Anti-Inflammatory Properties In Vitro and Hypoglycaemic Effects of Phenolics from Cultivated Fruit Body of Phellinus baumii in Type 2 Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PPE
2.2. Measurement of Anti-Inflammatory Activity In Vitro
2.3. Animal Breeding Conditions and Treatments
2.4. Determination of Serum and Tissue Biochemical Index in Mice
2.5. Oral Glucose Tolerance Test
2.6. Quantitative Real-Time PCR
2.7. Hematoxylin and Eosin Staining
2.8. Statistical Analysis
3. Results and Discussion
3.1. Anti-Inflammatory Properties of PPE
3.2. Effect of PPE on the Body Weight Gain and Blood Glucose Level
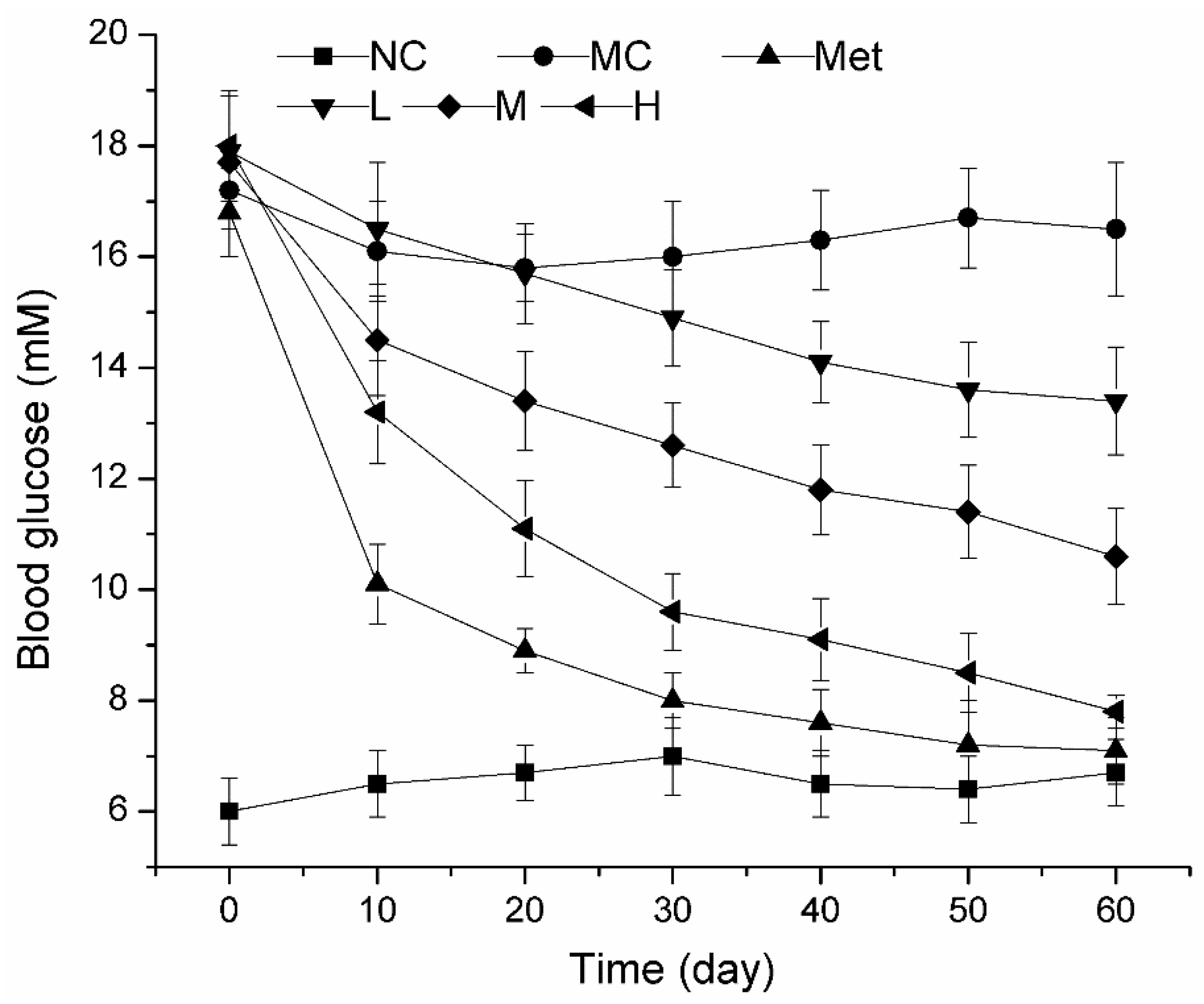
3.3. Dynamic Changes of Blood Glucose during PPE Treatment in Mice
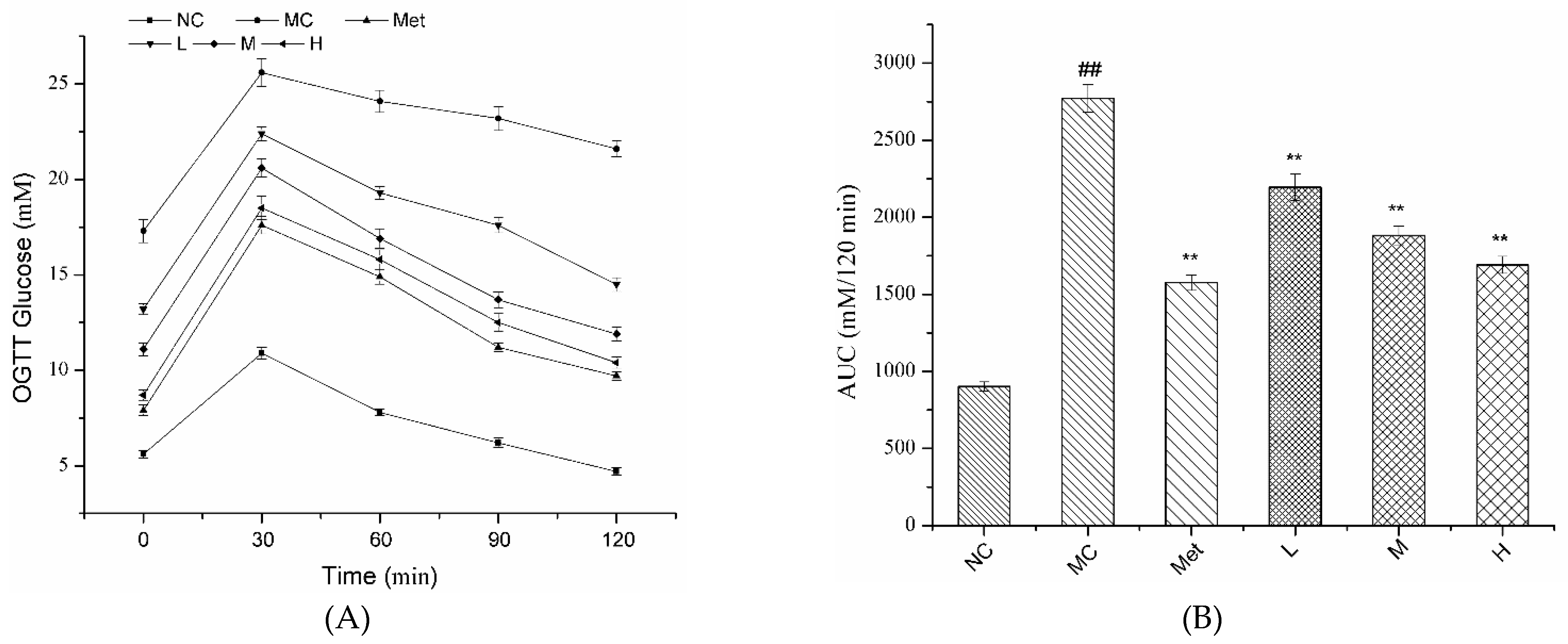
3.4. Effects of PPE on OGTT in Mice
3.5. Effects of PPE on Insulin and Insulin Resistance Index in Mice
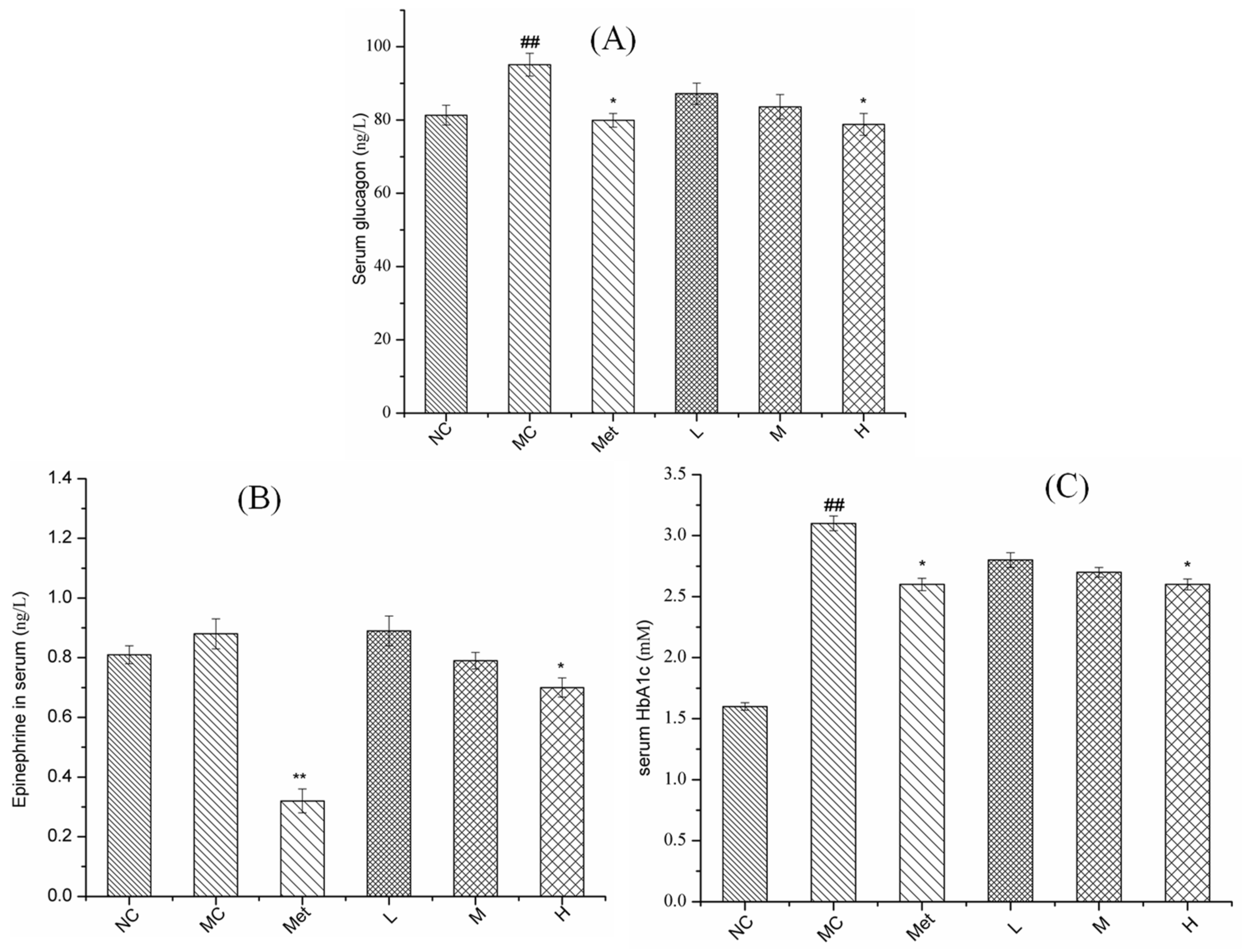
3.6. Effects of PPE on Serum Glucose Metabolism in Mice
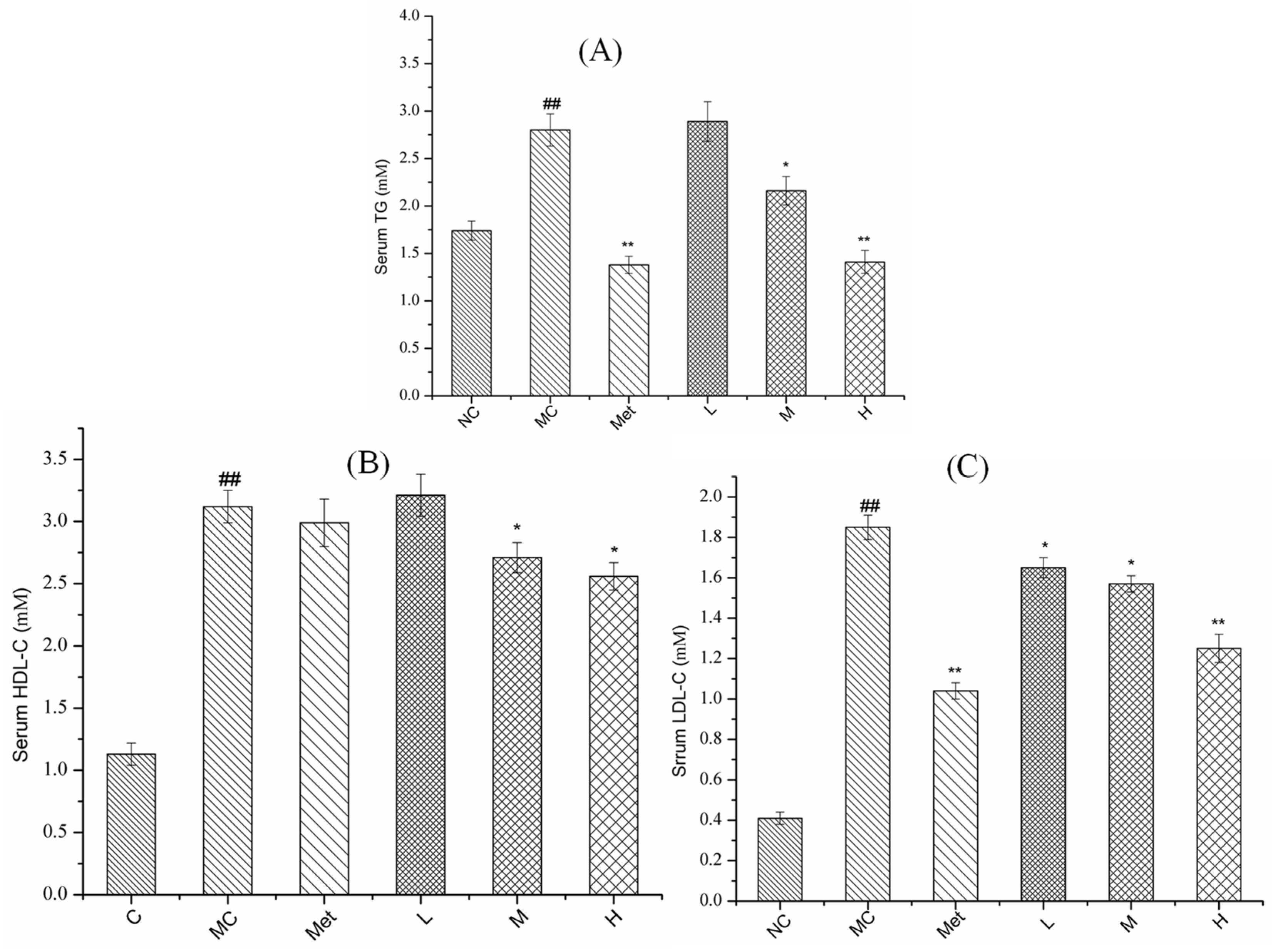
3.7. Effects of PPE on Serum Lipid Metabolism in Mice
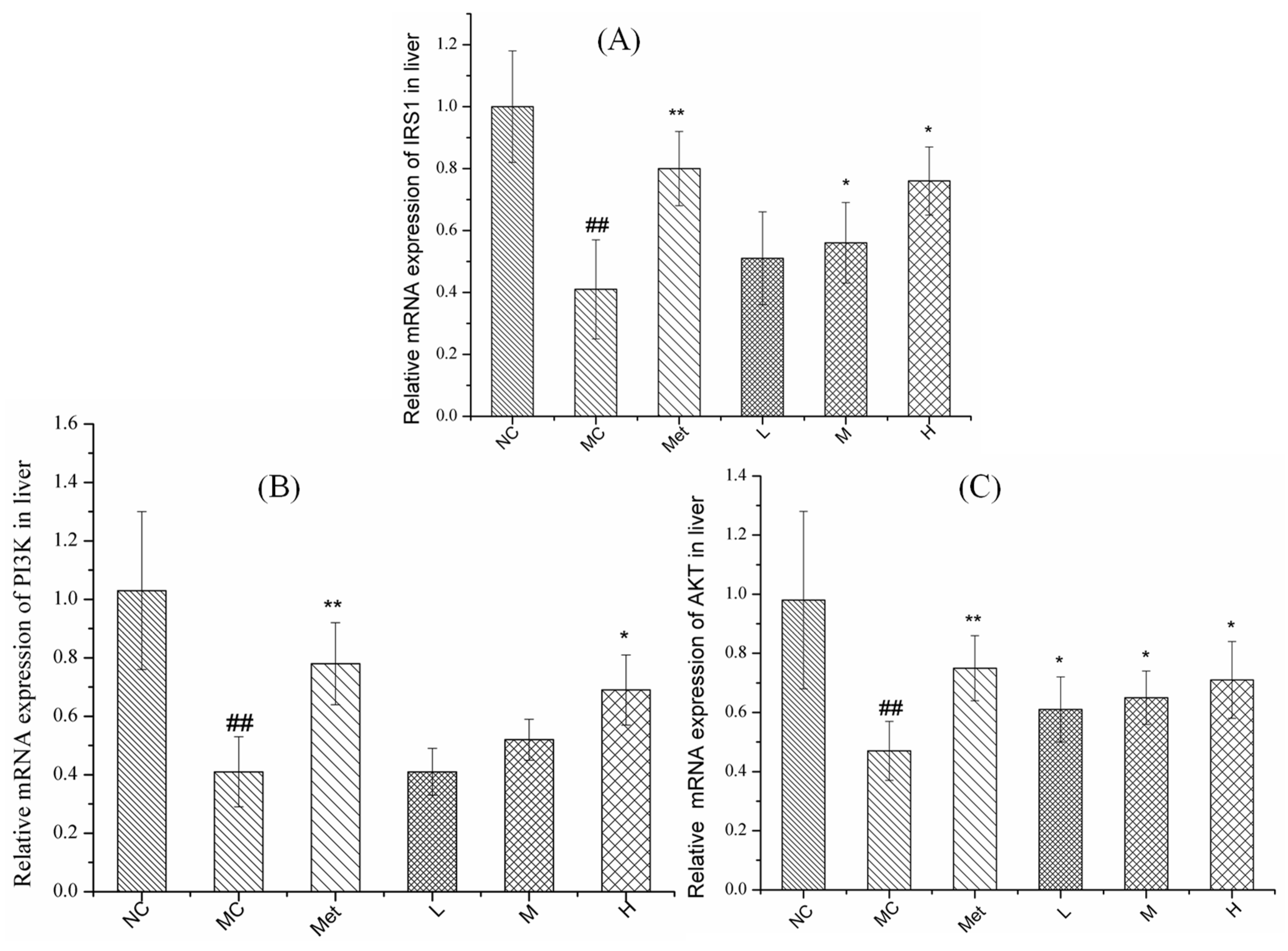
3.8. Effects of PPE on Gene Expression in the IRS1/PI3K/AKT Pathway in Mouse Liver
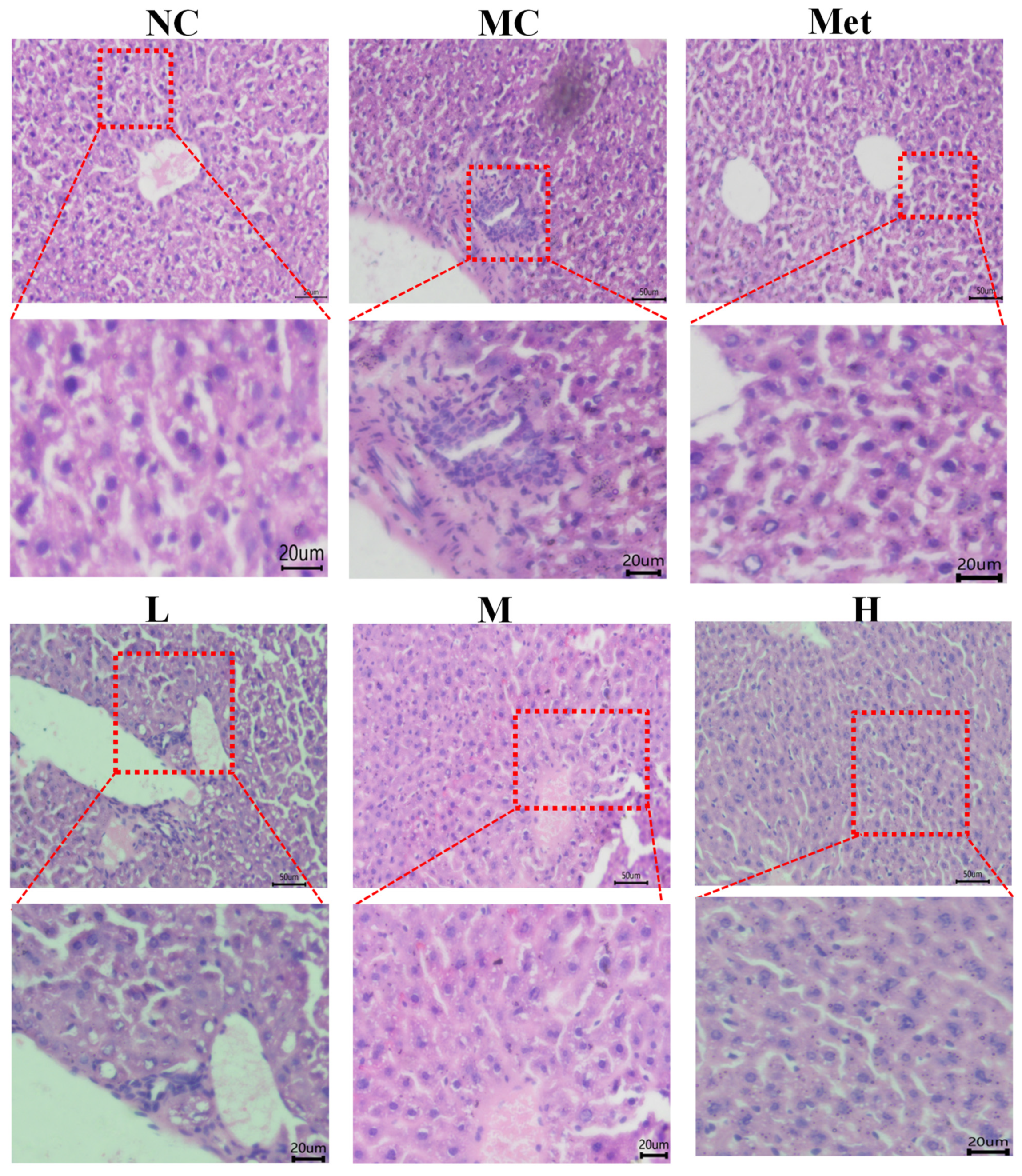
3.9. Effects of PPE on the Histomorphology of Mouse Liver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Zhu, Y.; Sidell, M.A.; Arterburn, D.; Daley, M.F.; Desai, J.; Fitzpatrick, S.L.; Horberg, M.A.; Koebnick, C.; McCormick, E.; Oshiro, C.; et al. Racial/Ethnic Disparities in the Prevalence of Diabetes and Prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) Multisite Cohort of Adults in the US. Diabetes Care 2019, 42, 2211–2219. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergstrom, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Backhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Umpierrez, G.E.; Murphy, M.B.; Kreisberg, R.A. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2006, 29, 2739–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.H.; Lee, Y.S.; Ku, S.; Lee, H.J. Phellinus baumii enhances the immune response in cyclophosphamide-induced immunosuppressed mice. Nutr. Res. 2020, 75, 15–31. [Google Scholar] [CrossRef]
- Liu, M.-M.; Zeng, P.; Li, X.-T.; Shi, L.-G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Lee, I.-K.; Choi, H.J.; Yun, B.-S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Han, M.-S.; Lee, M.-S.; Kim, Y.-S.; Yun, B.-S. Styrylpyrones from the medicinal fungus Phellinus baumii and their antioxidant properties. Bioorg. Med. Chem. Lett. 2010, 20, 5459–5461. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Wu, F.-H.; Yang, Y.; Wu, N.; Zhang, J.; Feng, N.; Tang, C.-H. Hypoglycemic Effect of Ethanol and Ethyl Acetate Extract of Phellinus baumii Fruiting Body in Streptozotocin-Induced Diabetic Mice. Evid.-Based Complementary Altern. Med. eCAM 2015, 2015, 783460. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-K.; Yun, B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Jang, T.S.; Kang, K.S.; Nam, J.W.; Lee, H.J.; Kim, K.H. Anti-inflammatory phenolic metabolites from the edible fungus Phellinus baumii in LPS-stimulated RAW264.7 cells. Molecules 2017, 22, 1583. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Ying, Y.; Li, Y.; Cai, M.; Guan, R.; Hu, J.; Sun, P. Cultivated Fruit Body of Phellinus baumii: A Potentially Sustainable Antidiabetic Resource. ACS Omega 2020, 5, 8596–8604. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.C.; Zhang, H.N.; Zhang, L.; Feng, J.; Wang, W.H.; Zhang, Z.; Musa, A.; Wu, D.; Yang, Y. Antioxidant and neuroprotector influence of endo-polyphenol extract from magnesium acetate multi-stage addition in the oak bracket medicinal mushroom, Phellinus baumii (Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Fan, M.-X.; Wu, J.-L.; Li, N.; Guo, M.-Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019, 277, 706–712. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of d-pinitol on insulin resistance through the PI3K/Akt signal-ing pathway in type 2 diabetes mellitus rats. J. Agric. Food Chem. 2015, 63, 6019–6026. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, L.; Cheng, Q.; Ji, B.; Yang, M.; Sanidad, K.Z.; Wang, C.; Zhou, F. Structurally Different Flavonoid Subclasses Attenuate High-Fat and High-Fructose Diet Induced Metabolic Syndrome in Rats. J. Agric. Food Chem. 2018, 66, 12412–12420. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus plantarum X1 with al-pha-glucosidase inhibitory activity ameliorates type 2 diabetes in mice. RSC Adv. 2016, 6, 3536–63547. [Google Scholar]
- Tian, B.; Zhao, J.; An, W.; Zhang, J.; Cao, X.; Mi, J.; Zhao, J.; Zhang, Y.; Li, J. Lycium ruthenicum diet alters the gut microbiota and partially enhances gut barrier function in male C57BL/6 mice. J. Funct. Foods 2019, 52, 516–528. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; Xie, X.; Chen, T.; Yin, Y.; Zhai, R.; Wang, X.; An, W.; Li, J. Anthocyanins from the fruits of Lycium ru-thenicum Murray improve high-fat diet-induced insulin resistance by ameliorating inflammation and oxidative stress in mice. Food Funct. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hoseth, E.Z.; Ueland, T.; Dieset, I.; Birnbaum, R.; Shin, J.H.; Kleinman, J.E.; Hyde, T.M.; Mørch, R.H.; Hope, S.; Lekva, T.; et al. A Study of TNF Pathway Activation in Schizophrenia and Bipolar Disorder in Plasma and Brain Tissue. Schizophr. Bull. 2017, 43, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Qiao, Y.C.; Xu, Y.; Ling, W.; Pan, Y.H.; Huang, Y.C.; Geng, L.J.; Zhao, H.L.; Zhang, X.X. Serum TNF-α concen-trations in type 2 diabetes mellitus patients and diabetic nephropathy patients: A systematic review and meta-analysis. Immunol. Lett. 2017, 186, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liao, Q.; Lyu, M.; Wong, Y.-K.; Zhang, X.; Zhou, J.; Ma, N.; Wang, J. The potential of artemisinins as anti-obesity agents via modulating the immune system. Pharmacol. Ther. 2020, 216, 107696. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. How Metabolism Generates Signals during Innate Immunity and Inflammation. J. Biol. Chem. 2013, 288, 22893–22898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Wu, C.; Wang, B.; Ouyang, Q. Big-data-driven safety decision-making: A conceptual framework and its influencing factors. Saf. Sci. 2018, 109, 46–56. [Google Scholar] [CrossRef]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 diabetes mellitus: From a metabolic disor-der to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef]
- Sun, X.J.; Kim, S.P.; Zhang, D.; Sun, H.; Cao, Q.; Lu, X.; Ying, Z.; Li, L.; Henry, R.R.; Ciaraldi, T.P.; et al. Deletion of interleukin 1 receptor-associated kinase 1 (Irak1) improves glucose tolerance primarily by increasing insulin sensi-tivity in skeletal muscle. J. Biol. Chem. 2017, 292, 12339–12350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, M.G.; Elayan, H.; Milic, M.; Sun, P.; Gharaibeh, M. Epinephrine and the Metabolic Syndrome. Curr. Hypertens. Rep. 2011, 14, 1–7. [Google Scholar] [CrossRef]
- Perry, R.J.; Zhang, D.; Guerra, M.T.; Brill, A.L.; Goedeke, L.; Nasiri, A.R.; Rabin-Court, A.; Wang, Y.; Peng, L.; Dufour, S.; et al. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nat. Cell Biol. 2020, 579, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Caro-Gómez, E.; Sierra, J.A.; Escobar, J.S.; Álvarez-Quintero, R.; Naranjo, M.; Medina, S.; Velásquez-Mejía, E.P.; Tabares-Guevara, J.H.; Jaramillo, J.C.; León-Varela, Y.M.; et al. Green coffee extract improves cardiometabolic parameters and modulates gut microbiota in high-fat-diet-fed apoE-/- mice. Nutrients 2019, 11, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; He, Z.; Wang, W.; Li, J.; Hu, A.; Li, L.; Yan, L.; Li, Z.; Yin, Q. Tangganjian decoction ameliorates type 2 diabetes mellitus and nonalcoholic fatty liver disease in rats by activating the IRS/PI3K/AKT signaling pathway. Biomed. Pharmacother. 2018, 106, 733–737. [Google Scholar] [CrossRef]
- Francés, D.E.; Ronco, M.T.; Monti, J.A.; Ingaramo, P.I.; Pisani, G.B.; Parody, J.P.; Pellegrino, J.M.; Sanz, P.M.; Carrillo, M.C.; Carnovale, C.E. Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical: New insights into the insulin effect. J. Endocrinol. 2010, 205, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Chen, X.X.; Tang, S.C.W.; Lao, L.X.; Sze, S.C.W.; Lee, K.F.; Zhang, K.Y.B. Edible plants from traditional Chinese med-icine is a promising alternative for the management of diabetic nephropathy. J. Funct. Food. 2015, 14, 12–22. [Google Scholar] [CrossRef]
- Hassan, A.; Tajuddin, N.; Shaikh, A. Retrospective Case Series of Patients with Diabetes or Prediabetes Who Were Switched from Omega-3-Acid Ethyl Esters to Icosapent Ethyl. Cardiol. Ther. 2015, 4, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.; Karim, S.; Ali, B.; Khan, S.; Ahmad, M.; Husain, A.; Mishra, R. PI3K signaling pathway targeting by using dif-ferent molecular approaches to treat cancer. J. Chin. Pharm. Sci. 2017, 26, 621–634. [Google Scholar] [CrossRef]
- Hu, X.; Wang, S.; Xu, J.; Wang, D.B.; Chen, Y.; Yang, G.Z. Triterpenoid saponins from Stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int. J. Mol. Sci. 2014, 15, 10446–10458. [Google Scholar] [CrossRef]


| Group | Body Weight of Mice (g) | ||||||
|---|---|---|---|---|---|---|---|
| 0 d | 10 d | 20 d | 30 d | 40 d | 50 d | 60 d | |
| NC | 25.2 ± 1.1 | 26.4 ± 1.0 | 28.7 ± 1.1 | 31.1 ± 1.5 | 33.0 ± 1.5 | 34.9 ± 1.2 | 35.9 ± 1.5 |
| MC | 28.2 ± 1.0 ## | 29.7 ± 1.2 ## | 32.4 ± 1.3 ## | 35.0 ± 1.4 ## | 38.3 ± 1.5 ## | 40.4 ± 1.6 ## | 42.1 ± 2.2 |
| Met | 28.4 ± 1.1 | 29.6 ± 1.2 | 32.3 ± 1.6 | 34.1 ± 1.7 | 36.2 ± 1.7 * | 38.4 ± 1.9 * | 39.8 ± 2.3 * |
| L | 27.7 ± 1.6 | 28.7 ± 1.7 | 31.5 ± 1.8 | 33.6 ± 1.9 | 36.1 ± 2.2 ** | 38.7 ± 2.7 * | 40.2 ± 3.1 |
| M | 28.3 ± 1.5 | 29.4 ± 1.8 | 32.4 ± 2.1 | 34.7 ± 2.2 | 36.8 ± 2.2 | 38.9 ± 2.1 | 40.4 ± 2.2 |
| H | 27.9 ± 1.5 | 28.9 ± 1.5 | 31.7 ± 2.0 | 33.9 ± 1.9 | 36.1 ± 2.0 ** | 38.2 ± 2.1 * | 39.3 ± 2.1 ** |
| Group | Body Weight Gain of Mice (g) | ||||||
| NC | 0 | 1.2 ± 0.6 | 3.5 ± 1.3 | 5.9 ± 1.8 | 7.8 ± 1.7 | 9.6 ± 1.54 | 10.7 ± 1.6 |
| MC | 0 | 1.5 ± 0.4 | 4.3 ± 0.8 | 6.8 ± 0.7 | 10.1 ± 0.8 ## | 12.3 ± 1.1 ## | 13.9 ± 1.6 ## |
| Met | 0 | 1.2 ± 0.3 | 3.9 ± 0.8 | 5.7 ± 0.8 * | 7.7 ± 0.9 ** | 10.0 ± 1.3 ** | 11.4 ± 1.6 ** |
| L | 0 | 0.9 ± 0.3 ** | 3.7 ± 0.8 | 5.9 ± 0.8 | 8.3 ± 1.1 ** | 11.0 ± 1.58 * | 12.5 ± 1.9 * |
| M | 0 | 1.1 ± 0.5 * | 4.0 ± 0.7 | 6.4 ± 1.0 | 8.5 ± 1.0 ** | 10.5 ± 1.3 ** | 12.0 ± 1.1 ** |
| H | 0 | 1.0 ± 0.6 * | 3.7 ± 1.0 | 6.0 ± 1.4 | 8.1 ± 1.7 ** | 10.2 ± 1.9 ** | 11.4 ± 2.1 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Zhang, S.; Geng, Y.; Tian, B.; Cai, M.; Guan, R.; Li, Y.; Ye, B.; Sun, P. Anti-Inflammatory Properties In Vitro and Hypoglycaemic Effects of Phenolics from Cultivated Fruit Body of Phellinus baumii in Type 2 Diabetic Mice. Molecules 2021, 26, 2285. https://doi.org/10.3390/molecules26082285
Yang K, Zhang S, Geng Y, Tian B, Cai M, Guan R, Li Y, Ye B, Sun P. Anti-Inflammatory Properties In Vitro and Hypoglycaemic Effects of Phenolics from Cultivated Fruit Body of Phellinus baumii in Type 2 Diabetic Mice. Molecules. 2021; 26(8):2285. https://doi.org/10.3390/molecules26082285
Chicago/Turabian StyleYang, Kai, Su Zhang, Yan Geng, Baoming Tian, Ming Cai, Rongfa Guan, Yougui Li, Bangwei Ye, and Peilong Sun. 2021. "Anti-Inflammatory Properties In Vitro and Hypoglycaemic Effects of Phenolics from Cultivated Fruit Body of Phellinus baumii in Type 2 Diabetic Mice" Molecules 26, no. 8: 2285. https://doi.org/10.3390/molecules26082285







