Occurrence of Cis-11,12-Methylene-Hexadecanoic Acid in the Red Alga Solieria pacifica (Yamada) Yoshida
Abstract
1. Introduction
2. Results and Discussion
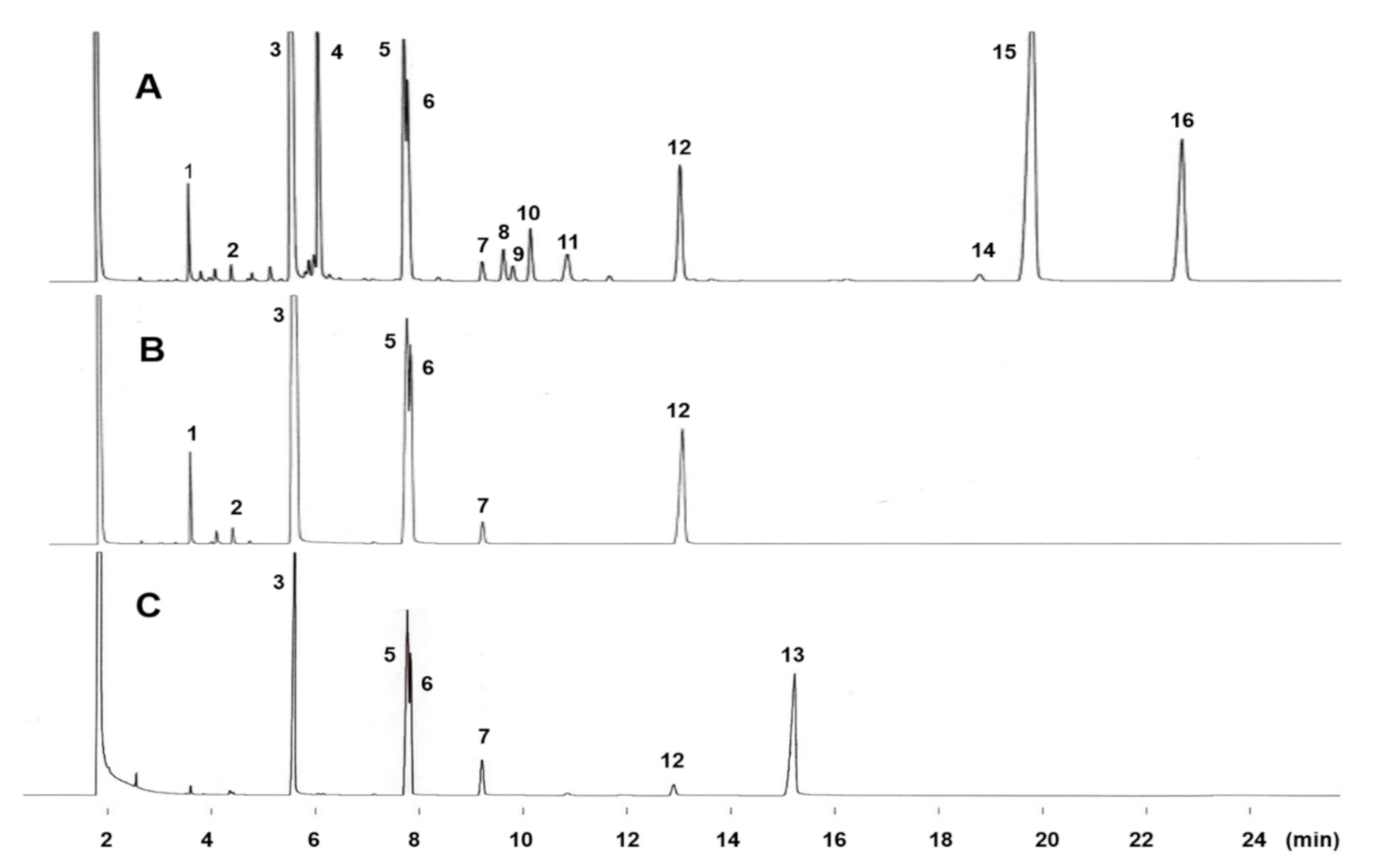
2.1. GC Elution Profiles
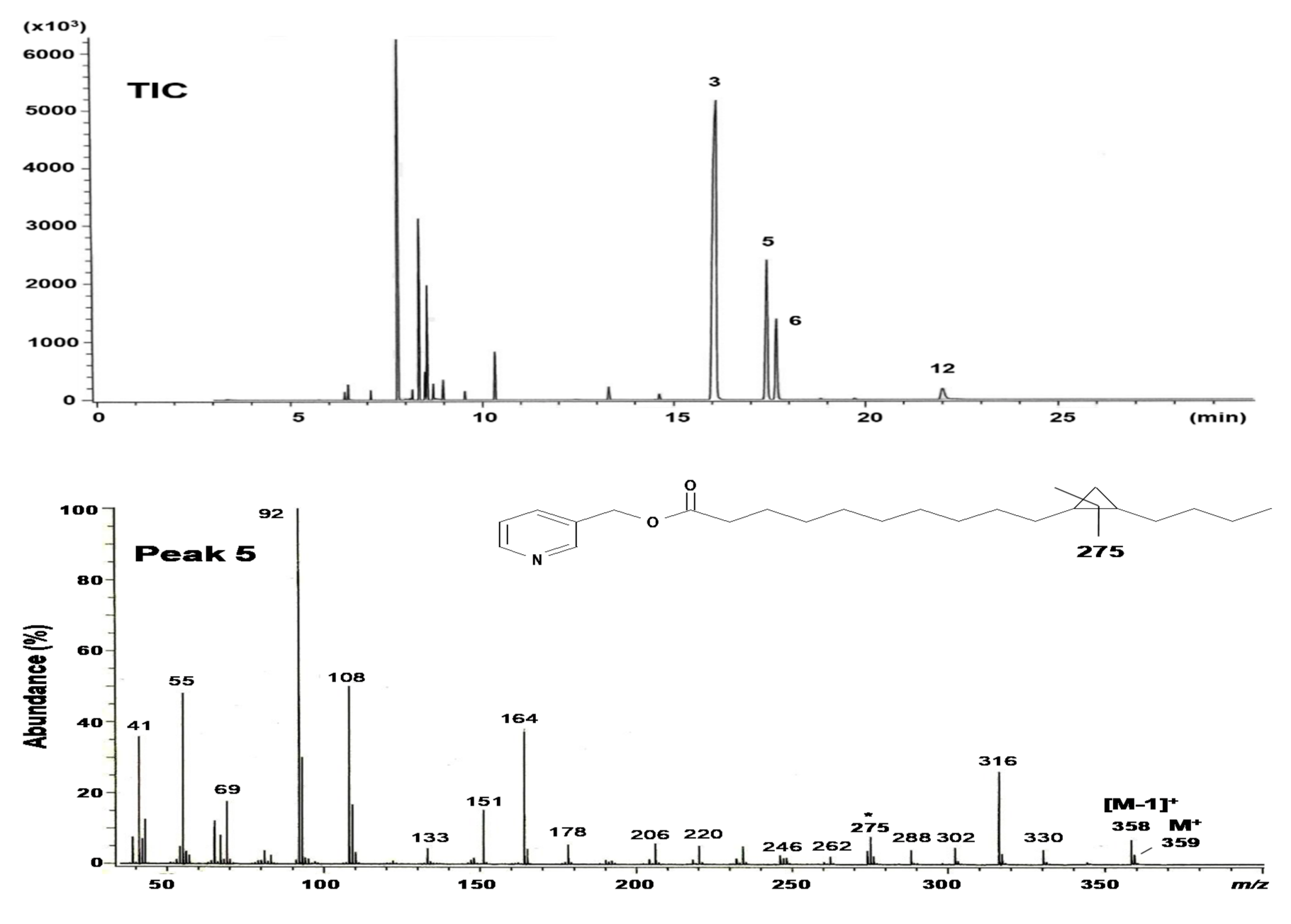
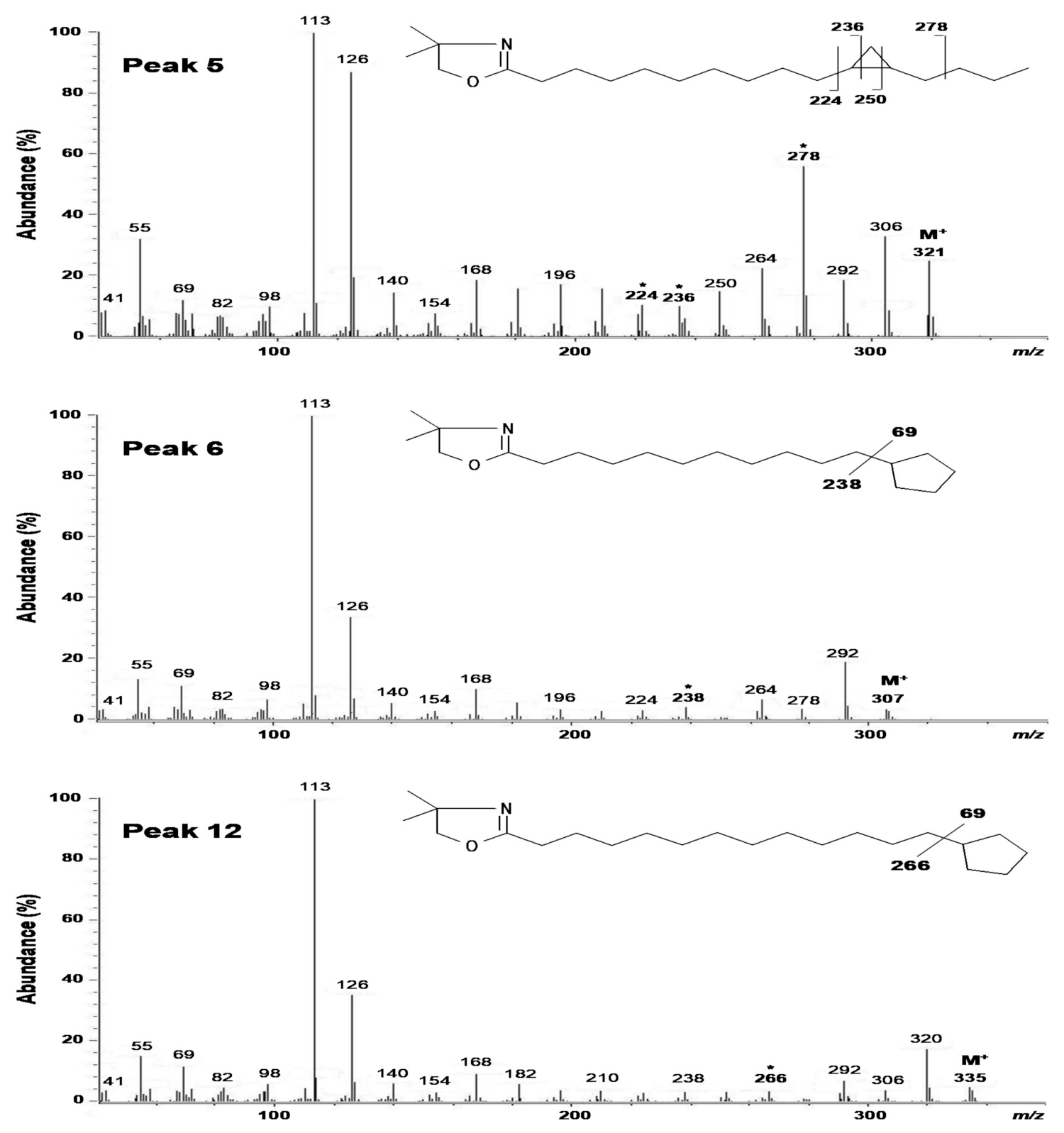
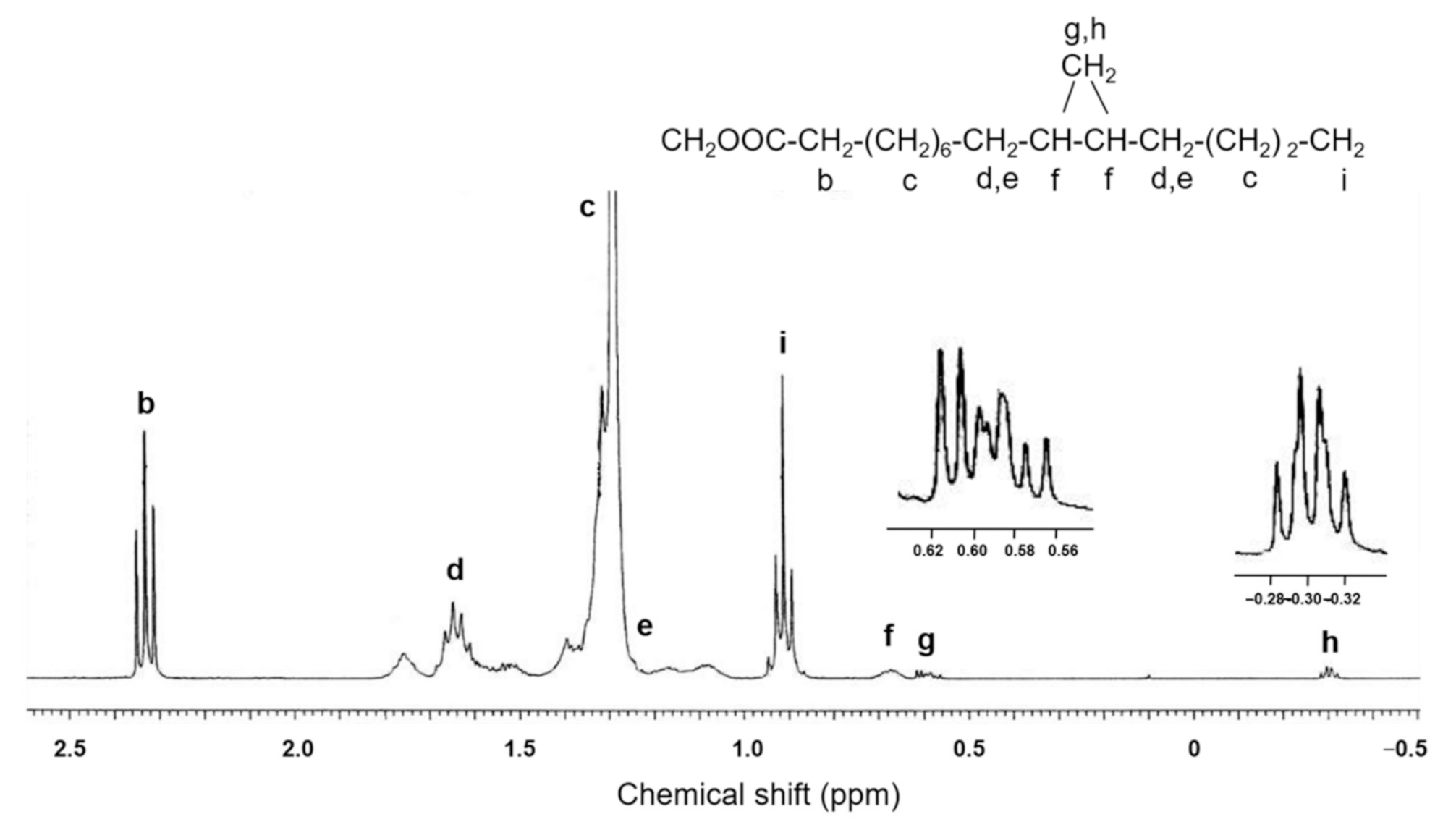
2.2. Structural Elucidation of cis-11,12-Methylene-Hexadecanoic Acid (Peak 5)
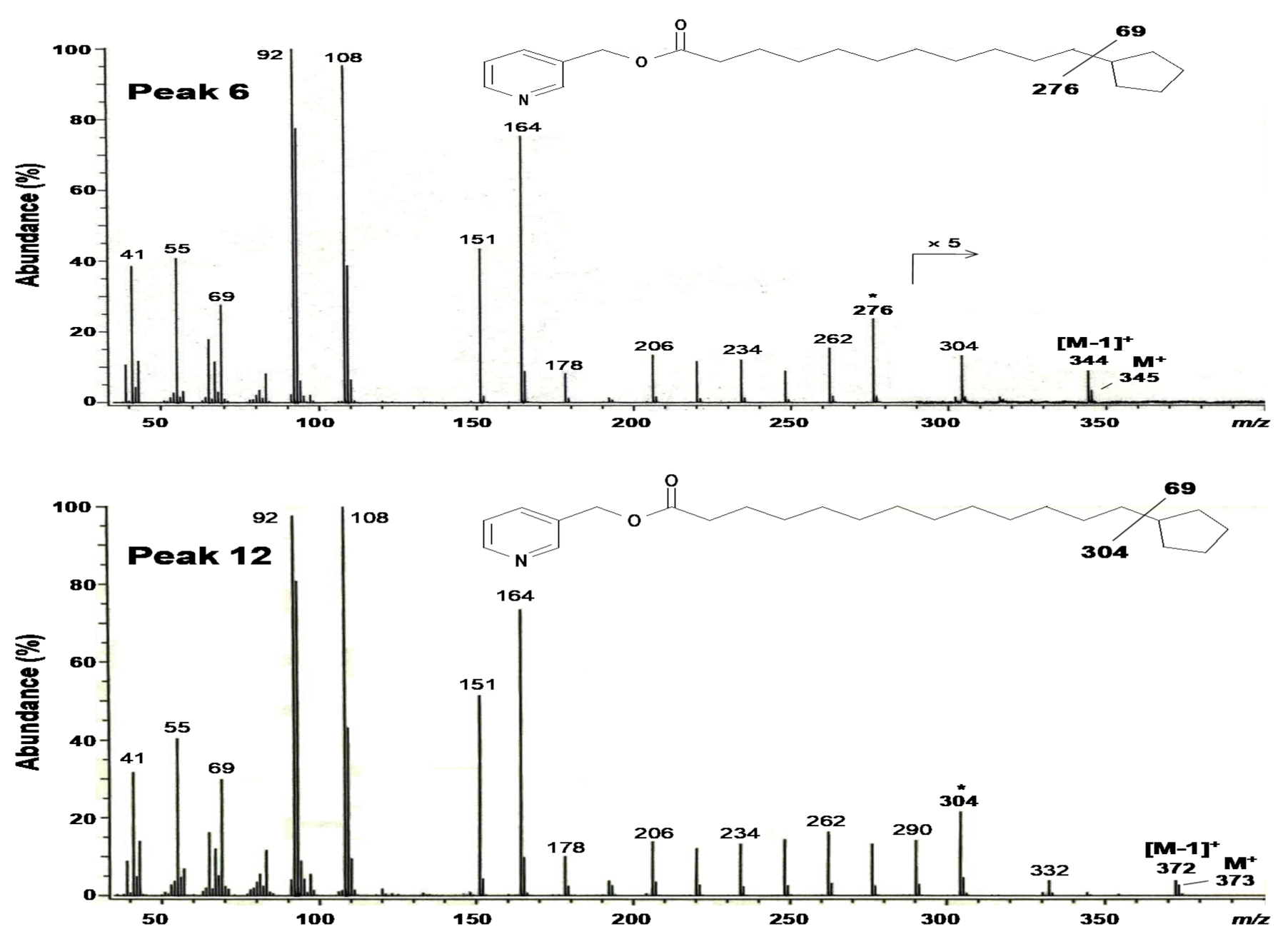
2.3. Structural Elucidation of 11-Cyclopentylundecanoic Acid (Peak 6), 13-Cyclopentyltridecanoic Acid (Peak 12), and cis-11-Hexadecenoic Acid (Peak 4)
2.4. Fatty Acid Composition
3. Materials and Methods
3.1. Samples, Lipid Extraction, and Analysis of Fatty Acids
3.2. Silver Ion-TLC (Ag+-TLC)
3.3. Hydrogenation
3.4. Preparation of Picolinyl Ester Derivatives
3.5. Preparation of 4,4-Dimethyloxazoline Derivatives
3.6. H-NMR
3.7. FT-IR
3.8. GC-MS
3.9. Mass Spectrum Data of Picolinyl Ester Derivatives
3.9.1. cis-11,12-Methylene-Hexadecanoic Acid Picolinyl Ester
3.9.2. 11-Cyclopenylundecanoic Acid Picolinyl Ester
3.9.3. 13-Cyclopenyltridecanoic Acid Picolinyl Ester
3.9.4. cis-11-Hexadecenoic Acid Picolinyl Ester
3.10. Mass Spectrum Data of DMOX Derivatives
3.10.1. cis-11,12-Methylene-Hexadecanoic Acid DMOX
3.10.2. 11-Cyclopenylundecanoic Acid DMOX
3.10.3. 13-Cyclopenyltridecanoic Acid DMOX
3.11. NMR Spectrum Data of Saturated FAME Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chiovitti, A.; Bacic, A.; Craik, D.J.; Munro, S.L.A.; Kraft, G.T.; Liao, M.L. Cell-wall polysaccharides from Australian red algae of the family Solieriaceae (Gigartinales, Rhodophyta): Novel, highly pyruvated carrageenans from the genus Callophycus. Carbohydr. Res. 1997, 299, 229–243. [Google Scholar] [CrossRef]
- Chiovitti, A.; Bacic, A.; Kraft, G.T.; Craik, D.J.; Liao, M.L. Pyruvated carrageenans from Solieria robusta and its adelphoparasite Tikvahiella candida. Hydrobiologia 1999, 398, 401–409. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the Pacific Coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Marseglia, A.; Caligiani, A.; Comino, L.; Righi, F.; Quarantelli, A.; Palla, G. Cyclopropyl and ω-cyclohexyl fatty acids as quality markers of cow milk and cheese. Food Chem. 2013, 140, 711–716. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Palla, G. An overview of the presence of cyclopropane fatty acids in milk and dairy products. J. Agric. Food Chem. 2014, 62, 7828–7832. [Google Scholar] [CrossRef]
- Lolli, V.; Dall’Asta, M.; Del Rio, D.; Palla, G.; Caligiani, A. Presence of cyclopropane fatty acids in foods and estimation of dietary intake in the Italian population. Int. J. Food Sci. Nutr. 2019, 70, 467–473. [Google Scholar] [CrossRef]
- Lolli, V.; Zanardi, E.; Moloney, A.P.; Caligiani, A. An overview on cyclic fatty acids as biomarkers of quality and authenticity in the meat sector. Foods 2020, 9, 1756. [Google Scholar] [CrossRef]
- Miralles, J.; Aknin, M.; Micouin, L.; Gaydou, E.M.; Kornprobst, J.M. Cyclopentyl and ω-5 monounsaturated fatty acids from red algae of the Solieriaceae. Phytochemistry 1990, 29, 2161–2163. [Google Scholar] [CrossRef]
- Christie, W.W.; Brechany, E.Y.; Shukla, V.K.S. Analysis of seed oils containing cyclopentenyl fatty acids by combined chromatographic procedures. Lipids 1989, 24, 116–120. [Google Scholar] [CrossRef]
- Dobson, G.; Christie, W.W. Structural analysis of fatty acids by mass spectrometry of picolinyl esters and dimethyloxazoline derivatives. TrAC Trends Anal. Chem. 1996, 15, 130–137. [Google Scholar] [CrossRef]
- Harvey, D.J. Picolinyl derivatives for the characterization of cyclopropane fatty acids by mass spectrometry. Biomed. Mass. Spectrom. 1984, 11, 187–192. [Google Scholar] [CrossRef]
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef]
- Harvey, D.J.; Christie, W.W. Advances in Lipid Methodology One; The Oily Press: Dundee, Scotland, 1992; pp. 19–80. ISBN 13-9780951417119. [Google Scholar]
- Zhang, J.Y.; Yu, Q.T.; Huang, Z.H. 2-Substituted 4,4-dimethyloxazolines as useful derivatives for the localization of cyclopropane ring in long-chain fatty acids. J. Mass Spectrom. Soc. Jpn. 1987, 35, 23–30. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.Y.; Yu, Q.T.; Liu, B.N.; Huang, Z.H. Chemical modification in mass spectrometry IV. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for double bond location of long-chain olefinic acids. Biomed. Environ. Mass Spectrom. 1988, 15, 33–44. [Google Scholar] [CrossRef]
- Christie, W.W.; Gunstone, F.D.; Ismail, I.A.; Wade, L. Fatty acids, Part 17. The synthesis and chromatographic and spectroscopic properties of the cyclopropane esters derived from all the methyl octadecenoates (Δ2–Δ17). Chem. Phys. Lipids 1968, 2, 196–202. [Google Scholar] [CrossRef]
- Knothe, G. NMR characterization of dihydrosterculic acid and its methyl ester. Lipids 2006, 41, 393–396. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, H.Y.; Yu, Q.T.; Yu, X.J.; Liu, B.N.; Huang, Z.H. The structures of cyclopentenyl fatty acids in the seed oils of Flacourtiaceae species by GC-MS of their 4,4-dimethyloxazoline derivatives. J. Am. Oil Chem. Soc. 1989, 66, 242–246. [Google Scholar] [CrossRef]
- Hamilton, J.T.G.; Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids 2000, 105, 93–104. [Google Scholar] [CrossRef]
- Illijas, M.I.; Indy, J.R.; Yasui, H.; Itabashi, Y. Lipid class and fatty acid composition of a little-known and rarely collected alga Exophyllum wentii Weber-van Bosse from Bali Island, Indonesia. J. Oleo Sci. 2009, 58, 103–110. [Google Scholar] [CrossRef]
- Cosper, C.I.; Ackman, R.G. Occurrence of cis-9,10-methylenehexadecanoic and cis-9,10-methyleneoctadecanoic acids in the lipids of immature and mature Fundulus heteroclitus (L.), and in Roe. Comp. Biochem. Physiol. 1983, 75, 649–654. [Google Scholar] [CrossRef]
- Elvert, M.; Boetius, A.; Jorgensen, B.B. Characterization of specific membrane fatty acids as chemotaxonomic markers for sulfate-reducing bacteria involved in anaerobic oxidation of methane. Geomicrobiol. J. 2003, 20, 403–419. [Google Scholar] [CrossRef]
- Grogan, D.W.; Cronan, J.E., Jr. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 1997, 61, 429–441. Available online: https://pubmed.ncbi.nlm.nih.gov/9409147 (accessed on 25 March 2021). [CrossRef]
- Fish, W.R.; Holz, G.G., Jr.; Beach, D.H.; Owen, E.; Anekwe, G.E. The cyclopropane fatty acid of trypanosomatids. Mol. Biochem. Parasitol. 1981, 3, 103–115. [Google Scholar] [CrossRef]
- Van der Horst, D.J.; Oudejans, R.C.H.M.; Zandee, D.I. Occurrence of cyclopropane fatty acids in females and eggs of the millipede Graphidostreptus tumuliporus (karsch) (Myriapoda: Diplopoda), as contrasted with their absence in the males. Comp. Biochem. Physiol. 1972, 41, 417–423. [Google Scholar] [CrossRef][Green Version]
- Yano, I.; Morris, L.J.; Nichols, B.W.; Jams, A.T. The biosynthesis of cyclopropane and cyclopropene fatty acids in higher plants (Malvaceae). Lipids 1972, 7, 35–45. [Google Scholar] [CrossRef]
- Spencer, G.F.; Payne-Wahl, K.; Plattner, R.D.; Kleiman, R. Lactobacillic acid and methyl-branched olefinic acids in Byrsocarpus coccineus seed oil. Lipids 1979, 14, 72–74. [Google Scholar] [CrossRef]
- Christie, W.W.; Dobson, G. Formation of cyclic fatty acids during the frying process. Eur. J. Lipid Sci. Technol. 2000, 102, 515–520. [Google Scholar] [CrossRef]
- Berdeaux, O.; Gregoire, S.; Fournier, C.; Christie, W.W.; Lambelet, P.; Sébédio, J.L. Detection of lactobacillic acid in low erucic rapeseed oil-A note of caution when quantifying cyclic fatty acid monomers in vegetable oils. Chem. Phys. Lipids 2010, 163, 698–702. [Google Scholar] [CrossRef]
- Saito, H.; Xue, C.; Yamashiro, R.; Moromizato, S.; Itabashi, Y. High polyunsaturated fatty acid levels in two subtropical macroalgae, Cladosiphon okamuranus and Caulerpa lentillifera. J. Phycol. 2010, 46, 665–673. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Stransky, K.; Jursik, T.; Vitek, A.; Skorepa, J. An improved method of characterizing fatty acids by equivalent chain length values. J. High Res. Chromatogr. 1992, 15, 730–740. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis: Isolation, Separating, Identification and Lipidomic Analysis, 4th ed.; The Oily Press: Bridgwater, UK, 2010; pp. 145–158. ISBN 978-0-9552512-4-5. [Google Scholar]
- Destaillats, F.; Angers, P. One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J. Am. Oil Chem. Soc. 2002, 79, 253–256. [Google Scholar] [CrossRef]
- Fay, L.; Richli, U. Location of double bonds in polyunsaturated fatty acids by gas chromatography-mass spectrometry after 4,4-dimethyloxazoline derivatization. J. Chromatogr. A 1991, 541, 89–98. [Google Scholar] [CrossRef]

| Fatty Acid | ECL a | wt % |
|---|---|---|
| 12:0 | 12.00 | 0.05 ± 0.0 |
| 14:0 | 14.00 | 1.18 ± 0.2 |
| Iso 15:0 | 14.60 | 0.27 ± 0.0 |
| 15:0 | 15.00 | 0.29 ± 0.0 |
| Iso 16:0 | 15.37 | 0.17 ± 0.0 |
| Anteiso 16:0 | 15.67 | 0.36 ± 0.0 |
| 16:0 | 16.00 | 28.36 ± 1.4 |
| Iso 17:0 | 16.53 | 0.19 ± 0.0 |
| 17:0 | 17.00 | 0.54 ± 0.1 |
| cy17:0 b | 17.39 | 7.89 ± 0.8 |
| cy16:0 c | 17.44 | 7.04 ± 0.7 |
| 18:0 | 18.00 | 0.56 ± 0.1 |
| cy18:0 d | 19.45 | 4.85 ± 0.5 |
| ΣSaturates | 51.75 ± 1.6 | |
| 14:1n-5 | 14.27 | 0.24 ± 0.0 |
| 16:1n-9 | 16.20 | 0.23 ± 0.0 |
| 16:1n-7 | 16.26 | 1.02 ± 0.1 |
| 16:1n-5 | 16.40 | 8.96 ± 0.7 |
| 18:1n-9 | 18.28 | 0.80 ± 0.1 |
| 18:1n-7 | 18.35 | 0.39 ± 0.0 |
| 18:1n-5 | 18.49 | 1.64 ± 0.3 |
| 20:1n-9 | 20.26 | 0.11 ± 0.0 |
| 20:1n-7 | 20.32 | 0.20 ± 0.0 |
| ΣMonoenes | 13.59 ± 0.9 | |
| 16:2n-6 | 16.65 | 0.14 ± 0.0 |
| 16:3n-3 | 17.30 | 0.03 ± 0.0 |
| 16:4n-3 | 17.74 | 0.04 ± 0.0 |
| 18:2n-6 | 18.77 | 0.27 ± 0.0 |
| 18:3n-6 | 19.02 | 0.20 ± 0.0 |
| 20:3n-6 | 20.91 | 0.39 ± 0.0 |
| 20:4n-6 | 21.12 | 23.69 ± 1.3 |
| 20:4n-3 | 21.29 | 0.05 ± 0.0 |
| 20:5n-3 | 21.68 | 9.14 ± 0.9 |
| ΣPolyenes | 33.95 ± 1.3 | |
| Others | 0.71 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-W.; Sim, J.-M.; Itabashi, Y.; Jung, M.-J.; Jun, J.-Y. Occurrence of Cis-11,12-Methylene-Hexadecanoic Acid in the Red Alga Solieria pacifica (Yamada) Yoshida. Molecules 2021, 26, 2286. https://doi.org/10.3390/molecules26082286
Kim G-W, Sim J-M, Itabashi Y, Jung M-J, Jun J-Y. Occurrence of Cis-11,12-Methylene-Hexadecanoic Acid in the Red Alga Solieria pacifica (Yamada) Yoshida. Molecules. 2021; 26(8):2286. https://doi.org/10.3390/molecules26082286
Chicago/Turabian StyleKim, Gwang-Woo, Jae-Man Sim, Yutaka Itabashi, Min-Jeong Jung, and Joon-Young Jun. 2021. "Occurrence of Cis-11,12-Methylene-Hexadecanoic Acid in the Red Alga Solieria pacifica (Yamada) Yoshida" Molecules 26, no. 8: 2286. https://doi.org/10.3390/molecules26082286
APA StyleKim, G.-W., Sim, J.-M., Itabashi, Y., Jung, M.-J., & Jun, J.-Y. (2021). Occurrence of Cis-11,12-Methylene-Hexadecanoic Acid in the Red Alga Solieria pacifica (Yamada) Yoshida. Molecules, 26(8), 2286. https://doi.org/10.3390/molecules26082286






