Synthesis, Complexation Properties, and Evaluation of New Aminodiphosphonic Acids as Vector Molecules for 68Ga Radiopharmaceuticals
Abstract
1. Introduction
2. Results and Discussion
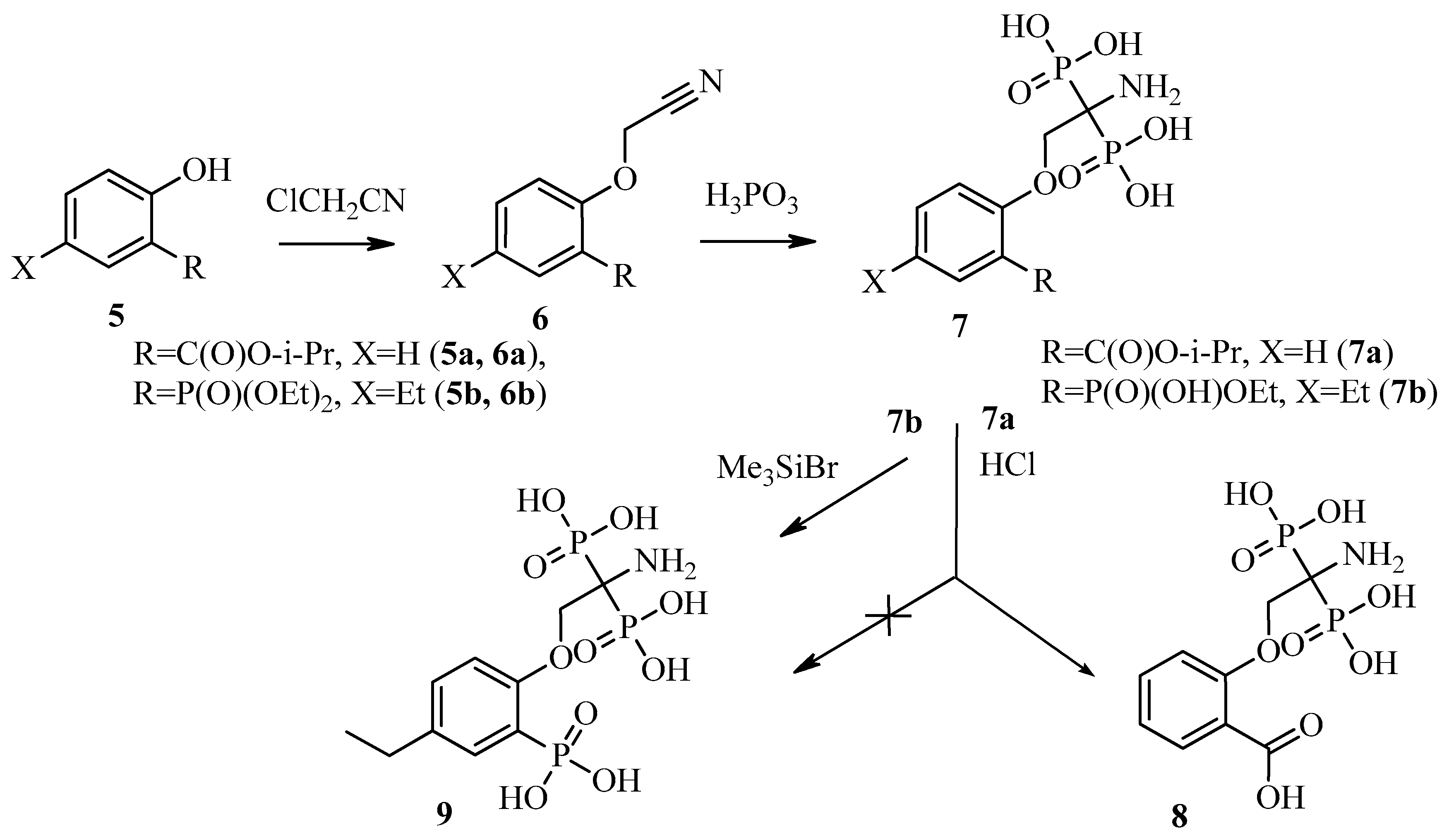
2.1. Synthesis
2.2. Stability Constants of Gallium(III) Complexes with 8 and 9
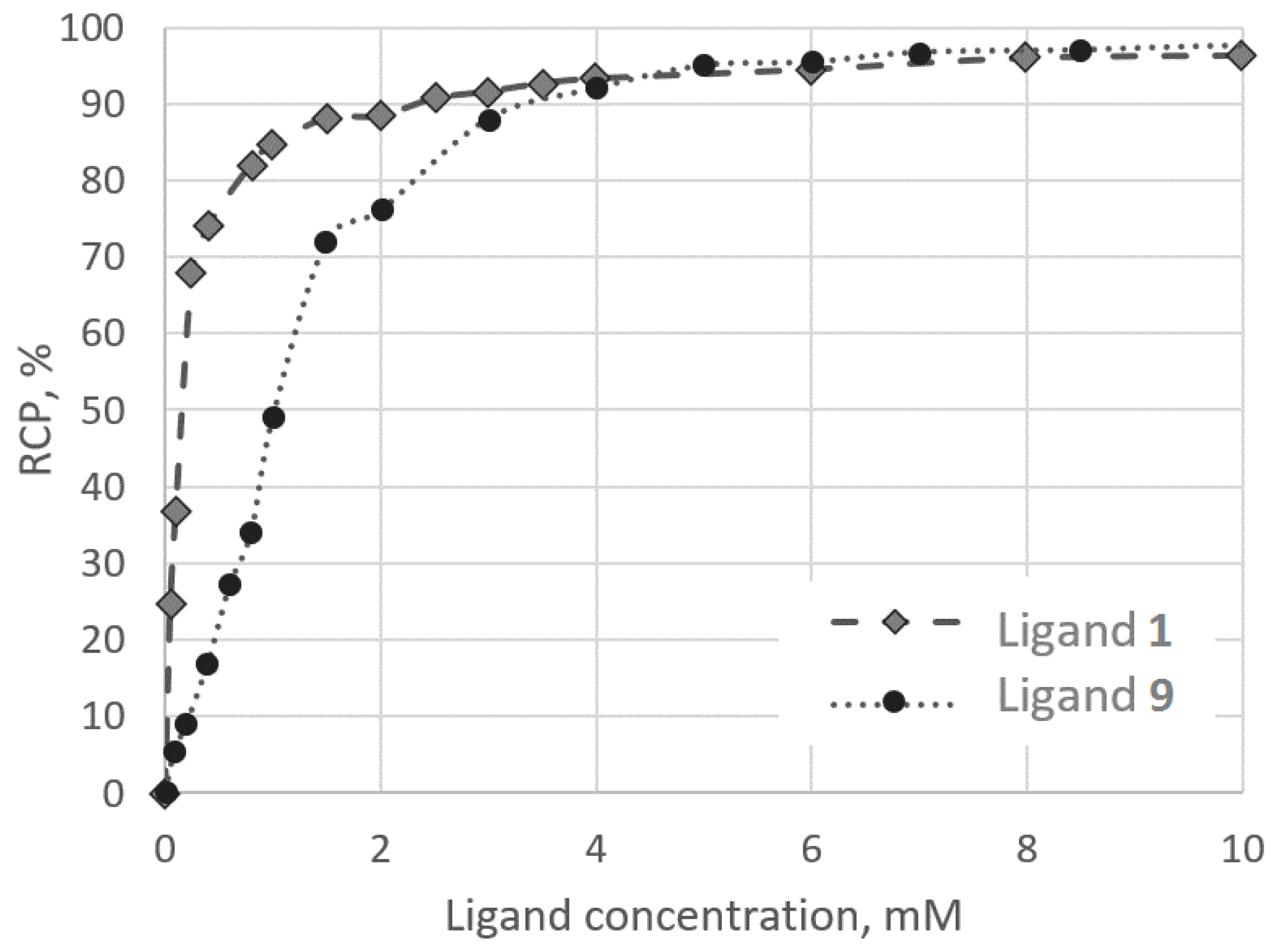
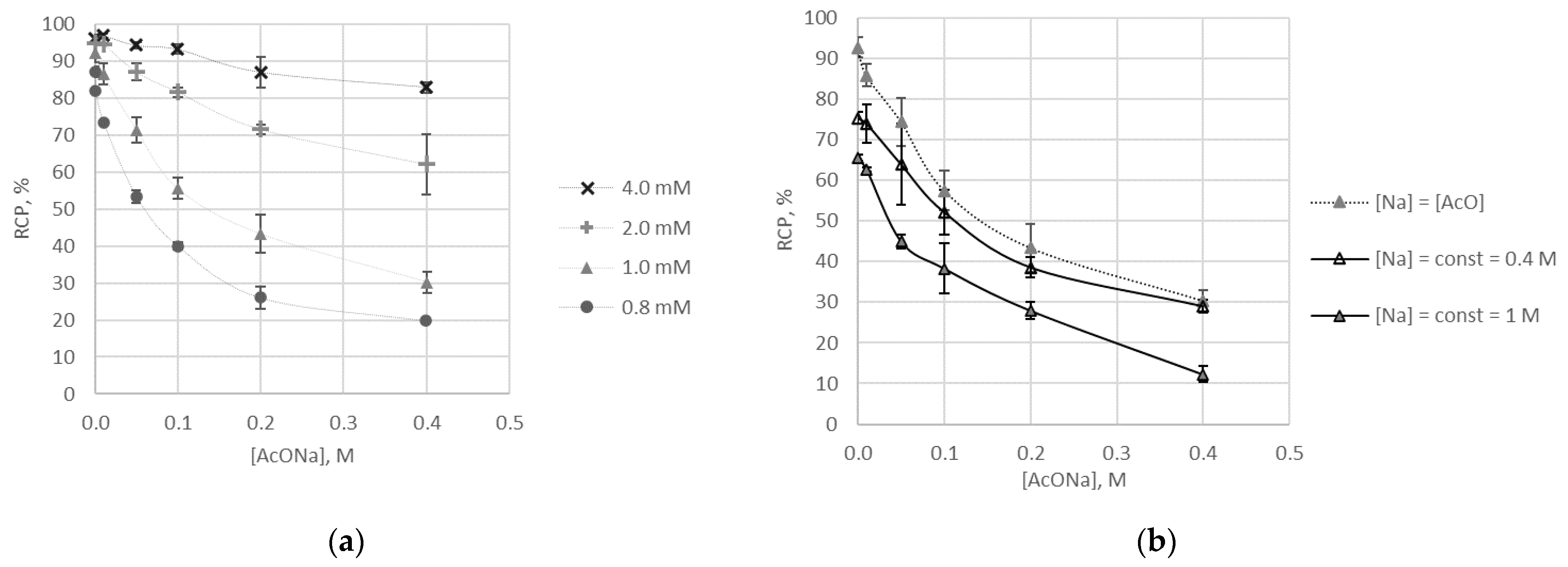
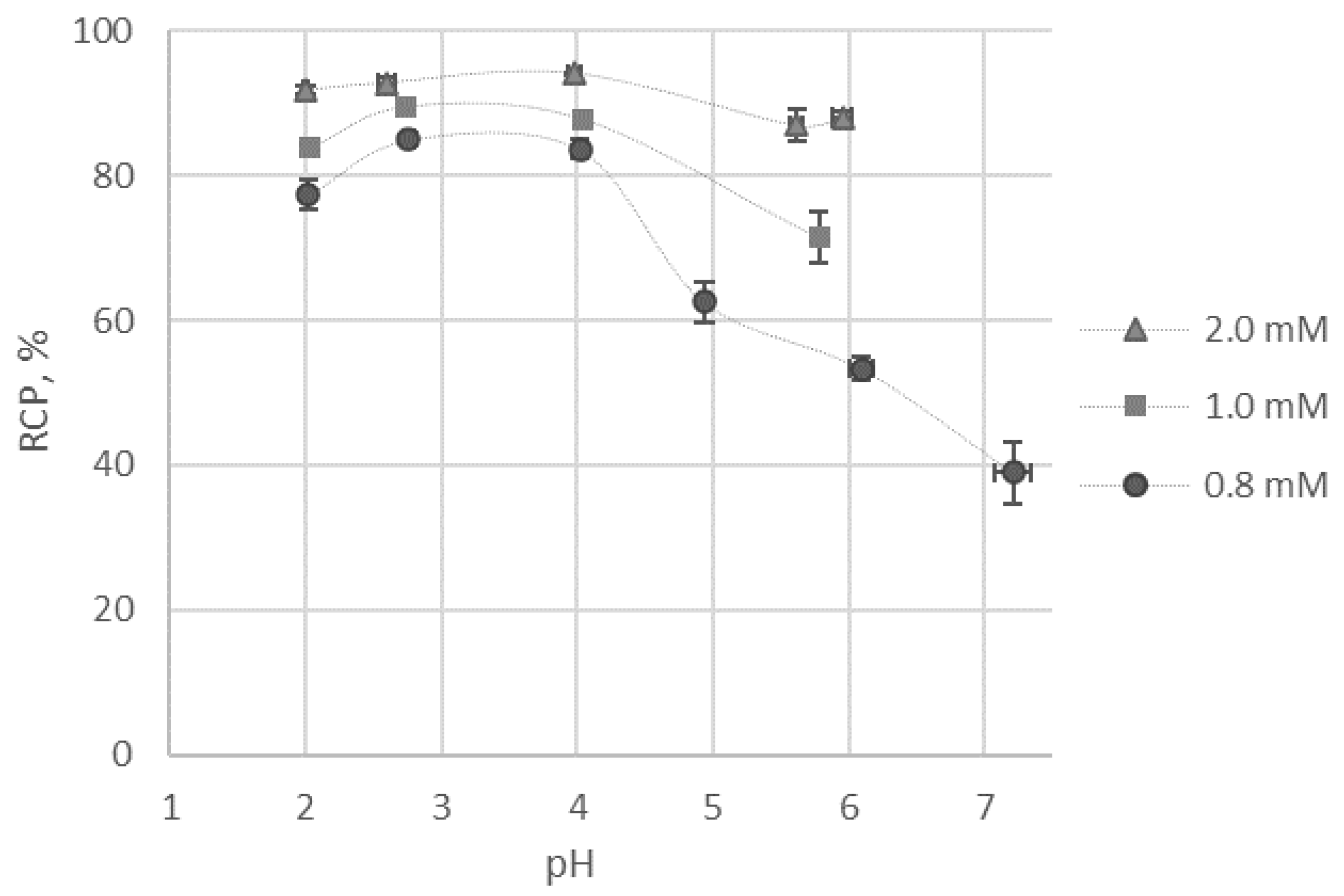
2.3. Radiolabelling and Stability
- no correlation for [68Ga]Ga-1;
- correlation with an extremum point for [68Ga]Ga-DOTA-conjugates (0.3 M of acetate corresponds to maximum labelling reaction yield) [27];
- continuous dependence for [68Ga]Ga-9 (with maximum labelling reaction yield corresponding to the minimal concentration of acetate).
2.4. Biodistribution of [68Ga]Ga-9
3. Materials and Methods
3.1. Synthesis
3.2. Stability Constant Measurements and Calculations
3.3. Radiolabelling, Stability, and Quality Control
3.4. Biodistribution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Roesch, F.; Riss, P.J. The Renaissance of the 68Ge/68Ga Radionuclide Generator Initiates New Developments in 68Ga Radiopharmaceutical Chemistry. Curr. Top. Med. Chem. 2010, 10, 1633–1668. [Google Scholar] [CrossRef]
- Velikyan, I. Prospective of 68Ga-Radiopharmaceutical development. Theranostics 2014, 4, 47–80. [Google Scholar] [CrossRef]
- Baum, R.P.; Rosch, F. Theranostics, Gallium-68, and Other radionuclides: A Pathway to Personalized Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Gourni, E.; Henriksen, G.; Gamez, P.; Caballero, A.B. Metal-based PSMA radioligands. Molecules 2017, 22, 523. [Google Scholar] [CrossRef]
- Pillai, M.R.A.; Nanabala, R.; Joy, A.; Sasikumar, A.; Knapp, F.F.R. Radiolabeled enzyme inhibitors and binding agents targeting PSMA: Effective theranostic tools for imaging and therapy of prostate cancer. Nucl. Med. Biol. 2016, 43, 692–720. [Google Scholar] [CrossRef]
- Zha, Z.; Wu, Z.; Choi, S.R.; Ploessl, K.; Smith, M.; Alexoff, D.; Zhu, L.; Kung, H.F. A New [68Ga]Ga-HBED-CC-Bisphosphonate as a Bone Imaging Agent. Mol. Pharm. 2020, 17, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Preliminary results of biodistribution and dosimetric analysis of [68 Ga]Ga-DOTA ZOL: A new zoledronate-based bisphosphonate for PET/CT diagnosis of bone diseases. Ann. Nucl. Med. 2019, 33, 404–413. [Google Scholar] [CrossRef]
- Meckel, M.; Kubíček, V.; Hermann, P.; Miederer, M.; Rösch, F. A DOTA based bisphosphonate with an albumin binding moiety for delayed body clearance for bone targeting. Nucl. Med. Biol. 2016, 43, 670–678. [Google Scholar] [CrossRef]
- Meckel, M.; Fellner, M.; Thieme, N.; Bergmann, R.; Kubicek, V.; Rösch, F. In vivo comparison of DOTA based 68Ga-labelled bisphosphonates for bone imaging in non-tumour models. Nucl. Med. Biol. 2013, 40, 823–830. [Google Scholar] [CrossRef]
- Kodina, G.E.; Malysheva, A.O.; Klement’eva, O.E. Osteotropic radiopharmaceuticals in Russian nuclear medicine techniques. Russ. Chem. Bull. Int. Ed. 2016, 65, 350–362. [Google Scholar] [CrossRef]
- Palma, E.; Correia, J.D.G.; Campello, M.P.C.; Santos, I. Bisphosphonates as radionuclide carriers for imaging or systemic therapy. Mol. Biosyst. 2011, 7, 2950–2966. [Google Scholar] [CrossRef]
- Romanenko, V.D.; Kukhar, V.P. 1-Amino-1,1-bisphosphonates. Fundamental syntheses and new developments. Arkivoc 2012, 2012, 127–166. [Google Scholar] [CrossRef]
- Chmielewska, E.; Kafarski, P. Synthetic procedures leading towards aminobisphosphonates. Molecules 2016, 21, 1474. [Google Scholar] [CrossRef]
- Mitrofanov, I.A.; Maruk, A.Y.; Larenkov, A.A.; Kodina, G.E.; Lunev, A.S.; Luneva, K.A.; Klementyeva, O.E.; Tsebrikova, G.S.; Baulin, V.E.; Ragulin, V.V.; et al. Evaluation of Applicability of Aminodiphosphonic Acids for the Development of Bone-Seeking 68Ga-Radiopharmaceuticals. Russ. J. Gen. Chem. 2020, 90, 390–397. [Google Scholar] [CrossRef]
- Tsebrikova, G.; Baulin, V.; Ragulin, V.; Solov’ev, V.; Maruk, A.; Lyamtseva, E.; Malysheva, A.; Larenkov, A.; Zhukova, M.; Lunev, A.; et al. New phosphonic acids as components of bone seeking radiopharmaceuticals. J. Label. Compd. Radiopharm. 2019, 62, S287–S289. [Google Scholar]
- Rasulova, N.; Sagdullaev, S.; Arybzhanov, D.; Khodjibekov, M.; Krylov, V.; Lyubshin, V. Optimal Timing of Bisphosphonate Administration in Combination with Samarium-153 Oxabifore in the Treatment of Painful Metastatic Bone Disease. World J. Nucl. Med. 2013, 12, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Tsebrikova, G.S.; Ragulin, V.V.; Baulin, V.E.; German, K.E.; Malysheva, A.O.; Klement’eva, O.E.; Kodina, G.E.; Larenkov, A.A.; Lyamtseva, E.A.; Taratonenkova, N.A.; et al. 2,5-Diamino-5,5-diphosphonovaleric Acid as a Ligand for an Osteotropic 188Re Radiopharmaceutical. Russ. J. Gen. Chem. 2018, 88, 1780–1785. [Google Scholar] [CrossRef]
- Teixeira, F.; Pérez, A.; Madden, W.; Hernández, L.; del Carpio, E.; Lubes, V. Speciation of the ternary complexes formed between copper(II), salicylic acid and small blood serum bioligands. J. Mol. Liq. 2016, 224, 346–350. [Google Scholar] [CrossRef]
- Baulin, V.E.; Kalashnikova, I.P.; Vikharev, Y.B.; Vikhareva, E.A.; Baulin, D.V.; Tsivadze, A.Y. Phosphoryl Analogs of Salicylic Acid: Synthesis and Analgesic and Anti-Inflammatory Activity. Russ. J. Gen. Chem. 2018, 88, 1786–1791. [Google Scholar] [CrossRef]
- Ragulin, V.V. Phosphorus-Containing Aminocarboxylic Acids: XV. α,ω-Diamino-ω,ω-diphosphonoalkylcarboxylic Acids. Russ. J. Gen. Chem. 2018, 88, 1045–1048. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Félix, V.; Lima, L.M.P.; Price, D.W. Metal complexes of cyclen and cyclam derivatives useful for medical applications: A discussion based on thermodynamic stability constants and structural data. Dalt. Trans. 2007, 2734–2745. [Google Scholar] [CrossRef]
- Popov, K.; Rönkkömäki, H.; Lajunen, L.H.J. Critical evaluation of stability constants of phosphonic acids (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 1641–1677. [Google Scholar] [CrossRef]
- Bollinger, J.E.; Roundhill, D.M. Complexation of Indium(III), Gallium(III), Iron(III), Gadolinium(III), and Neodymium(III) Ions with Amino Diphosphonic Acids in Aqueous Solution. Inorg. Chem. 1993, 32, 2821–2826. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Martell, A.E. Gallium Complexes of Multidentate Ligands in Aqueous Solution. Inorg. Chem. 1980, 19, 1646–1651. [Google Scholar] [CrossRef]
- Stein, B.W.; Morgenstern, A.; Batista, E.R.; Birnbaum, E.R.; Bone, S.E.; Cary, S.K.; Ferrier, M.G.; John, K.D.; Pacheco, J.L.; Kozimor, S.A.; et al. Advancing Chelation Chemistry for Actinium and Other +3 f-Elements, Am, Cm, and la. J. Am. Chem. Soc. 2019, 141, 19404–19414. [Google Scholar] [CrossRef] [PubMed]
- Arefyeva, E.; Larenkov, A.; Maruk, A.; Kodina, G. Effects of Buffering Agents on Gallium-68 Speciation in Radiopharmaceutical Related Preparations. J. Label. Compd. Radiopharm. 2021. In Press. [Google Scholar]
- Hacht, B. Gallium (III)-acetate speciation studies under physiological conditions. Open Sci. J. 2016, 1, 1–13. [Google Scholar] [CrossRef][Green Version]
- Clausén, M.; Öhman, L.O.; Kubicki, J.D.; Persson, P. Characterisation of gallium(III)-acetate complexes in aqueous solution: A potentiometric, EXAFS, IR and molecular orbital modelling study. J. Chem. Soc. Dalt. Trans. 2002, 2559–2564. [Google Scholar] [CrossRef]
- Skorik, N.A.; Artish, A.S. Stability of complexes of scandium, gallium, indium and thorium with anions of certain organic acids. Zhurnal Neorg. Khim. 1985, 30, 1994–1997. [Google Scholar]
- Petit, T.; Lange, K.M.; Conrad, G.; Yamamoto, K.; Schwanke, C.; Hodeck, K.F.; Dantz, M.; Brandenburg, T.; Suljoti, E.; Aziz, E.F. Probing ion-specific effects on aqueous acetate solutions: Ion pairing versus water structure modifications. Struct. Dyn. 2014, 1, 034901. [Google Scholar] [CrossRef] [PubMed]
- Bubenshchikov, V.B.; Maruk, A.Y.; Bruskin, A.B.; Kodina, G.E. Preparation and properties of 68Ga complexes with RGD peptide derivatives. Radiochemistry 2016, 58, 506–512. [Google Scholar] [CrossRef]
- Lunyov, A.S.; Clement’eva, О.Е.; Lunyova, К.А.; Zhukova, M.V.; Malysheva, А.О. Qualitative and quantitative comparisons of bone PET-imaging using 68Ga-oxabiphor and Na18F. Saratov J. Med. Sci. Res. 2017, 13, 886–891. [Google Scholar]
- Lunev, A.S.; Larenkov, A.A.; Petrosova, K.A.; Klementyeva, O.E.; Kodina, G.E. Fast PET imaging of inflammation using 68Ga-citrate with Fe-containing salts of hydroxy acids. EJNMMI Radiopharm. Chem. 2016, 1 (Suppl. S1), 10. [Google Scholar]
- Tsebrikova, G.S.; Barsamian, R.T.; Solov, V.P.; Kudryashova, Z.A.; Baulin, V.E.; Wang, Y.J.; Tsivadze, A.Y. Complexation of gallium(III) nitrate with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylenephosphonic acid). Russ. Chem. Bull. 2018, 67, 2184–2187. [Google Scholar] [CrossRef]
- Solov’ev, V.P.; Tsivadze, A.Y. Supramolecular complexes: Determination of stability constants on the basis of various experimental methods. Prot. Met. Phys. Chem. Surfaces 2015, 51, 1–35. [Google Scholar] [CrossRef]
- Solov’ev, V.P. The CHEMEQUI Program for Computations of Equilibrium Constants and Related Quantities from Experimental Results of UV–Vis, IR and NMR Spectroscopy, Calorimetry, Potentiometry and Conductometry. Available online: http://vpsolovev.ru/programs/chemequi/ (accessed on 8 December 2020).
- Ali, M.; Pant, M.; Abraham, A. A simplex differential evolution algorithm: Development and applications. Trans. Inst. Meas. Control 2012, 34, 691–704. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Benézéth, P.; Diakonov, I.I.; Pokrovski, G.S.; Dandurand, J.L.; Schott, J.; Khodakovsky, I.L. Gallium speciation in aqueous solution. Experimental study and modelling: Part 2. Solubility of α-GaOOH in acidic solutions from 150 to 250°C and hydrolysis constants of gallium (III) to 300 °C. Geochim. Cosmochim. Acta 1997, 61, 1345–1357. [Google Scholar] [CrossRef]
- Larenkov, A.A.; Maruk, A.Y.; Kodina, G.E. Intricacies of the Determination of the Radiochemical Purity of 68Ga Preparations: Possibility of Sorption of Ionic 68Ga Species on Reversed-Phase Columns. Radiochemistry 2018, 60, 625–633. [Google Scholar] [CrossRef]
- Maruk, A.Y.; Larenkov, A.A. Determination of ionic 68Ga impurity in radiopharmaceuticals: Major revision of radio-HPLC methods. J. Radioanal. Nucl. Chem. 2020, 323, 189–195. [Google Scholar] [CrossRef]
- GOST 33216-2014. Guidelines for Accommodation and Care of Animals. Species-Specific Provisions for Laboratory Rodents and Rabbits; Standardinform: Moscow, Russia, 2014. [Google Scholar]
- Moiseenko, V.M.; Blinov, N.N. Sovremennaya Taktika Lecheniya Bol’nykh so Zlokachestvennymi Novoobrazovaniyami s Metastazami v Kosti: Posobie Dlya Vrachei (Modern Tactics of Treating Patients with Malignant Neoplasms with Bone Metastases: A Manual for Doctors, in Russian); Ministry of Healthcare of the Russian Federation, Petrov Research Institute of Oncology: St. Petersburg, Russia, 1996. [Google Scholar]

| No. | Equilibrium | logK b | ||
|---|---|---|---|---|
| 8 | 9 | AEDP c | ||
| 1 | H + L = HL | 10.88 ± 0.19 | 11.82 ± 0.29 | 11.5 |
| 2 | HL + H = H2L | 9.11 ± 0.26 | 10.65 ± 0.43 | 8.58 |
| 3 | H2L + H = H3L | 5.65 ± 0.28 | 9.76 ± 0.45 | 5.37 |
| 4 | H3L + H = H4L | 3.47 ± 0.26 | 8.50 ± 0.45 | 1.50 |
| 5 | H4L + H = H5L | 2.99 ± 0.32 | 5.05 ± 0.46 | |
| 6 | H5L + H = H6L | 2.75 ± 0.48 | ||
| No. | Equilibrium | logK | |||
|---|---|---|---|---|---|
| 8 | 9 | EDDADPO b | EDTPO b | ||
| 1 | Ga + L = GaL | 26.63 ± 0.24 | 31.92 ± 0.32 | 26.82 | 31.83 |
| 2 | GaL + H = GaHL | 8.28 ± 0.40 | 8.90 ± 0.61 | 5.10 | 6.65 |
| 3 | GaHL + H = GaH2L | 4.60 ± 0.43 | 8.28 ± 0.68 | 2.24 | 5.10 |
| 4 | GaH2L + H = GaH3L | 3.25 ± 0.43 | 8.03 ± 0.65 | 3.29 | |
| 5 | GaH3L + H = GaH4L | ≈4.9 | 2.46 | ||
| Probe | [68Ga]Ga-Acetate | [68Ga]Ga-9 | ||
|---|---|---|---|---|
| Organ or Tissue | Time after Injection | |||
| 60 min | 120 min | 60 min | 120 min | |
| Blood a | 3.0 ± 0.4 | 4.5 ± 0.4 | 2.6 ± 0.3 | 2.5 ± 0.0 |
| Lung b | 3.3 ± 0.3 | 6.0 ± 0.3 | 3.0 ± 1.0 | 4.1 ± 1.4 |
| Liver b | 6.8 ± 0.9 | 11.2 ± 0.7 | 4.9 ± 0.7 | 6.5 ± 0.5 |
| Kidney b | 1.5 ± 0.1 | 2.6 ± 0.4 | 1.6 ± 0.2 | 1.9 ± 0.3 |
| Intestine b | 4.9 ± 0.7 | 7.7 ± 0.5 | 6.4 ± 1.4 | 4.6 ± 1.3 |
| Muscular tissue a | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| Hip normal a | 0.8 ± 0.3 | 1.7 ± 0.3 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| Fraction site a | 1.9 ± 0.5 | 3.9 ± 0.9 | 0.6 ± 0.1 | 0.9 ± 0.1 |
| Fraction site/intact bone | 2.4 | 2.3 | 1.2 | 1.8 |
| Probe | [68Ga]Ga-9 | [68Ga]Ga-Citrate | ||
|---|---|---|---|---|
| Organ or Tissue | Time after Injection | |||
| 60 min | 120 min | 60 min | 120 min | |
| Blood a | 5.4 ± 1.7 | 1.9 ± 1.0 | 3.1 ± 0.2 | 1.5 ± 0.4 |
| Lung b | 2.9 ± 0.9 | 3.5 ± 1.2 | 2.6 ± 1.3 | 4.9 ± 2.2 |
| Liver b | 7.1 ± 0.8 | 7.2 ± 0.9 | 6.9 ± 1.2 | 6.1 ± 0.8 |
| Kidney b | 1.8 ± 0.2 | 1.7 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.3 |
| Intestines b | 3.8 ± 0.3 | 5.9 ± 0.9 | 5.9 ± 0.7 | 8.0 ± 1.5 |
| Muscular tissue a | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.03 | 0.1 ± 0.1 |
| Pathology site a | 1.6 ± 0.9 | 1.1 ± 0.5 | 1.1 ± 0.2 | 0.8 ± 0.02 |
| Pathology site/muscular tissue | 5.6 | 5.8 | 2.7 | 8.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruk, A.Y.; Ragulin, V.V.; Mitrofanov, I.A.; Tsebrikova, G.S.; Solov’ev, V.P.; Lunev, A.S.; Lunyova, K.A.; Klementyeva, O.E.; Baulin, V.E.; Kodina, G.E.; et al. Synthesis, Complexation Properties, and Evaluation of New Aminodiphosphonic Acids as Vector Molecules for 68Ga Radiopharmaceuticals. Molecules 2021, 26, 2357. https://doi.org/10.3390/molecules26082357
Maruk AY, Ragulin VV, Mitrofanov IA, Tsebrikova GS, Solov’ev VP, Lunev AS, Lunyova KA, Klementyeva OE, Baulin VE, Kodina GE, et al. Synthesis, Complexation Properties, and Evaluation of New Aminodiphosphonic Acids as Vector Molecules for 68Ga Radiopharmaceuticals. Molecules. 2021; 26(8):2357. https://doi.org/10.3390/molecules26082357
Chicago/Turabian StyleMaruk, Alesya Ya., Valery V. Ragulin, Iurii A. Mitrofanov, Galina S. Tsebrikova, Vitaly P. Solov’ev, Alexandr S. Lunev, Kristina A. Lunyova, Olga E. Klementyeva, Vladimir E. Baulin, Galina E. Kodina, and et al. 2021. "Synthesis, Complexation Properties, and Evaluation of New Aminodiphosphonic Acids as Vector Molecules for 68Ga Radiopharmaceuticals" Molecules 26, no. 8: 2357. https://doi.org/10.3390/molecules26082357
APA StyleMaruk, A. Y., Ragulin, V. V., Mitrofanov, I. A., Tsebrikova, G. S., Solov’ev, V. P., Lunev, A. S., Lunyova, K. A., Klementyeva, O. E., Baulin, V. E., Kodina, G. E., & Tsivadse, A. Y. (2021). Synthesis, Complexation Properties, and Evaluation of New Aminodiphosphonic Acids as Vector Molecules for 68Ga Radiopharmaceuticals. Molecules, 26(8), 2357. https://doi.org/10.3390/molecules26082357






