How 10 at% Al Addition in the Ti-V-Zr-Nb High-Entropy Alloy Changes Hydrogen Sorption Properties
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef]
- Gao, M.C.; Miracle, D.B.; Maurice, D.; Yan, X.; Zhang, Y.; Hawk, J.A. High-Entropy Functional Materials. J. Mater. Res. 2018, 33, 3138–3155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A Novel Low-Density, High-Hardness, High-Entropy Alloy with Close-Packed Single-Phase Nanocrystalline Structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A Fracture-Resistant High-Entropy Alloy for Cryogenic Applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.P.; Chen, Y.Y.; Hsu, C.Y.; Yeh, J.W.; Shih, H.C. The Effect of Boron on the Corrosion Resistance of the High Entropy Alloys Al0.5CoCrCuFeNiB x. J. Electrochem. Soc. 2007, 154, C424. [Google Scholar] [CrossRef]
- Koželj, P.; Vrtnik, S.; Jelen, A.; Jazbec, S.; Jagličić, Z.; Maiti, S.; Feuerbacher, M.; Steurer, W.; Dolinšek, J. Discovery of a Superconducting High-Entropy Alloys. Phys. Rev. Lett. 2014, 113, 107001. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior Hydrogen Storage in High Entropy Alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, D.; Ek, G.; Cedervall, J.; Zlotea, C.; Møller, K.T.; Hansen, T.C.; Bednarčík, J.; Paskevicius, M.; Sørby, M.H.; Jensen, T.R.; et al. Structure and Hydrogenation Properties of a HfNbTiVZr High-Entropy Alloys. Inorg. Chem. 2018, 57, 2103–2110. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa, S.J.A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-Induced Phase Transition of MgZrTiFe0.5Co0.5Ni0.5 High Entropy Alloys. Int. J. Hydrog. Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, J.; Hu, J.; Zhang, J.; Mao, Y.; Xiao, H.; Zhou, X.; Zu, X. A Novel TiZrHfMoNb High-Entropy Alloy for Solar Thermal Energy Storage. Nanomaterials 2019, 9, 248. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.-P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen Sorption in TiZrNbHfTa High Entropy Alloys. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Mohammadi, A.; Li, Y.; Zepon, G.; Li, H.-W.; Edalati, K. Reversible Room Temperature Hydrogen Storage in High-Entropy Alloy TiZrCrMnFeNi. Scr. Mater. 2020, 178, 387–390. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Sarac, B.; Berdonosova, E.; Karazehir, T.; Lassnig, A.; Gammer, C.; Zadorozhnyy, M.; Ketov, S.; Klyamkin, S.; Eckert, J. Evaluation of Hydrogen Storage Performance of ZrTiVNiCrFe in Electrochemical and Gas-Solid Reactions. Int. J. Hydrog. Energy 2020, 45, 5347–5355. [Google Scholar] [CrossRef]
- Cardoso, K.R.; Roche, V.; Jorge, A.M., Jr.; Antiqueira, F.J.; Zepon, G.; Champion, Y. Hydrogen Storage in MgAlTiFeNi High Entropy Alloys. J. Alloys Compd. 2021, 858, 158357. [Google Scholar] [CrossRef]
- Strozi, R.B.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Synthesis and Hydrogen Storage Behavior of Mg–V–Al–Cr–Ni High Entropy Alloys. Int. J. Hydrog. Energy 2021, 46, 2351–2361. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sørby, M.H.; Hauback, B.C. Hydrogen Storage in High-Entropy Alloys with Varying Degree of Local Lattice Strain. Int. J. Hydrog. Energy 2019, 44, 29140–29149. [Google Scholar] [CrossRef] [Green Version]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sørby, M.H.; Sahlberg, M.; Hauback, B.C. Counting Electrons—A New Approach to Tailor the Hydrogen Sorption Properties of High-Entropy Alloys. Acta Mater. 2019, 175, 121–129. [Google Scholar] [CrossRef]
- Shen, H.; Hu, J.; Li, P.; Huang, G.; Zhang, J.; Zhang, J.; Mao, Y.; Xiao, H.; Zhou, X.; Zu, X.; et al. Compositional Dependence of Hydrogenation Performance of Ti-Zr-Hf-Mo-Nb High-Entropy Alloys for Hydrogen/Tritium Storage. J. Mater. Sci. Technol. 2020, 55, 116–125. [Google Scholar] [CrossRef]
- Silva, B.H.; Zlotea, C.; Champion, Y.; Botta, W.J.; Zepon, G. Design of TiVNb-(Cr, Ni or Co) Multicomponent Alloys with the Same Valence Electron Concentration for Hydrogen Storage. J. Alloys Compd. 2021, 865, 158767. [Google Scholar] [CrossRef]
- Ek, G.; Nygård, M.M.; Pavan, A.F.; Montero, J.; Henry, P.F.; Sørby, M.H.; Witman, M.; Stavila, V.; Zlotea, C.; Hauback, B.C.; et al. Elucidating the Effects of the Composition on Hydrogen Sorption in TiVZrNbHf-Based High-Entropy Alloys. Inorg. Chem. 2021, 60, 1124–1132. [Google Scholar] [CrossRef]
- Montero, J.; Zlotea, C.; Ek, G.; Crivello, J.-C.; Laversenne, L.; Sahlberg, M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules 2019, 24, 2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aylward, G.; Findlay, T. SI Chemical Data, 3rd ed.; Wiley: Hoboken, NJ, USA, 1995; Volume 72, p. A109. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy: Challenges and Prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Burzo, E. (Ed.) Hydrogen Storage Materials; Advanced Materials and Technologies; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- King, D.J.M.; Cheung, S.T.Y.; Humphry-Baker, S.A.; Parkin, C.; Couet, A.; Cortie, M.B.; Lumpkin, G.R.; Middleburgh, S.C.; Knowles, A.J. High Temperature, Low Neutron Cross-Section High-Entropy Alloys in the Nb-Ti-V-Zr System. Acta Mater. 2019, 166, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Zlotea, C.; Oumellal, Y.; Msakni, M.; Bourgon, J.; Bastide, S.; Cachet-Vivier, C.; Latroche, M. First Evidence of Rh Nano-Hydride Formation at Low Pressure. Nano Lett. 2015, 15, 4752–4757. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Oikawa, K.; Kamiyama, T.; Akiba, E. Crystal Structure of Two Hydrides Formed from a Ti–V–Mn BCC Solid Solution Alloy Studied by Time-of-Flight Neutron Powder Diffraction—A NaCl Structure and a CaF2 Structure. J. Alloys Compd. 2001, 316, 284–289. [Google Scholar] [CrossRef]
- Sakaki, K.; Kim, H.; Asano, K.; Nakamura, Y. Hydrogen Storage Properties of Nb-Based Solid Solution Alloys with a BCC Structure. J. Alloys Compd. 2020, 820, 153399. [Google Scholar] [CrossRef]
- Montero, J.; Ek, G.; Laversenne, L.; Nassif, V.; Zepon, G.; Sahlberg, M.; Zlotea, C. Hydrogen Storage Properties of the Refractory Ti–V–Zr–Nb–Ta Multi-Principal Element Alloys. J. Alloys Compd. 2020, 835, 155376. [Google Scholar] [CrossRef]
- Montero, J.; Ek, G.; Sahlberg, M.; Zlotea, C. Improving the Hydrogen Cycling Properties by Mg Addition in Ti-V-Zr-Nb Refractory High Entropy Alloys. Scr. Mater. 2021, 194, 113699. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis. In Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr; FULLPROF: Toulouse, France, 1990; Volume 127. [Google Scholar]
- Zlotea, C.; Chevalier-César, C.; Léonel, E.; Leroy, E.; Cuevas, F.; Dibandjo, P.; Vix-Guterl, C.; Martens, T.; Latroche, M. Synthesis of Small Metallic Mg-Based Nanoparticles Confined in Porous Carbon Materials for Hydrogen Sorption. Faraday Discuss. 2011, 151, 117–131. [Google Scholar] [CrossRef]
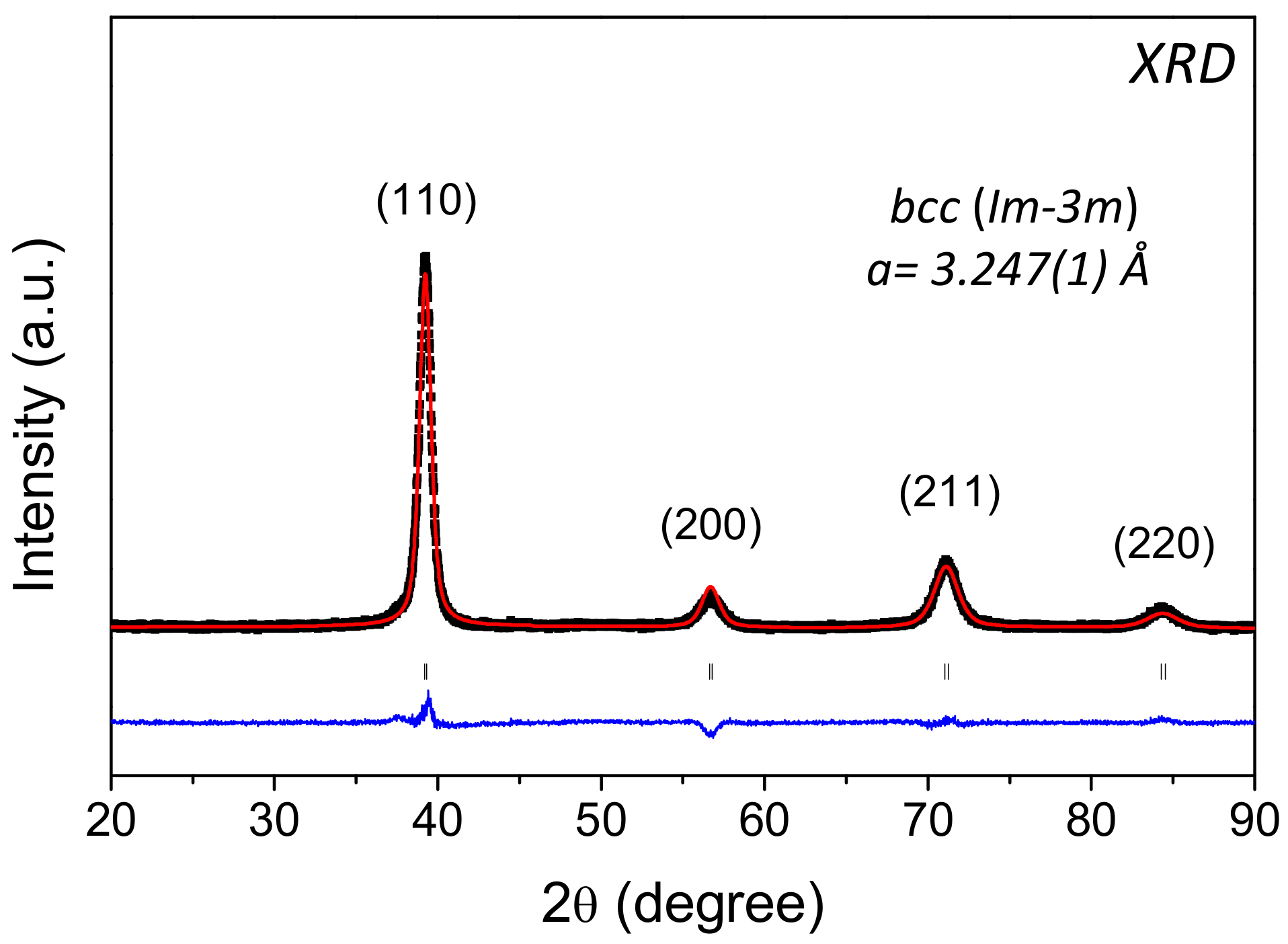



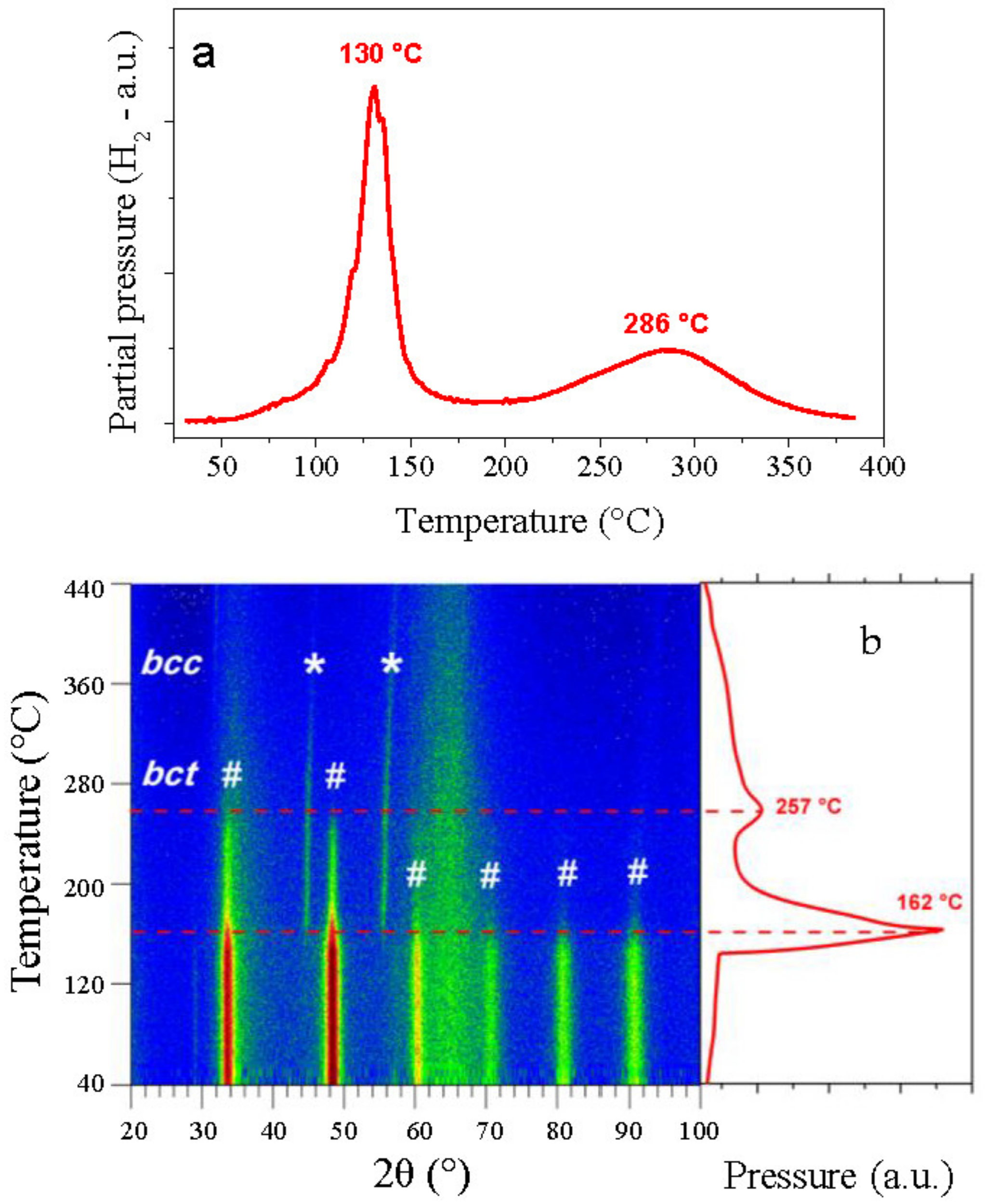

| Composition | Phase | Hydrogenation Cycle | Structure | Space Group | Lattice Parameters (Å) |
|---|---|---|---|---|---|
| Al0.10Ti0.30V0.25Zr0.10Nb0.25 | as-cast | - | bcc | Im-3m | abcc = 3.247(1) |
| Al0.10Ti0.30V0.25Zr0.10Nb0.25H1.6 | hydride | 1st | bct | I4/mmm | abct = 3.137(1) cbct = 4.374(1) |
| Al0.10Ti0.30V0.25Zr0.10Nb0.25 | desorbed | 20th | bcc | Im-3m | abcc = 3.244(1) |
| Al0.10Ti0.30V0.25Zr0.10Nb0.25H1.5 | hydride | 20th | bct | I4/mmm | abct = 3.197(1) cbct = 4.368(1) |
| Element | Nominal (at.%) | Measured (at.%) | ||
|---|---|---|---|---|
| Average | Al, Zr-Rich Region | Al, Zr-Poor Region | ||
| Al (K) | 10 | 10.0 (0.6) | 12.1 (0.6) | 9.2 (0.4) |
| Ti (K) | 30 | 29.5 (0.9) | 27.8 (0.9) | 29.8 (0.6) |
| V (K) | 25 | 24.6 (1.2) | 22.6 (1.2) | 24.7 (0.7) |
| Zr (L) | 10 | 10.8 (2.5) | 17.5 (2.5) | 8.3 (0.6) |
| Nb (L) | 25 | 25.1 (1.6) | 19.1 (1.6) | 28.0 (0.5) |
| Alloy | Reference | Initial Storage Capacity | Reversible Storage Capacity | ||
|---|---|---|---|---|---|
| H/M | wt.% | H/M | wt.% | ||
| Similar Ti-V-Zr-Nb HEAs | |||||
| Ti0.325V0.275Zr0.125Nb0.275 | [22] | 1.8 | 2.7 | 1.5 | 2.25 |
| Al0.10Ti0.30V0.25Zr0.10Nb0.25 | present work | 1.6 | 2.6 | 1.5 | 2.45 |
| Mg0.10Ti0.30V0.25Zr0.10Nb0.25 | [31] | 1.7 | 2.7 | 1.5 | 2.4 |
| Ta0.10Ti0.30V0.25Zr0.10Nb0.25 | [30] | 2.0 | 2.5 | 1.7 | 2.2 |
| Other equimolar HEAs | |||||
| TiVZrNbHf | [8] | 2.5 | 2.7 | - | - |
| TiZrNbHfTa | [12] | 2.0 | 1.7 | - | - |
| TiVZrNb | [17] | 2.0 | 2.7 | - | - |
| TiVNb | [18] | 2.0 | 3.0 | 0 | 0 |
| TiVNbTa | [18] | 1.9 | 2.0 | 0 | 0 |
| TiVNbCr | [18] | 2.0 | 3.1 | 1.2 | 2 |
| Intermetallics | |||||
| TiFe | [25] | 1.0 | 1.9 | 1.0 | 1.9 |
| YFe3 | [25] | 1.2 | 1.9 | 1.2 | 1.9 |
| LaNi5 | [25] | 1.1 | 1.5 | 1.1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, J.; Ek, G.; Laversenne, L.; Nassif, V.; Sahlberg, M.; Zlotea, C. How 10 at% Al Addition in the Ti-V-Zr-Nb High-Entropy Alloy Changes Hydrogen Sorption Properties. Molecules 2021, 26, 2470. https://doi.org/10.3390/molecules26092470
Montero J, Ek G, Laversenne L, Nassif V, Sahlberg M, Zlotea C. How 10 at% Al Addition in the Ti-V-Zr-Nb High-Entropy Alloy Changes Hydrogen Sorption Properties. Molecules. 2021; 26(9):2470. https://doi.org/10.3390/molecules26092470
Chicago/Turabian StyleMontero, Jorge, Gustav Ek, Laetitia Laversenne, Vivian Nassif, Martin Sahlberg, and Claudia Zlotea. 2021. "How 10 at% Al Addition in the Ti-V-Zr-Nb High-Entropy Alloy Changes Hydrogen Sorption Properties" Molecules 26, no. 9: 2470. https://doi.org/10.3390/molecules26092470
APA StyleMontero, J., Ek, G., Laversenne, L., Nassif, V., Sahlberg, M., & Zlotea, C. (2021). How 10 at% Al Addition in the Ti-V-Zr-Nb High-Entropy Alloy Changes Hydrogen Sorption Properties. Molecules, 26(9), 2470. https://doi.org/10.3390/molecules26092470






