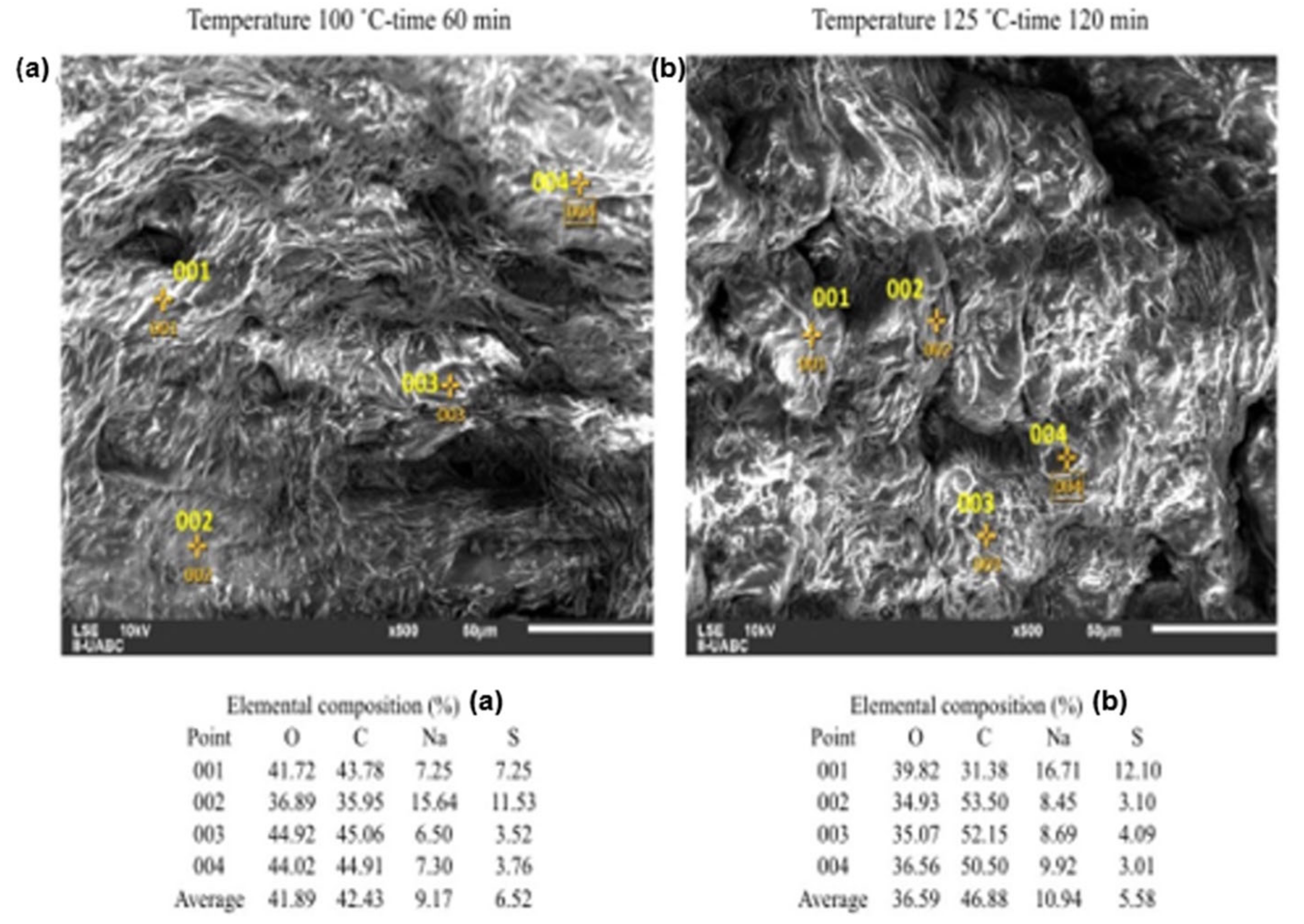
2.3. SEM-EDS Analyses
3. Discussion
4. Materials and Methods
4.1. Proximate and Chemical Analysis of Orange Peel
4.2. Design of Experiments for the Orange Peel Diluted Acid Hydrolysis
4.3. SEM-EDS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Food and Agriculture Organization FAOSTAT. Food Production. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 24 June 2020).
- Sistema de Información Agroalimentaria y Pesquera (SIAP). Cierre de la Producción por Estado. 2020. Available online: http://infosiap.siap.gob.mx:8080/agricola_siap_gobmx/AvanceNacionalCultivo.do (accessed on 24 June 2020).
- Gaind, S. Exploitation of orange peel for fungal solubilization of rock phosphate by solid state fermentation. Waste Biomass Valor. 2016, 8, 1351–1360. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A. Techno-economic and environmental assessment of p-Cymene and pectin production from orange peel. Waste Biomass Valor. 2015, 6, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Siles, J.A.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef]
- Aboagye, D.; Banadda, N.; Kiggundu, N.; Kabenge, I. Assessment of orange peel waste availability in Ghana and potential bio-oil yield using fast pyrolysis. Renew. Sust. Energ. Rev. 2017, 70, 814–821. [Google Scholar] [CrossRef]
- Maschke, R.W.; Geipel, K.; Bley, T. Modeling of plant in vitro cultures: Overview and estimation of biotechnological processes. Biotechno. Bioeng. 2015, 112, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santi, G.; Crognale, S.; Annibale, A.D.; Petruccioli, M.; Ruzzi, M.; Valentini, R.; Moresi, M. Orange peel pretreatment in a novel lab-scale direct steam-injection apparatus for ethanol production. Biomass Bioenerg. 2014, 61, 146–156. [Google Scholar] [CrossRef]
- Molina-Calle, M.; Priego-Capote, F.; Luque de Castro, M.D. Development and application of a quantitative method for determination of flavonoids in orange peel: Influence of sample pretreatment on composition. Talanta 2015, 144, 349–355. [Google Scholar] [CrossRef]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452. [Google Scholar] [CrossRef]
- Sarkar, O.; Katakojwala, R.; Mohan, S.V. Low Carbon Hydrogen production from waste based Biorefinery System and Environmental Sustainability Assessment. Green Chem. 2021, 23, 561–574. [Google Scholar] [CrossRef]
- Deng, J.-J.; Zhang, M.-S.; Li, Z.-W.; Lu, D.L.; Mao, H.-H.; Zhu, M.-J.; Li, J.-Z.; Luo, X.-C. One-step processing of shrimp shell waste with a chitinase fused to a carbohydrate-binding module. Green Chem. 2020, 22, 6862–6873. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Campbell, H.E.; García, C.; Coronado, M.A.; León, J.A.; Sagaste, C.A.; Pérez, L.J. Extraction and Characterization of Orange Peel Essential Oil from Mexico and United States of America. J. Essent. Oil. Bear. Plants 2017, 20, 897–914. [Google Scholar] [CrossRef]
- Chen, W.-H.; Jang, M.-F.; Jheng, S.-L.; Lo, C.-J.; Wang, W. Cellulosic sugars from biomass: Effect of acid presoaking on pretreatment efficiency and operating cost estimation for sugar production. Bioresour. Technol. Rep. 2019, 7, 100259. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Wu, Z.-Y.; Sriariyanun, M. Evaluation of Macaranga tanarius as a biomass feedstock for fermentable sugars production. Bioresour. Technol. 2019, 294, 122195. [Google Scholar] [CrossRef] [PubMed]
- Kassaye, S.; Pant, K.K.; Jain, S. Hydrolysis of cellulosic bamboo biomass into reducing sugars via a combined alkaline solution and ionic liquid pretreatment steps. Renew. Energ. 2017, 104, 177–184. [Google Scholar] [CrossRef]
- Khamis, N.A.; Shamsudin, S.; Rahman, N.S.A.; Kasim, K.F. Effects of autohydrolysis on rice biomass for reducing sugars production. Mater. Today Proc. 2019, 16, 2078–2087. [Google Scholar] [CrossRef]
- Láinez, M.; Héctor, A.; Ruiz, H.A.; Castro-Luna, A.A.; Martínez-Hernández, S. Release of simple sugars from lignocellulosic biomass of Agave salmiana leaves subject to sequential pretreatment and enzymatic saccharification. Biomass Bioenerg. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Martínez, C.M.; Adamovic, T.; Cantero, D.A.; Cocero, M.J. Scaling up the production of sugars from agricultural biomass by ultrafast hydrolysis in supercritical water. J. Supercrit. Fluid. 2019, 143, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Timung, R.; Goud, V.V. Subcritical water hydrolysis of spent Java Citronella biomass for production of reducing sugar. Mater. Today Proc. 2018, 5, 23128–23135. [Google Scholar] [CrossRef]
- Elliston, A.; Collins, S.R.A.; Faulds, C.B.; Roberts, I.N.; Waldron, K.W. Biorefining of waste paper biomass: Increasing the concentration of glucose by optimizing enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2014, 172, 3621–3634. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment-a review. Biotechnol. Bioeng. 2012, 6, 1430–1442. [Google Scholar] [CrossRef] [Green Version]
- Murthy, G.S.; Johnston, D.B.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Design and Evaluation of an Optimal Controller for Simultaneous Saccharification and Fermentation Process. Appl. Biochem. Biotechnol. 2012, 166, 87–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerurkar, M.; Joshi, M.; Adivarekar, R. Bioscouring of cotton using lipase from marine bacteria bacillus sonorensis. Appl. Biochem. Biotechnol. 2015, 175, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Buyukkileci, A.O.; Fernandez, M.M.L.; Tari, C. Utilization of orange peel, a food industrial waste, in the production of exo-polygalacturonase by pellet forming Aspergillus sojae. Bioprocess. Biosyst. Eng. 2015, 38, 749–760. [Google Scholar] [CrossRef] [Green Version]
- Tai, C.; Keshwani, D. Impact of pretreatment with dilute sulfuric acid under moderate temperature on hydrolysis of corn stover with two enzyme systems. Appl. Biochem. Biotechnol. 2014, 172, 2628–2639. [Google Scholar] [CrossRef]
- Chaiprapat, S.; Wongchana, S.; Loykulnant, S.; Kongkaew, C.; Charnnok, B. Evaluating sulfuric acid reduction, substitution, and recovery to improve environmental performance and biogas productivity in rubber latex industry. Process. Saf. Environ. 2015, 94, 420–429. [Google Scholar] [CrossRef]
- Lindgren, M.; Siljander, S.; Suihkonen, R.; Pohjanne, P.; Vuorinen, J. Erosion-corrosion resistance of various stainless steel grades in high-temperature sulfuric acid solution. Wear 2016, 364–365, 10–21. [Google Scholar] [CrossRef]
- Jin, M.; Sarks, C.; Bals, B.D.; Posawatz, N.; Gunawan, C.; Dale, B.E.; Balan, V. Toward high solids loading process for lignocellulosic biofuel production at a low cost. Biotechnol. Bioeng. 2016, 5, 980–988. [Google Scholar] [CrossRef]
- Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. Available online: https://www.nrel.gov/docs/gen/fy08/42621.pdf (accessed on 20 August 2018).
- ASTM International. ASTM E872-82–Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM International. ASTM E830-87–Standard Test Method for Ash in the Analysis Sample of Refuse Derived Fuel; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- TAPPI. Preparation of Wood for Chemical Analysis Test Method T264 cm-07 (TAPPI); TAPPI: Peachtree Corners, GA, USA, 2007. [Google Scholar]
- TAPPI. Water Solubility of Wood and Pulp Test Method T207 cm-99 (TAPPI); TAPPI: Peachtree Corners, GA, USA, 1999. [Google Scholar]
- ASTM International. ASTM D1106-96–Standard Test Method for Acid-Insoluble Lignin in Wood; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- ASTM International. ASTM D1104-56–Method of Test for Hollocelulose in Wood; ASTM International: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Rowell, R.M.; Pettersen, R.; Tshabalala, M.A. Handbook of Wood Chemistry and Wood Composites, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2013; pp. 33–74. [Google Scholar]
- Chong, Y.; Yan, A.; Yang, X.; Cai, Y.; Chen, J. An optimum fermentation model established by genetic algorithm for biotransformation from crude polydatin to resveratrol. Appl. Biochem. Biotechnol. 2012, 166, 446–457. [Google Scholar] [CrossRef]
- Dai, J.-Y.; Zhao, P.; Cheng, X.-L.; Xiu, Z.-L. Enhanced production of 2,3-Butanediol from sugarcane molasses. Appl. Biochem. Biotechnol. 2015, 175, 3014–3024. [Google Scholar] [CrossRef]
- Bustamante, J.; Stempvoort, S.; García-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis). Flavour Fragr. J. 2004, 20, 80–85. [Google Scholar] [CrossRef]
- Ji, W.; Shen, Z.; Wen, Y. Hydrolysis of wheat straw by dilute sulfuric acid in a continuous mode. Chem. Eng. J. 2015, 260, 20–27. [Google Scholar] [CrossRef]
- García, J.F.M.; Sánchez, S.; Cuevas, M. Evaluation of the effect of the dilute acid hydrolysis on sugars release from olive prunings. Renew. Energ. 2013, 51, 382–387. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Julio-Altamiranda, Y.T.; Mercado-Pacheco, J.D.; Sánchez-Tuirán, E.L.; González-Delgado, A.D. Evaluation of mechanical-green solvent pretreatment of oil palm wastes for reducing sugars production in North-Colombia. Sustain. Chem. Pharm. 2020, 16, 100256. [Google Scholar] [CrossRef]
- Manmai, N.; Unpaprom, Y.; Ponnusamy, V.K.; Ramaraj, R. Bioethanol production from the comparison between optimization of sorghum stalk and sugarcane leaf for sugar production by chemical pretreatment and enzymatic degradation. Fuel 2020, 278, 118262. [Google Scholar] [CrossRef]
- Martins, E.H.; Ratuchne, A.; Machado, G.O.; Knob, A. Canola meal as a promising source of fermentable sugars: Potential of the Penicillium glabrum crude extract for biomass hydrolysis. Biocatal. Agric. Biotechnol. 2020, 27, 101713. [Google Scholar] [CrossRef]
- Wang, H.; Srinivasan, R.; Yu, F.; Steele, P.; Li, Q.; Mitchell, B.; Samala, A. Effect of acid, steam explosion, and size reduction pretreatments on Bio-oil production from sweetgum, switchgrass, and corn stover. Appl. Biochem. Biotechnol. 2012, 167, 285–287. [Google Scholar] [CrossRef] [PubMed]
| Analysis | Composition (% in Weight) | Standard Deviation | |
|---|---|---|---|
| Proximate | Moisture | 73.530% | 0.477% |
| Volatiles | 99.261% | 0.074% | |
| Ash | 0.052% | 0.004% | |
| Fixed carbon | 0.687% | 0.078% | |
| Chemical | Acetone extractables | 6.821% | 0.604% |
| Hot water extractables | 40.399% | 2.595% | |
| Lignin determination | 19.801% | 3.595% | |
| Holocellulose determination | 78.110% | 4.404% | |
| Cellulose determination | 69.096% | 9.015% | |
| Hemicellulose determination | 5.433% | 5.433% | |
| Run | H2SO4 Concentration (%v/v) (A) | Temperature (°C) (B) | Time (h) (C) | Glucose (g/L) | Average Glucose (g/L) | Standard Deviation (Glucose) | Fructose (g/L) | Average Fructose (g/L) | Standard Deviation (Fructose) |
|---|---|---|---|---|---|---|---|---|---|
| 1,2 | 0.5 | 100 | 1 | 11.302 10.016 | 10.659 | 0.909 | 4.651 5.092 | 4.871 | 0.312 |
| 3,4 | 0.5 | 100 | 2 | 7.940 8.821 | 8.380 | 0.623 | 3.367 3.499 | 3.433 | 0.093 |
| 5,6 | 0.5 | 125 | 1 | 13.843 17.009 | 15.426 | 2.239 | 5.242 8.936 | 7.089 | 2.612 |
| 7,8 | 0.5 | 125 | 2 | 24.585 19.189 | 21.887 | 3.816 | 9.709 8.862 | 9.285 | 0.599 |
| 9,10 | 1 | 100 | 1 | 8.212 7.998 | 8.105 | 0.151 | 4.231 3.184 | 3.707 | 0.740 |
| 11,12 | 1 | 100 | 2 | 9.500 10.309 | 9.904 | 0.572 | 3.580 5.600 | 4.59 | 1.428 |
| 13,14 | 1 | 125 | 1 | 9.686 11.373 | 10.529 | 1.193 | 4.049 5.090 | 4.569 | 0.736 |
| 15,16 | 1 | 125 | 2 | 16.727 15.826 | 16.276 | 0.637 | 6.803 7.908 | 7.355 | 0.781 |
| 17,18 | 1.5 | 100 | 1 | 7.080 6.039 | 6.559 | 0.736 | 3.880 2.377 | 3.128 | 1.063 |
| 19,20 | 1.5 | 100 | 2 | 3.397 4.192 | 3.794 | 0.562 | 1.768 1.742 | 1.755 | 0.018 |
| 21,22 | 1.5 | 125 | 1 | 3.064 4.274 | 3.669 | 0.856 | 1.829 2.015 | 1.922 | 0.132 |
| 23,24 | 1.5 | 125 | 2 | 8.604 8.709 | 8.656 | 0.074 | 3.600 3.704 | 3.652 | 0.074 |
| Variation Source | Glucose | Fructose | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Square Sum | Freedom Degrees | Mean Square | F0 | F Critic | Square Sum | Freedom Degrees | Mean Square | F0 | F | |
| Critic | ||||||||||
| Acid concentration (A) | 292.829 | 2 | 146.415 | 71.54 | 3.89 | 52.911 | 2 | 26.456 | 26.06 | 3.89 |
| Temperature (B) | 140.568 | 1 | 140.568 | 68.68 | 4.75 | 25.911 | 1 | 25.577 | 25.20 | 4.75 |
| Time (C) | 32.441 | 1 | 32.441 | 15.85 | 4.75 | 3.813 | 1 | 3.813 | 3.76 | 4.75 |
| AB | 67.025 | 2 | 33.512 | 16.37 | 3.89 | 13.803 | 2 | 6.902 | 6.80 | 3.89 |
| AC | 7.251 | 2 | 3.625 | 1.77 | 3.89 | 3.267 | 2 | 1.634 | 1.61 | 3.89 |
| BC | 69.629 | 1 | 69.629 | 34.02 | 4.75 | 12.447 | 1 | 12.448 | 12.26 | 4.75 |
| ABC | 6.403 | 2 | 3.201 | 1.56 | 3.89 | 0.787 | 2 | 0.393 | 0.39 | 3.89 |
| Error | 24.560 | 12 | 2.047 | 12.181 | 12 | 1.015 | ||||
| Total | 640.705 | 23 | 124.786 | 23 | ||||||
| Sample | Stage | Analysis | Conditions and Equipment | Reference |
|---|---|---|---|---|
| Orange peel | Proximate analysis | Moisture | 45 °C for 48 h inside a muffle | [30] |
| Volatiles | 950 °C for 7 min without air | [31] | ||
| Ash | 580 °C for 4 h inside a muffle | [32] | ||
| Fixed carbon | The difference in % of the sample, moisture, volatiles and ash analysis | [32] | ||
| Chemical analysis | Acetone extractable | 8 h Soxhlet extraction with acetone | [33] | |
| Water extractable | 3 h boiling water with condenser reflux | [34] | ||
| Lignin % | 4 h, 15 mL of H2SO4, stirring and 560 mL of distilled water | [35] | ||
| Holocellulose % | 150 mL water, 0.2 mL of acetic acid and 1 g of sodium chlorite per hour for 4 h. | [36] | ||
| Cellulose % | 25 mL of NaOH at 17.5 %, 100 mL of NaOH at 8.3%, 10 mL of acetic acid and water for 105 min. | [37] | ||
| Essential oil extraction | Hydrodistillation | 65 g of orange peel, 90 min, orange peel grinding of 1 min, 500 mL of water | [13] | |
| Hydrolysis | Diluted acid hydrolysis | H2SO4 concentration, time and temperature according to the factorial design | [38,39] | |
| pH stabilization | NaOH at 0.5 N until a pH of 4.8–5.2 was reached | [42] | ||
| Orange peel drying | 65 °C for 24 h | |||
| SEM-EDS | SEM micrography | JEOL JSM-6010LA SEM, working distance 11 mm, 10 kV, 50 Pa, 300x, 500x | [48] | |
| EDS analysis | ||||
| Hydrosylate | Reducing sugars determination | 3,5 DNS reagent | 2.5 g of 3,5-Dinitric salicylic acid, 7.5 g of mixed potassium sodium tartrate, 4 g of NaOH in 250 mL of water | [44,45,47] |
| Calibration curve | Glucose and fructose calibration curves prepared from 0.2 g/L to 2 g/L with water | [43,44] | ||
| Sample preparation | 1:10 dilution of the hydrosylate in distillated water and 1:1 diluted hydrosylate with DNS reagent | |||
| Reducing sugars quantification | Perkin-Elmer Lambda 25 UV-Vis Spectrometer at 575 nm measured in quartz cell | [43,44,47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. https://doi.org/10.3390/molecules26051348
Ayala JR, Montero G, Coronado MA, García C, Curiel-Alvarez MA, León JA, Sagaste CA, Montes DG. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules. 2021; 26(5):1348. https://doi.org/10.3390/molecules26051348
Chicago/Turabian StyleAyala, José R., Gisela Montero, Marcos A. Coronado, Conrado García, Mario A. Curiel-Alvarez, José A. León, Carlos A. Sagaste, and Daniela G. Montes. 2021. "Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars" Molecules 26, no. 5: 1348. https://doi.org/10.3390/molecules26051348
APA StyleAyala, J. R., Montero, G., Coronado, M. A., García, C., Curiel-Alvarez, M. A., León, J. A., Sagaste, C. A., & Montes, D. G. (2021). Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules, 26(5), 1348. https://doi.org/10.3390/molecules26051348







