Analgesic and Anticancer Activity of Benzoxazole Clubbed 2-Pyrrolidinones as Novel Inhibitors of Monoacylglycerol Lipase
Abstract
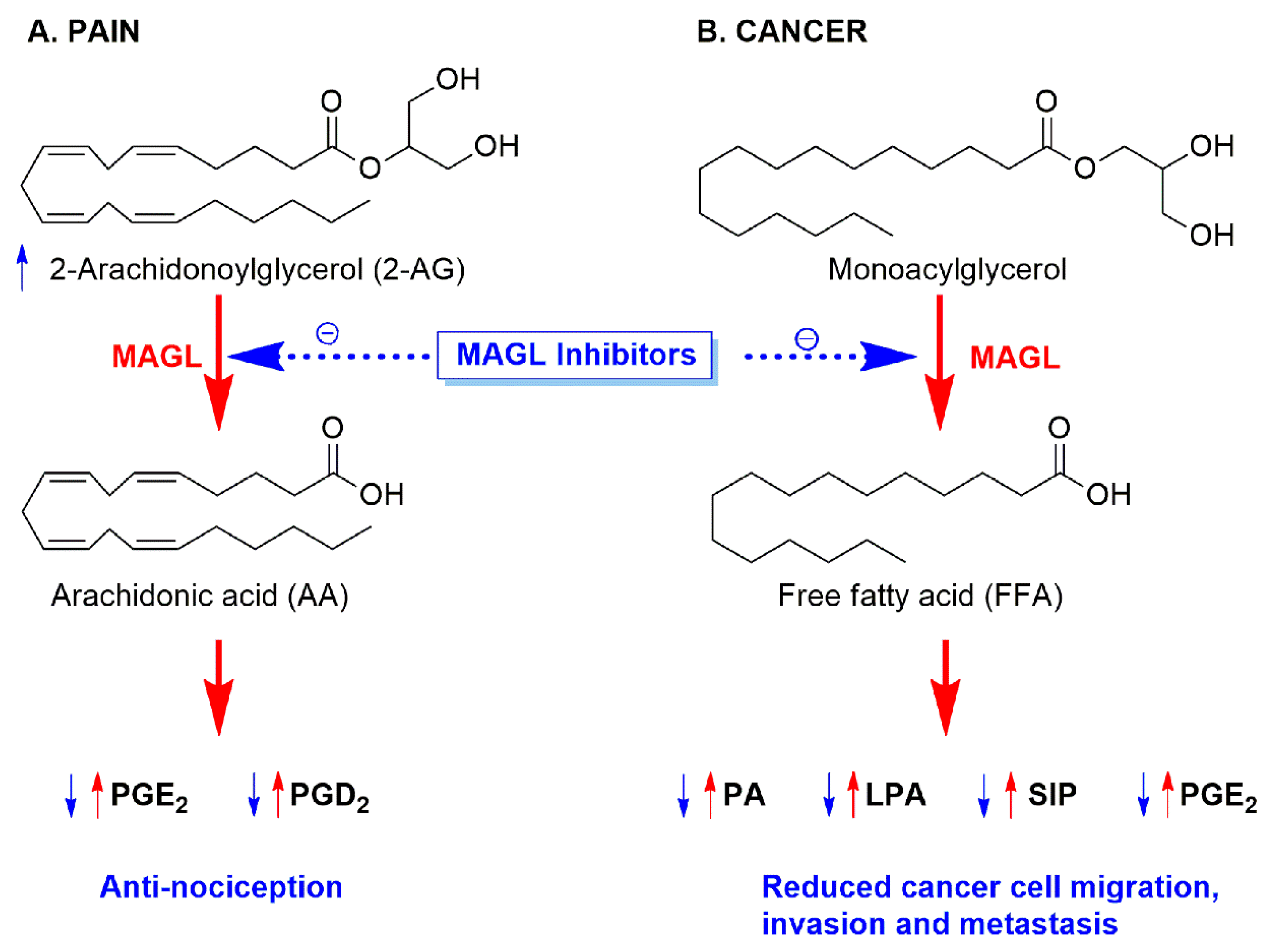
1. Introduction
2. Results
2.1. Chemistry
2.2. Human MAGL Assay
2.3. Human FAAH Assay
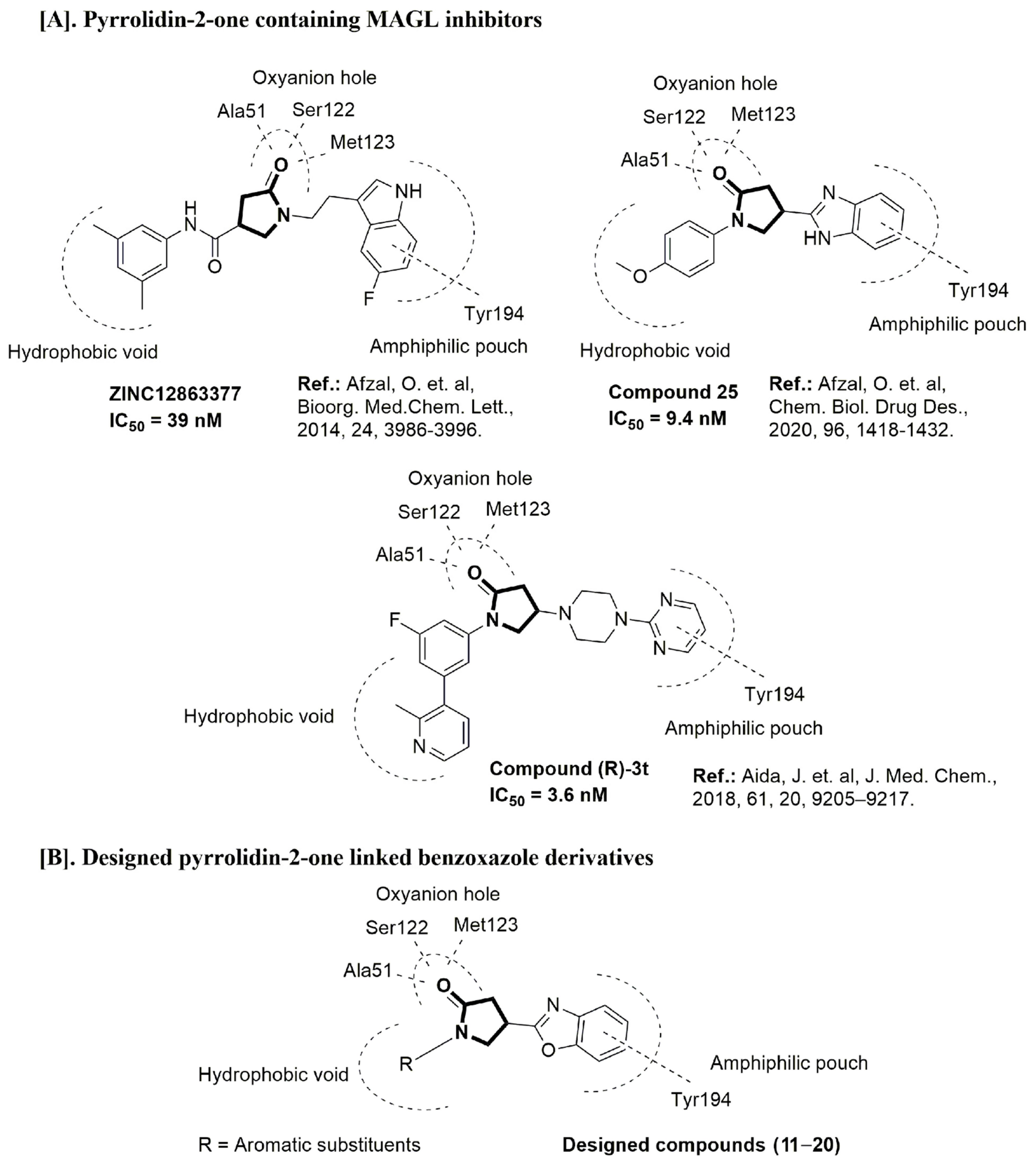
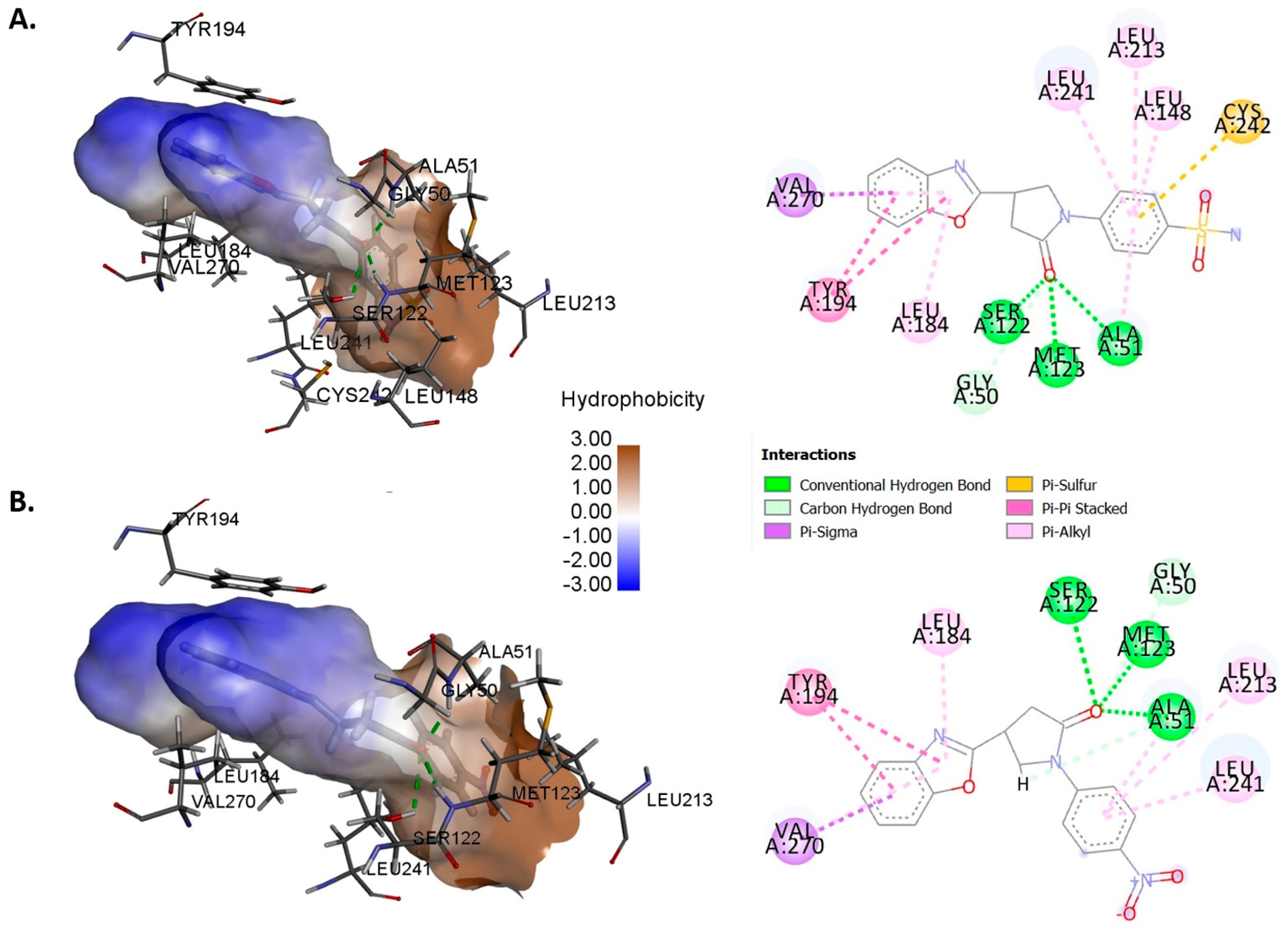
2.4. Molecular Docking Study
2.5. Pharmacokinetic and Physicochemical Characteristics
2.6. In Silico Absorption and Toxicity Profile
2.7. Analgesic Activity
2.8. Anticancer Activity
3. Discussion
4. Experimental
4.1. Chemistry
4.1.1. Synthesis of 1-(Aryl Substituted)-5-Oxopyrrolidine-3-Carboxylic Acids (1–10)
Method-1 (for compound 1)
Method-2 (for compounds 2–10)
4.1.2. Synthesis of 4-(Benzoxazolyl)-1-(Aryl Substituted)Pyrrolidin-2-Ones (11–20)
4.2. Human MAGL Assay
4.3. Human FAAH Assay
4.4. Molecular Docking Study
4.5. Physicochemical and Pharmacokinetic Characteristics
4.6. In Silico Absorption and Toxicity Profile
4.7. Analgesic Activity
4.8. Anticancer Screening: Sulforhodamine B Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Di Marzo, V.; Bisogno, T.; De Petrocellis, L. the biosynthesis, fate and pharmacological properties of endocannabinoids. Handb. Exp. Pharmacol. 2005, 168, 147–185. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K. 2- Arachidonoylglycerol: A possible endogenous can-nabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Endocannabinoid Research Group; Bisogno, T.; Di Marzo, V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int. J. Obes. 2006, 30, S7–S12. [Google Scholar] [CrossRef]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. 2011, 204, 267–276. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Long, J.Z.; O’Neal, S.T.; Abdullah, R.A.; Poklis, J.L.; Boger, D.; Cravatt, B.F.; Lichtman, A.H. Blockade of endo-cannabinoid-degrading enzymes attenuates neuropathic pain. J. Pharmacol. Exp. Ther. 2009, 330, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, S.G.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J. Pain 2010, 11, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Suplita, R.L.; Bolton, N.M.; Neely, M.H.; Fegley, D.; Mangieri, R.; Krey, J.F.; Walker, J.M.; Holmes, P.V.; Crystal, J.D.; et al. An endocannabinoid mechanism for stress-induced analgesia. Nat. Cell Biol. 2005, 435, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Connell, K.; Bolton, N.; Olsen, D.; Piomelli, D.; Hohmann, A.G. Role of the basolateral nucleus of the amygdala in endocan-nabinoid-mediated stress-induced analgesia. Neurosci. Lett. 2006, 397, 180–184. [Google Scholar] [CrossRef]
- Guindon, J.; Guijarro, A.; Piomelli, D.; Hohmann, A.G. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br. J. Pharmacol. 2010, 163, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Lai, Y.; Takacs, S.M.; Bradshaw, H.B.; Hohmann, A.G. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 2013, 67, 94–109. [Google Scholar] [PubMed]
- Aaltonen, N.; Kedzierska, E.; Orzelska-Górka, J.; Lehtonen, M.; Navia-Paldanius, D.; Jakupovic, H.; Savinainen, J.R.; Neva-lainen, T.; Laitinen, J.T.; Parkkari, T.; et al. In vivo characterization of the ultrapotent monoacylglycerol lipase inhibitor {4-[bis-(benzo[d][1,3]dioxol-5-yl)methyl]-piperidin-1-yl}(1H-1,2,4-triazol-1-yl)methanone (JJKK-048). J. Pharm. Exp. Ther. 2016, 359, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Nomura, D.K.; Lombardi, D.P.; Chang, J.W.; Niessen, S.; Ward, A.M.; Long, J.Z.; Hoover, H.H.; Cravatt, B.F. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem. Biol. 2011, 18, 846–856. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Wu, Y.; Wang, D.; Feng, G.; Tang, Y.-P.; Teng, Z.; Chen, C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s Disease. Cell Rep. 2012, 2, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- McAllister, L.A.; Butler, C.R.; Mente, S.; O’Neil, S.V.; Fonseca, K.R.; Piro, J.R.; Cianfrogna, J.A.; Foley, T.L.; Gilbert, A.M.; Harris, A.R.; et al. Discovery of trifluoromethyl glycol carbamates as potent and selective covalent monoacylglycerol lipase (MAGL) inhibitors for treatment of neuroinflammation. J. Med. Chem. 2018, 61, 3008–3026. [Google Scholar] [CrossRef] [PubMed]
- Cisar, J.S.; Weber, O.D.; Clapper, J.R.; Blankman, J.L.; Henry, C.L.; Simon, G.M.; Alexander, J.P.; Jones, T.K.; Ezekowitz, R.A.B.; O’Neill, G.P.; et al. Identification of ABX-1431, a selective inhibitor of monoacylglycerol lipase and clinical candidate for treatment of neurological disorders. J. Med. Chem. 2018, 61, 9062–9084. [Google Scholar] [CrossRef]
- Maione, S.; Morera, E.; Marabese, I.; Ligresti, A.; Luongo, L.; Ortar, G.; DiMarzo, V. Antinociceptive effects of tetrazole inhib-itors of endocannabinoid inactivation: Cannabinoid and noncannabinoid receptor-mediated mechanisms. Br. J. Pharmacol. 2008, 155, 775–782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fowler, C.J. Monoacylglycerol lipase—A target for drug development? Br. J. Pharmacol. 2012, 166, 1568–1585. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.M.; Nomura, D.K. Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci. 2013, 92, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Masquelier, J.; Muccioli, G.G. Controlling 2-arachidonoylglycerol metabolism as an anti-inflammatory strategy. Drug Discov. Today 2014, 19, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M. A new age for MAGL. Chem. Biol. 2010, 17, 4–6. [Google Scholar] [CrossRef][Green Version]
- Long, J.Z.; Nomura, D.K.; Cravatt, B.F. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem. Biol. 2009, 16, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.-W.; Cravatt, B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef]
- Nomura, D.K.; Morrison, B.E.; Blankman, J.L.; Long, J.Z.; Kinsey, S.G.; Marcondes, M.C.G.; Ward, A.M.; Hahn, Y.K.; Lichtman, A.H.; Conti, B.; et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 2011, 334, 809–813. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, B.; Seviour, E.G.; Tao, K.-X.; Liu, X.-H.; Ling, Y.; Chen, J.-Y.; Wang, G.-B. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011, 307, 6–17. [Google Scholar] [CrossRef]
- King, A.R.; Duranti, A.; Tontini, A.; Rivara, S.; Rosengarth, A.; Clapper, J.R.; Astarita, G.; Geaga, J.A.; Luecke, H.; Mor, M.; et al. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem. Biol. 2007, 14, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; LaBar, G.; Lambert, D.M. CAY10499, a novel monoglyceride lipase inhibitor evidenced by an expeditious MGL assay. ChemBioChem 2008, 9, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Minkkilä, A.; Savinainen, J.R.; Käsnänen, H.; Xhaard, H.; Nevalainen, T.; Laitinen, J.T.; Poso, A.; Leppänen, J.; Saario, S.M. Screening of various hormone-sensitive lipase inhibitors as endocannabinoid-hydrolyzing enzyme inhibitors. ChemMedChem 2009, 4, 1253–1259. [Google Scholar] [CrossRef]
- Long, J.Z.; Jin, X.; Adibekian, A.; Li, W.; Cravatt, B.F. Characterization of tunable piperidine and piperazine carbamates as inhibitors of endocannabinoid hydrolases. J. Med. Chem. 2010, 53, 1830–1842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertrand, T.; Augé, F.; Houtmann, J.; Rak, A.; Vallée, F.; Mikol, V.; Berne, P.; Michot, N.; Cheuret, D.; Hoornaert, C.; et al. Structural basis for human monoglyceride lipase inhibition. J. Mol. Biol. 2010, 396, 663–673. [Google Scholar] [CrossRef]
- Chang, J.W.; Niphakis, M.J.; Lum, K.M.; Cognetta, A.B.; Wang, C.; Matthews, M.L.; Niessen, S.; Buczynski, M.W.; Parsons, L.H.; Cravatt, B.F. Remarkably selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem. Biol. 2012, 19, 579–588. [Google Scholar] [CrossRef]
- Morera, L.; LaBar, G.; Ortar, G.; Lambert, D.M. Development and characterization of endocannabinoid hydrolases FAAH and MAGL inhibitors bearing a benzotriazol-1-yl carboxamide scaffold. Bioorg. Med. Chem. 2012, 20, 6260–6275. [Google Scholar] [CrossRef]
- Aaltonen, N.; Savinainen, J.R.; Ribas, C.R.; Rönkkö, J.; Kuusisto, A.; Korhonen, J.; Navia-Paldanius, D.; Häyrinen, J.; Takabe, P.; Käsnänen, H.; et al. Piperazine and piperidine triazole ureas as ultrapotent and highly selective inhibitors of monoacylglycerol lipase. Chem. Biol. 2013, 20, 379–390. [Google Scholar] [CrossRef]
- Aida, J.; Fushimi, M.; Kusumoto, T.; Sugiyama, H.; Arimura, N.; Ikeda, S.; Sasaki, M.; Sogabe, S.; Aoyama, K.; Koike, T. Design, synthesis, and evaluation of piperazinyl pyrrolidin-2-ones as a novel series of reversible monoacylglycerol lipase inhibitors. J. Med. Chem. 2018, 61, 9205–9217. [Google Scholar] [CrossRef]
- Granchi, C.; Lapillo, M.; Glasmacher, S.; Bononi, G.; Licari, C.; Poli, G.; El Boustani, M.; Caligiuri, I.; Rizzolio, F.; Gertsch, J.; et al. Optimization of a benzoylpiperidine class identifies a highly potent and selective reversible monoacylglycerol lipase (MAGL) inhibitor. J. Med. Chem. 2019, 62, 1932–1958. [Google Scholar] [CrossRef]
- Castelli, R.; Scalvini, L.; Vacondio, F.; Lodola, A.; Anselmi, M.; Vezzosi, S.; Carmi, C.; Bassi, M.; Ferlenghi, F.; Rivara, S. Benzisothiazolinone derivatives as potent allosteric monoacylglycerol lipase inhibitors that functionally mimic sulfenylation of regulatory cysteines. J. Med. Chem. 2020, 63, 1261–1280. [Google Scholar] [CrossRef]
- LaBar, G.; Bauvois, C.; Borel, F.; Ferrer, J.-L.; Wouters, J.; Lambert, D.M. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. ChemBioChem 2009, 11, 218–227. [Google Scholar] [CrossRef]
- Schalk-Hihi, C.; Schubert, C.; Alexander, R.; Bayoumy, S.; Clemente, J.C.; Deckman, I.; DesJarlais, R.L.; Dzordzorme, K.C.; Flores, C.M.; Grasberger, B.; et al. Crystal structure of a soluble form of human monoglyceride lipase in complex with an inhibitor at 1.35 Å resolution. Protein Sci. 2011, 20, 670–683. [Google Scholar] [CrossRef]
- Scalvini, L.; Piomelli, D.; Mor, M. Monoglyceride lipase: Structure and inhibitors. Chem. Phys. Lipids 2016, 197, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Kumar, R.; Firoz, A.; Jaggi, M.; Bawa, S. Docking based virtual screening and molecular dynamics study to identify potential monoacylglycerol lipase inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 3986–3996. [Google Scholar] [CrossRef]
- Afzal, O.; Akhtar, S.; Kumar, S.; Ali, R.; Jaggi, M.; Bawa, S. Hit to lead optimization of a series of N-[4-(1,3-benzothiazol-2-yl)phenyl]acetamides as monoacylglycerol lipase inhibitors with potential anticancer activity. Eur. J. Med. Chem. 2016, 121, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Kumar, S.; Shalmali, N.; Afzal, O.; Azim, S.; Chanana, D.; Alam, O.; Paudel, Y.N.; Sharma, M.; Bawa, S. Development of thiazole-5-carboxylate derivatives as selective inhibitors of monoacylglycerol lipase as target in cancer. Mini Rev. Med. Chem. 2019, 19, 410–423. [Google Scholar] [CrossRef]
- Altamimi, A.S.A.; Bawa, S.; Athar, F.; Hassan, Q.; Riadi, Y.; Afzal, O. Pyrrolidin-2-one linked benzofused heterocycles as novel small molecule monoacylglycerol lipase inhibitors and antinociceptive agents. Chem. Biol. Drug Des. 2020, 96, 1418–1432. [Google Scholar] [CrossRef]
- Mickevicius, M.; Beresnevicius, Z.J.; Mickevicius, V.; Mikulskiene, G. Condensation products of 1-aryl-4-carboxy-2-pyrroli-dinones with o-diaminoarenes, o-aminophenol, and their structural studies. Heteroat. Chem. 2006, 17, 47–56. [Google Scholar] [CrossRef]
- Granchi, C.; Rizzolio, F.; Palazzolo, S.; Carmignani, S.; Macchia, M.; Saccomanni, G.; Manera, C.; Martinelli, A.; Minutolo, F.; Tuccinardi, T. Structural optimization of 4-chlorobenzoylpiperidine derivatives for the development of potent, reversible, and selective monoacylglycerol lipase (MAGL) inhibitors. J. Med. Chem. 2016, 59, 10299–10314. [Google Scholar] [CrossRef]
- Mor, M.; Rivara, S.; Lodola, A.; Plazzi, P.V.; Tarzia, G.; Duranti, A.; Tontini, A.; Piersanti, G.; Kathuria, S.; Piomelli, D. Cy-clohexylcarbamic acid 3’- or 4’-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: Synthesis, quantitative structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2004, 47, 4998–5008. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Herbertz, T.; Hudkins, R.L.; Dorsey, B.D.; Mallamo, J.P. Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem. Neurosci. 2012, 3, 50–68. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2018, 35, 1067–1069. [Google Scholar] [CrossRef]
- Coderre, T.J.; Vaccarino, A.L.; Melzack, R. Central nervous system plasticity in the tonic pain response to subcutaneous for-malin injection. Brain Res. 1990, 535, 155–158. [Google Scholar] [CrossRef]
- Laughlin, T.M.; Tram, K.V.; Wilcox, G.L.; Birnbaum, A.K. Comparison of antiepileptic drugs tiagabine, lamotrigine, and gabapentin in mouse models of acute, prolonged, and chronic nociception. J. Pharmacol. Exp. Ther. 2002, 302, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.R.; A Schepartz, S.; A Chabner, B. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]


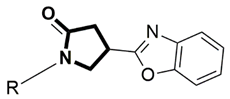 | |||
| Compound | R | hMAGL; IC50 | hFAAH; IC50 |
|---|---|---|---|
| 11 | 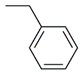 | >100 μM | ND |
| 12 | 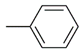 | >100 μM | ND |
| 13 | 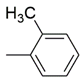 | 85 ± 1.5 μM | ND |
| 14 | 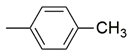 | 72 ± 2.1 μM | ND |
| 15 | 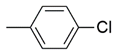 | 65 ± 3.2 nM | 28 ± 1.8 μM |
| 16 | 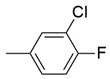 | 34 ± 1.7 nM | 25 ± 2.3 μM |
| 17 |  | 62 ± 2.7 μM | ND |
| 18 |  | 42 ± 1.5 nM | 37 ± 2.2 μM |
| 19 | 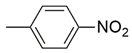 | 8.4 ± 1.9 nM | 55 ± 2.7 μM |
| 20 | 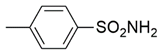 | 7.6 ± 0.8 nM | 68 ± 2.1 μM |
| CAY10499 | -- | 415 ± 3.2 nM | -- |
| JZL184 | -- | 10 ± 0.8 nM | -- |
| URB597 | -- | -- | 5 ± 0.6 nM |
| S. No. | Property | Description | Range of Properties in CNS Drugs | Compound 19 | Compound 20 | |||
|---|---|---|---|---|---|---|---|---|
| QL | PL | PU | QU | |||||
| 1 | #stars | drug likeness penalty; the higher the value, the less drug-like the molecule | 0 | 0 | 0 | 3 | 0 | 0 |
| 2 | #amine | no. of basic amines | 0 | 1 | 1 | 2 | 0 | 0 |
| 3 | #amidine | no. of amidines groups | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | #acid | no. of carboxylic acid groups | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | #amide | no. of amides groups | 0 | 0 | 0 | 1 | 0 | 0 |
| 6 | #rotor | no. of rotatable bonds (without CX3, alkene, amide, small ring) | 0 | 3 | 6 | 8 | 1 | 2 |
| 7 | CNS | a qualitative CNS activity parameter | −2 | 0 | 1 | 2 | −2 | −2 |
| 8 | dipole | computed dipole moment | 0.67 | 1.1 | 3.9 | 8.9 | 9.47 | 10.22 |
| 9 | SASA | solvent accessible surface area | 348 | 487 | 620 | 798 | 584.85 | 617.51 |
| 10 | FOSA | SASA on saturated carbon and attached hydrogen | 16 | 178 | 314 | 464 | 91.99 | 91.96 |
| 11 | FISA | SASA on N, O, and H attached to heteroatoms | 0 | 0 | 64 | 176 | 167.04 | 210.06 |
| 12 | PISA | π component of SASA | 0 | 160 | 292 | 343 | 325.81 | 313.57 |
| 13 | WPSA | weakly polar component of the SASA (halogens, P, and S) | 0 | 0 | 0 | 126 | 0 | 1.94 |
| 14 | volume | solvent accessible volume (Å3) | 492 | 830 | 1104 | 1388 | 1002.52 | 1065.46 |
| 15 | donorHB | estimated no. of hydrogen bonds that would be donated to the solvent water | 0 | 0 | 1 | 3 | 0 | 2 |
| 16 | accptHB | estimated no. of hydrogen bonds that would be accepted from the solvent water | 1 | 2.8 | 5.2 | 8.3 | 6 | 9.5 |
| 17 | glob | a globularity descriptor (1 for a sphere) | 0.77 | 0.82 | 0.88 | 0.93 | 0.82 | 0.81 |
| 18 | QPpolrz | predicted polarizability (Å3) | 14 | 28 | 38 | 49 | 36.43 | 38.19 |
| 19 | QPlogPo/w | octanol−water logP | −0.16 | 2.5 | 4.7 | 6.0 | 2.13 | 0.90 |
| 20 | QPlogS | solubility in log(moles/liter) | −6.5 | −4.6 | −2.5 | −0.42 | −3.99 | −3.99 |
| 21 | CIQPlogS | log of conformation-independent solubility | −6.3 | −4.2 | −2.3 | 0.36 | −4.16 | −3.77 |
| 22 | QPPCaco | apparent Caco-2 cell permeability | 0 | 0 | 810 | 3269 | 258.09 | 100.92 |
| 23 | QPlogBB | brain/blood partition coefficient | −1.2 | −0.06 | 0.75 | 1.2 | −1.12 | −1.65 |
| 24 | QPPMDCK | predicted apparent MDCK cell permeability (nm/s) | 0 | 0 | 634 | 5899 | 114.43 | 42.50 |
| 25 | QPlogKhsa | prediction of binding to human serum albumin | −1 | 0.04 | 0.78 | 1.04 | −0.11 | −0.34 |
| 26 | HumanOralAbsorption | Human oral absorption | 2 | 3 | 3 | 3 | 3 | 3 |
| 27 | PercentHuman OralAbsorption | Percent of human oral absorption | 61 | 95 | 100 | 100 | 82.63 | 68.11 |
| 28 | TPSA | van der Waals surface area of polar nitrogen and oxygen atoms | 3.8 | 12 | 54 | 109 | 98.65 | 119.08 |
| 29 | #NandO | no. of N and O atoms | 1 | 2 | 4 | 7 | 7 | 7 |
| 30 | RuleOfFive | no. of violations of Lipinski’s rule of five | 0 | 0 | 0 | 1 | 0 | 0 |
| 31 | RuleOfThree | no. of violations of Jorgensen’s rule of three | 0 | 0 | 0 | 1 | 0 | 0 |
| 32 | #in34 | no. of atoms in three- or four-membered rings | 0 | 0 | 0 | 0 | 0 | 0 |
| 33 | #in56 | no. of atoms in five- or six-membered rings | 5 | 11 | 17 | 24 | 20 | 20 |
| 34 | #noncon | no. of atoms not able to form conjugation in nonaromatic rings | 0 | 0 | 4 | 10 | 3 | 3 |
| 35 | #nonHatm | no. of non-H atoms | 8 | 19 | 25 | 30 | 24 | 25 |
| Compound | BBB | HIA | HOB | AMES test | Carcinogenicity | Rat Acute Toxicity (LD50, mol/kg) |
|---|---|---|---|---|---|---|
| 19 | Yes | Yes | Yes | Mutagenic | Non-carcinogen | 2.30 |
| 20 | Yes | Yes | Yes | Non-Mutagenic | Non-carcinogen | 2.21 |
| Panel | Cell Line | Compound 19 (NSC: 778839) | Compound 20 (NSC: 778842) | ||
|---|---|---|---|---|---|
| % G | % GI | % G | % GI | ||
| Leukemia | CCRF-CEM | 93.58 | 6.42 | 94.48 | 5.52 |
| HL-60(TB) | 100.09 | −0.09 | 96.15 | 3.85 | |
| K-562 | 98.41 | 1.59 | 98.83 | 1.17 | |
| MOLT-4 | 93.64 | 6.36 | 92.47 | 7.53 | |
| RPMI-8226 | 101.28 | −1.28 | 103.53 | −3.53 | |
| SR | 88.92 | 11.08 | 93.20 | 6.80 | |
| Non-Small Cell Lung Cancer | A549/ATCC | 100.60 | −0.60 | 95.28 | 4.72 |
| HOP-62 | 85.04 | 14.96 | 81.97 | 18.03 | |
| HOP-92 | 104.72 | −4.72 | 77.78 | 22.22 | |
| NCI-H226 | 98.63 | 1.37 | 92.75 | 7.25 | |
| NCI-H23 | 93.07 | 6.93 | 92.19 | 7.81 | |
| NCI-H322M | 94.18 | 5.82 | 99.10 | 0.90 | |
| NCI-H460 | 102.33 | −2.33 | 104.20 | −4.20 | |
| Colon Cancer | COLO 205 | 103.79 | −3.79 | 104.05 | −4.05 |
| HCC-2998 | 102.05 | −2.05 | 100.24 | −0.24 | |
| HCT-116 | 102.14 | −2.14 | 95.78 | −4.22 | |
| HCT-15 | 98.10 | 1.9 | 101.16 | −1.16 | |
| HT29 | 99.25 | 0.75 | 103.23 | −3.23 | |
| KM12 | 105.43 | −5.43 | 101.16 | −1.16 | |
| SW-620 | 102.52 | −2.52 | 102.95 | −2.95 | |
| CNS Cancer | SF-268 | 91.85 | 8.15 | 87.43 | 12.57 |
| SF-295 | 98.21 | 1.79 | 93.71 | 6.29 | |
| SF-539 | 95.59 | 4.41 | 87.58 | 12.42 | |
| SNB-19 | 99.29 | 0.71 | 97.02 | 2.98 | |
| SNB-75 | 64.51 | 35.49 | 68.12 | 31.88 | |
| U251 | 100.97 | −0.97 | 95.21 | 4.79 | |
| Melanoma | LOX IMVI | 89.06 | 10.94 | 92.99 | 7.01 |
| MALME-3M | 88.53 | 11.47 | 93.29 | 6.71 | |
| M14 | 101.37 | −1.37 | 98.58 | 1.42 | |
| MDA-MB-435 | 95.05 | 4.95 | 100.55 | −0.55 | |
| SK-MEL-2 | 102.31 | −2.31 | 111.54 | −11.54 | |
| SK-MEL-28 | 111.25 | −11.25 | 101.79 | −1.79 | |
| SK-MEL-5 | 98.72 | 1.28 | 98.82 | −1.18 | |
| UACC-257 | 106.92 | −6.92 | 110.78 | −10.78 | |
| UACC-62 | 97.59 | 2.41 | 92.78 | 7.22 | |
| Ovarian Cancer | IGROV1 | 104.14 | −4.14 | 101.63 | −1.63 |
| OVCAR-3 | 98.56 | 1.44 | 98.53 | 1.47 | |
| OVCAR-4 | 106.27 | −6.27 | 99.47 | 0.53 | |
| OVCAR-5 | 98.30 | 1.70 | 92.08 | 7.92 | |
| OVCAR-8 | 101.95 | −1.95 | 97.49 | 2.51 | |
| NCI/ADR-RES | 98.37 | 1.63 | 101.45 | −1.45 | |
| SK-OV-3 | 88.34 | 11.66 | 94.77 | 5.23 | |
| Renal Cancer | 786-0 | 104.06 | −4.06 | 98.99 | 1.01 |
| A498 | 113.46 | −13.46 | 113.94 | −13.94 | |
| ACHN | 91.70 | 8.3 | 89.48 | 10.52 | |
| CAKI-1 | 97.19 | 2.81 | 92.35 | 7.65 | |
| SN12C | 97.21 | 2.79 | 95.50 | 4.50 | |
| TK-10 | 110.14 | −10.14 | 114.82 | −14.82 | |
| UO-31 | 78.82 | 21.18 | 70.05 | 29.95 | |
| Prostate Cancer | PC-3 | 91.14 | 8.86 | 88.11 | 11.89 |
| DU-145 | 110.09 | −10.09 | 111.62 | −11.62 | |
| Breast Cancer | MCF7 | 99.19 | 0.81 | 92.32 | 7.68 |
| MDA-MB-231/ATCC | 88.41 | 11.59 | 80.11 | 19.89 | |
| HS 578T | 101.81 | −1.81 | 104.19 | −4.19 | |
| T-47D | 80.01 | 19.99 | 83.24 | 16.24 | |
| MDA-MB-468 | 98.03 | 1.97 | 100.34 | −0.34 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, O.; Altamimi, A.S.A.; Shahroz, M.M.; Sharma, H.K.; Riadi, Y.; Hassan, M.Q. Analgesic and Anticancer Activity of Benzoxazole Clubbed 2-Pyrrolidinones as Novel Inhibitors of Monoacylglycerol Lipase. Molecules 2021, 26, 2389. https://doi.org/10.3390/molecules26082389
Afzal O, Altamimi ASA, Shahroz MM, Sharma HK, Riadi Y, Hassan MQ. Analgesic and Anticancer Activity of Benzoxazole Clubbed 2-Pyrrolidinones as Novel Inhibitors of Monoacylglycerol Lipase. Molecules. 2021; 26(8):2389. https://doi.org/10.3390/molecules26082389
Chicago/Turabian StyleAfzal, Obaid, Abdulmalik Saleh Alfawaz Altamimi, Mir Mohammad Shahroz, Hemant Kumar Sharma, Yassine Riadi, and Md Quamrul Hassan. 2021. "Analgesic and Anticancer Activity of Benzoxazole Clubbed 2-Pyrrolidinones as Novel Inhibitors of Monoacylglycerol Lipase" Molecules 26, no. 8: 2389. https://doi.org/10.3390/molecules26082389
APA StyleAfzal, O., Altamimi, A. S. A., Shahroz, M. M., Sharma, H. K., Riadi, Y., & Hassan, M. Q. (2021). Analgesic and Anticancer Activity of Benzoxazole Clubbed 2-Pyrrolidinones as Novel Inhibitors of Monoacylglycerol Lipase. Molecules, 26(8), 2389. https://doi.org/10.3390/molecules26082389








