Terminal and Internal Alkyne Complexes and Azide-Alkyne Cycloaddition Chemistry of Copper(I) Supported by a Fluorinated Bis(pyrazolyl)borate
Abstract
:1. Introduction
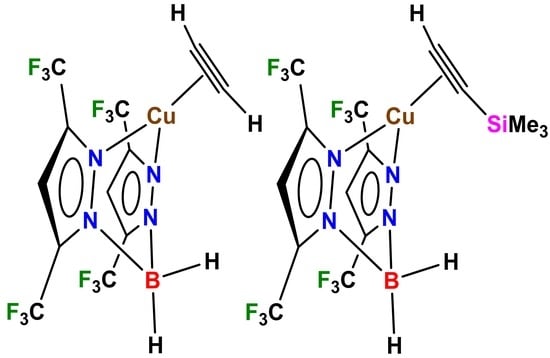
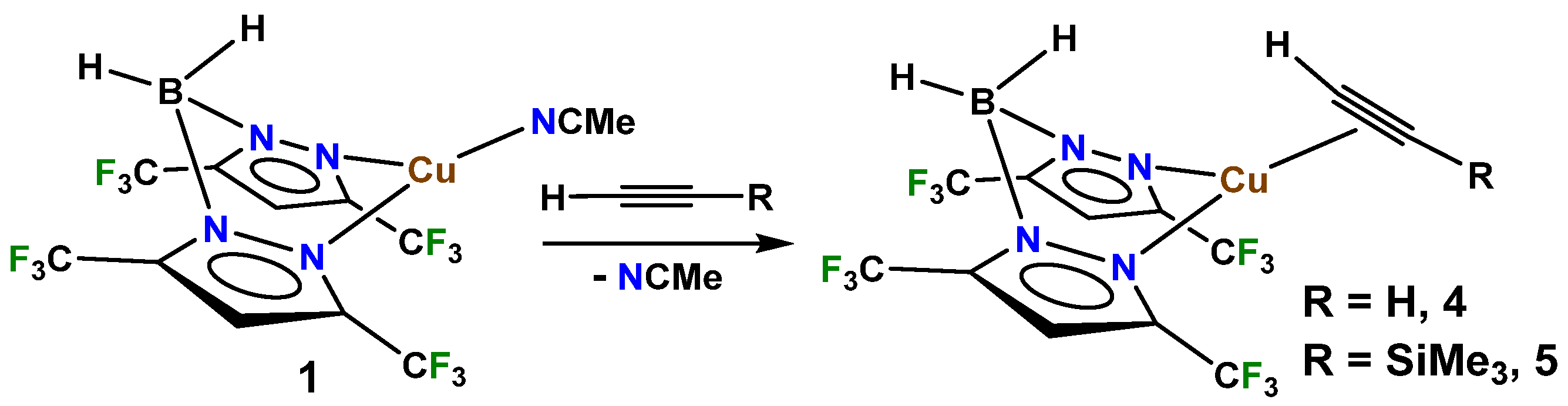
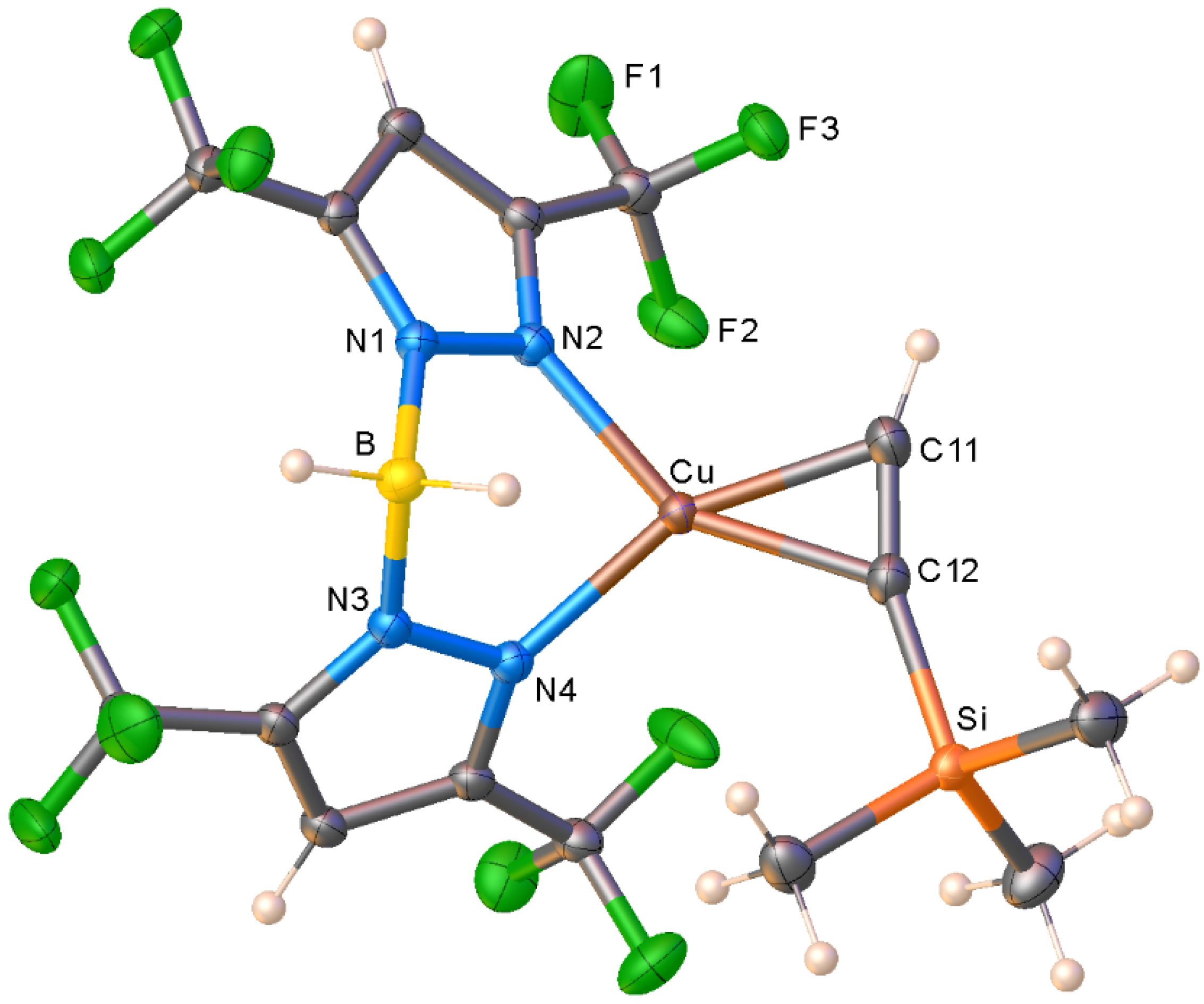
2. Results and Discussion
3. Experimental Details
- General method I for the synthesis of triazoles:
- 2.
- General method II for the synthesis of triazoles:
- 3.
- General method III for the synthesis of triazoles
4. X-ray Data Collection and Structure Determinations
5. Theoretical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. Cu(I)-Catalyzed Huisgen Azide-Alkyne 1,3-Dipolar Cycloaddition Reaction in Nucleoside, Nucleotide, and Oligonucleotide Chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, J.S.S.; Zeni, G. A decade of advances in the reaction of nitrogen sources and alkynes for the synthesis of triazoles. Coord. Chem. Rev. 2020, 409, 213217. [Google Scholar] [CrossRef]
- Leophairatana, P.; Samanta, S.; De Silva, C.C.; Koberstein, J.T. Preventing Alkyne–Alkyne (i.e., Glaser) Coupling Associated with the ATRP Synthesis of Alkyne-Functional Polymers/Macromonomers and for Alkynes under Click (i.e., CuAAC) Reaction Conditions. J. Am. Chem. Soc. 2017, 139, 3756–3766. [Google Scholar] [CrossRef] [PubMed]
- Noonikara-Poyil, A.; Ridlen, S.G.; Dias, H.V.R. Isolable Copper(I) η2-Cyclopropene Complexes. Inorg. Chem. 2020, 59, 17860–17865. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Leslie, D.; Coleman, M.G.; Mack, J. Recyclable heterogeneous metal foil-catalyzed cyclopropenation of alkynes and diazoacetates under solvent-free mechanochemical reaction conditions. Chem. Sci. 2018, 9, 4650–4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Requejo, M.M.; Mairena, M.A.; Belderrain, T.R.; Nicasio, M.C.; Trofimenko, S.; Perez, P.J. A family of highly active copper(I)-homoscorpionate catalysts for the alkyne cyclopropenation reaction. Chem. Commun. 2001, 1804–1805. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lin, Y.; Huang, L.; Sun, Z.; Yang, Y.; Zhou, X.; Vovk, E.; Liu, X.; Huang, X.; Sun, M.; et al. Copper Catalysts in Semihydrogenation of Acetylene: From Single Atoms to Nanoparticles. ACS Catal. 2020, 10, 3495–3504. [Google Scholar] [CrossRef]
- Redfern, L.R.; Lo, W.-S.; Dillingham, I.J.; Eatman, J.G.; Mian, M.R.; Tsung, C.-K.; Farha, O.K. Enhancing Four-Carbon Olefin Production from Acetylene over Copper Nanoparticles in Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 31496–31502. [Google Scholar] [CrossRef]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-metal-catalyzed addition of heteroatom-hydrogen bonds to alkynes. Chem. Rev. 2004, 104, 3079–3159. [Google Scholar] [CrossRef]
- Whyte, A.; Torelli, A.; Mirabi, B.; Zhang, A.; Lautens, M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622. [Google Scholar] [CrossRef]
- Fujihara, T.; Xu, T.; Semba, K.; Terao, J.; Tsuji, Y. Copper-Catalyzed Hydrocarboxylation of Alkynes Using Carbon Dioxide and Hydrosilanes. Angew. Chem. Int. Ed. 2011, 50, 523–527. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; He, Z.; Guan, Q.; Li, W. Demystifying the mechanism of NMP ligands in promoting Cu-catalyzed acetylene hydrochlorination: Insights from a density functional theory study. Inorg. Chem. Front. 2020, 7, 3204–3216. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. Metal-catalyzed and metal-free alkyne hydrothiolation: Synthetic aspects and application trends. Eur. J. Org. Chem. 2014, 2014, 3955–3969. [Google Scholar] [CrossRef]
- Grirrane, A.; Alvarez, E.; Garcia, H.; Corma, A. Synthesis, Structure, Reactivity and Catalytic Implications of a Cationic, Acetylide-Bridged Trigold-JohnPhos Species. Chem.-Eur. J. 2020, 26, 8810–8818. [Google Scholar] [CrossRef] [PubMed]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic coupling: A powerful tool in molecular construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Chernyak, N.; Gevorgyan, V. General and Efficient Copper-Catalyzed Three-Component Coupling Reaction towards Imidazoheterocycles. One-Pot Synthesis of Alpidem and Zolpidem. Angew. Chem. Int. Ed. 2010, 49, 2743–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.; Dong, J.; Liu, L.; Sun, M.; Qiu, R.; Zhou, Y.; Yin, S.-F. Copper Catalysis for Selective Heterocoupling of Terminal Alkynes. J. Am. Chem. Soc. 2016, 138, 12348–12351. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Zhang, Y.-F.; Ma, C.-L.; Gu, Q.-S.; Wang, F.-L.; Li, Z.-L.; Jiang, S.-P.; Liu, X.-Y. A general asymmetric copper-catalysed Sonogashira C(sp3)-C(sp) coupling. Nat. Chem. 2019, 11, 1158–1166. [Google Scholar] [CrossRef]
- Hamada, T.; Ye, X.; Stahl, S.S. Copper-Catalyzed Aerobic Oxidative Amidation of Terminal Alkynes: Efficient Synthesis of Ynamides. J. Am. Chem. Soc. 2008, 130, 833–835. [Google Scholar] [CrossRef]
- Hay, A.S. Oxidative couplings of acetylenes. II. J. Org. Chem. 1962, 27, 3320–3321. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y. Copper- and copper–N-heterocyclic carbene-catalyzed C─H activating carboxylation of terminal alkynes with CO2 at ambient conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 20184–20189. [Google Scholar] [CrossRef] [Green Version]
- Manjolinho, F.; Arndt, M.; Goossen, K.; Goossen, L.J. Catalytic C-H Carboxylation of Terminal Alkynes with Carbon Dioxide. ACS Catal. 2012, 2, 2014–2021. [Google Scholar] [CrossRef]
- Tachiyama, T.; Yoshida, M.; Aoyagi, T.; Fukuzumi, S. Mechanistic study on dimerization of acetylene with a Nieuwland catalyst. Appl. Organomet. Chem. 2008, 22, 205–210. [Google Scholar] [CrossRef]
- Bakhoda, A.; Okoromoba, O.E.; Greene, C.; Boroujeni, M.R.; Bertke, J.A.; Warren, T.H. Three-Coordinate Copper(II) Alkynyl Complex in C–C Bond Formation: The Sesquicentennial of the Glaser Coupling. J. Am. Chem. Soc. 2020, 142, 18483–18490. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, C.; Pinaud, N.; Astruc, D. Recyclable Catalytic Dendrimer Nanoreactor for Part-Per-Million CuI Catalysis of “Click” Chemistry in Water. J. Am. Chem. Soc. 2014, 136, 12092–12098. [Google Scholar] [CrossRef]
- Fang, Y.; Bao, K.; Zhang, P.; Sheng, H.; Yun, Y.; Hu, S.-X.; Astruc, D.; Zhu, M. Insight into the Mechanism of the CuAAC Reaction by Capturing the Crucial Au4Cu4–π-Alkyne Intermediate. J. Am. Chem. Soc. 2021, 143, 1768–1772. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Bonson, S.E.; Zimmerman, S.C. A Bioorthogonal Small Molecule Selective Polymeric “Clickase”. J. Am. Chem. Soc. 2020, 142, 13966–13973. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, A.K.; Bose, P.; Jaiswal, M.K.; Rajkhowa, S.; Singh, A.S.; Hotha, S.; Mishra, N.; Tiwari, V.K. Cu(I)-Catalyzed Click Chemistry in Glycoscience and Their Diverse Applications. Chem. Rev. 2021, 121, 7638–7956. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, X.; Chai, Y.; Wu, G.; Li, W.; Li, J.; da Silva, I.; Manuel, P.; Cheng, Y.; Daemen, L.L.; et al. Efficient Separation of Acetylene and Carbon Dioxide in a Decorated Zeolite. Angew. Chem. Int. Ed. 2021, 133, 6600–6606. [Google Scholar] [CrossRef]
- Chen, S.; Behera, N.; Yang, C.; Dong, Q.; Zheng, B.; Li, Y.; Tang, Q.; Wang, Z.; Wang, Y.; Duan, J. A chemically stable nanoporous coordination polymer with fixed and free Cu2+ ions for boosted C2H2/CO2 separation. Nano Res. 2020, 14, 546–553. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Xiang, S.; Wu, H.; Fronczek, F.R.; Zhou, W.; Krishna, R.; O’Keeffe, M.; Chen, B. High Separation Capacity and Selectivity of C2 Hydrocarbons over Methane within a Microporous Metal-Organic Framework at Room Temperature. Chem.-Eur. J. 2012, 18, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiang, S.; Zhang, W.; Zhang, Z.; Wang, L.; Bai, J.; Chen, B. A new MOF-505 analog exhibiting high acetylene storage. Chem. Commun. 2009, 48, 7551–7553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Hu, M.-C.; Li, S.-N.; Jiang, Y.-C.; Qu, P.; Zhai, Q.-G. Assembly of [Cu2(COO)4] and [M3(μ3-O)(COO)6] (M = Sc, Fe, Ga, and In) building blocks into porous frameworks towards ultra-high C2H2/CO2 and C2H2/CH4 separation performance. Chem. Commun. 2018, 54, 2012–2015. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Jakob, A.; Milde, B. Copper(I) Alkyne and Alkynide Complexes. Organometallics 2012, 31, 7661–7693. [Google Scholar] [CrossRef]
- Perez, P.J.; Dias-Requejo, M.M. Copper Organometallics, in Comprehensive Organometallic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2007; Volume 2, pp. 153–195. [Google Scholar]
- Koehler, K.; Eichhorn, J.; Meyer, F.; Vidovic, D. Dicopper(I) Oxalate Complexes Stabilized by Lewis Bases: Potential Precursors for Copper Deposition. Organometallics 2003, 22, 4426–4432. [Google Scholar] [CrossRef]
- Doppelt, P.; Baum, T.H. Alkyne complexes of copper(I) (1,1,1,5,5,5-hexafluoro-2,4-pentanedionato): Syntheses and characterization of (η2-bis(trimethylsilyl)acetylene) copper(I) (hfac), (μ-η2-bis(trimethylsilyl)acetylene) bis(copper(I) (hfac)) and a series of (η2-alkyne) Cu(hfac) complexes. J. Organomet. Chem. 1996, 517, 53–62. [Google Scholar]
- Dias, H.V.R.; Richey, S.A.; Diyabalanage, H.V.K.; Thankamani, J. Copper(I) complexes supported by a heavily fluorinated bis(pyrazolyl)borate: Syntheses and characterization of [H2B(3,5-(CF3)2Pz)2]CuL (where L = PPh3, NCCH3, HCCPh, H2CCHPh) and {[H2B(3,5-(CF3)2Pz)2]Cu}2(1,5-COD). J. Organomet. Chem. 2005, 690, 1913–1922. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Flores, J.A.; Wu, J.; Kroll, P. Monomeric Copper(I), Silver(I), and Gold(I) Alkyne Complexes and the Coinage Metal Family Group Trends. J. Am. Chem. Soc. 2009, 131, 11249–11255. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Dash, C.; Celik, M.A.; Yousufuddin, M.; Frenking, G.; Dias, H.V.R. Tris(alkyne) and Bis(alkyne) Complexes of Coinage Metals: Synthesis and Characterization of (cyclooctyne)3M+ (M = Cu, Ag) and (cyclooctyne)2Au+ and Coinage Metal (M = Cu, Ag, Au) Family Group Trends. Organometallics 2013, 32, 3135–3144. [Google Scholar] [CrossRef]
- Das, A.; Dash, C.; Yousufuddin, M.; Dias, H.V.R. Coordination and Ligand Substitution Chemistry of Bis(cyclooctyne)copper(I). Organometallics 2014, 33, 1644–1650. [Google Scholar] [CrossRef]
- Parasar, D.; Almotawa, R.M.; Jayaratna, N.B.; Ceylan, Y.S.; Cundari, T.R.; Omary, M.A.; Dias, H.V.R. Synthesis, Photophysical Properties, and Computational Analysis of Di- and Tetranuclear Alkyne Complexes of Copper(I) Supported by a Highly Fluorinated Pyrazolate. Organometallics 2018, 37, 4105–4118. [Google Scholar] [CrossRef]
- Parasar, D.; Ponduru, T.T.; Noonikara-Poyil, A.; Jayaratna, N.B.; Dias, H.V.R. Acetylene and terminal alkyne complexes of copper(I) supported by fluorinated pyrazolates: Syntheses, structures, and transformations. Dalton Trans. 2019, 48, 15782–15794. [Google Scholar] [CrossRef]
- Lakhi, J.S.; Patterson, M.R.; Dias, H.V.R. Coinage metal metallacycles involving a fluorinated 3,5-diarylpyrazolate. New J. Chem. 2020, 44, 14814–14822. [Google Scholar] [CrossRef]
- Patterson, M.; Dias, H.V.R. Tetranuclear and trinuclear copper(I) pyrazolates as catalysts in copper mediated azide-alkyne cycloadditions (CuAAC). Dalton Trans. 2022, 51, 375–383. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Polach, S.A.; Wang, Z. Coinage metal complexes of 3,5-bis(trifluoromethyl)pyrazolate ligand. Synthesis and characterization of {[3,5-(CF3)2Pz]Cu}3 and {[3,5-(CF3)2Pz]Ag}3. J. Fluorine Chem. 2000, 103, 163–169. [Google Scholar] [CrossRef]
- Lang, H.; Koehler, K.; Blau, S. η2-Alkyne copper(I) and silver(I) compounds; from polymeric [MIR]n to monomeric [MIR] units (M = Cu, Ag). Coord. Chem. Rev. 1995, 143, 113–168. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Bauer, H.; Faust, J.; Rolf, F.; Johannes, F.; Krüerke, U.; Kunz, M.; Somer, H.M. (Eds.) Cu Organocopper Compounds: Compounds with Ligands Bonded by Two C. Atoms; Springer: Berlin/Heidelberg, Germany, 1987; Volume 4, pp. 1–101. [Google Scholar]
- Jardine, F.H. Copper(I) Complexes. In Advances in Inorganic Chemistry and Radiochemistry; Emeléus, H.J., Sharpe, A.G., Eds.; Academic Press: Cambridge, MA, USA, 1975; Volume 17, pp. 115–163. [Google Scholar]
- Thompson, J.S.; Whitney, J.F. Copper(I) complexes with unsaturated small molecules. Preparation and structural characterization of copper(I)-di-2-pyridylamine complexes with olefins, acetylene, and carbon monoxide. Inorg. Chem. 1984, 23, 2813–2819. [Google Scholar] [CrossRef]
- Munakata, M.; Kitagawa, S.; Kawada, I.; Maekawa, M.; Shimono, H. Synthesis, formation constants and structures of ternary copper(I) complexes with 1,10-phenanthroline and alkynes. J. Chem. Soc. Dalton Trans. 1992, 2225–2230. [Google Scholar] [CrossRef]
- Thompson, J.S.; Whitney, J.F. Preparation and structural characterization of acetylene(2,2’-dipyridylamine)copper(I) tetrafluoroborate. J. Am. Chem. Soc. 1983, 105, 5488–5490. [Google Scholar] [CrossRef]
- Mykhalichko, B.M.; Mys’kiv, M.G.; Davydov, V.N. Acetylene as a bridging π-ligand. Synthesis and crystal structure of NH4[Cu8Cl9(C2H2)4]·2/5{[Cu(H2O)2]+[CuCl2]−·H2O}. Zh. Neorg. Khim. 1999, 44, 411–414. [Google Scholar]
- Mykhalichko, B.M.; Mys’kiv, M.G.; Aksel’rud, L.G. Acetylene as a bridging Π-ligand: Synthesis and crystal structure of NH4Cu3Cl4.C2H2. Koord. Khim. 1993, 19, 722–726. [Google Scholar]
- Osechkin, S.I.; Mys’kiv, M.G.; Zavalii, P.Y.; Sobolev, A.N. X-ray investigation of copper(I) chloride π-complexes with bidentate acetylene in the CuCl-HCl-C2H2-KCl-H2O system. Metalloorg. Khim. 1991, 4, 997–1003. [Google Scholar]
- Hyman, M.R.; Arp, D.J. Quantification and removal of some contaminating gases from acetylene used to study gas-utilizing enzymes and microorganisms. Appl. Environ. Microbiol. 1987, 53, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Trotuş, I.-T.; Zimmermann, T.; Schüth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef]
- ACS. Spectroscopy Resources, ACS Division of Organic Chemistry. Available online: https://organicchemistrydata.org/links/#spectroscopy_resources (accessed on 7 December 2021).
- Fast, H.; Welsh, H.L. High-resolution Raman spectra of acetylene, acetylene-d1, and acetylene-d2. J. Mol. Spectrosc. 1972, 41, 203–221. [Google Scholar] [CrossRef]
- Chatt, J.; Duncanson, L.A. Olefin coördination compounds. III. Infrared spectra and structure: Attempted preparation of acetylene compounds. J. Chem. Soc. 1953, 2939–2947. [Google Scholar] [CrossRef]
- Dewar, M.J.S. A review of the π-complex theory. Bull. Soc. Chim. Fr. 1951, 18, C71–C79. [Google Scholar]
- Dias, H.V.R.; Wang, Z.; Jin, W. Synthesis and Chemistry of [Hydrotris(3,5-bis(trifluoromethyl)pyrazolyl)borato]silver(I) Complexes. Inorg. Chem. 1997, 36, 6205–6215. [Google Scholar] [CrossRef]
- Wieder, N.L.; Carroll, P.J.; Berry, D.H. Structure and Reactivity of Acetylene Complexes of Bis(imino)pyridine Ruthenium(0). Organometallics 2011, 30, 2125–2136. [Google Scholar] [CrossRef]
- Bustelo, E.; Carbó, J.J.; Lledós, A.; Mereiter, K.; Puerta, M.C.; Valerga, P. First X-ray Characterization and Theoretical Study of π-Alkyne, Alkynyl-Hydride, and Vinylidene Isomers for the Same Transition Metal Fragment [Cp*Ru(PEt3)2]+. J. Am. Chem. Soc. 2003, 125, 3311–3321. [Google Scholar] [CrossRef] [PubMed]
- Pörschke, K.R.; Tsay, Y.-H.; Krüger, C. Ethynebis(triphenylphosphane)nickel(0). Angew. Chem. Int. Ed. 1985, 24, 323–324. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C74862&Mask=800 (accessed on 7 December 2021).
- McMullan, R.K.; Kvick, Å.; Popelier, P. Structures of cubic and orthorhombic phases of acetylene by single-crystal neutron diffraction. Acta Crystallogr. Sect. B 1992, 48, 726–731. [Google Scholar] [CrossRef]
- Albright, T.A.; Hoffmann, R.; Thibeault, J.C.; Thorn, D.L. Ethylene complexes. Bonding, rotational barriers, and conformational preferences. J. Am. Chem. Soc. 1979, 101, 3801–3812. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamamoto, H.; Arai, T.; Nakamura, A.; Chen, J.; Kai, Y.; Kasai, N. Facile synthesis and stereochemistry of alkyne complexes of Cp2MH and Cp2MCH2CH2R (M = niobium, tantalum). Organometallics 1991, 10, 4058–4066. [Google Scholar] [CrossRef]
- Hill, A.F.; Tshabang, N.; Willis, A.C. Poly(methimazolyl)borate alkyne complexes of molybdenum and tungsten. Eur. J. Inorg. Chem. 2007, 3781–3785. [Google Scholar] [CrossRef]
- Kiplinger, J.L.; Arif, A.M.; Richmond, T.G. Influence of π-Conflict on Structure and Reactivity. Comparative Study of η2-Nitriles and η2-Alkynes as Four-Electron Donor Ligands in Tungsten(II) Fluoride Carbonyl Systems. Organometallics 1997, 16, 246–254. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. A theoretical study of the ethylene-metal bond in complexes between copper(1+), silver(1+), gold(1+), platinum(0) or platinum(2+) and ethylene, based on the Hartree-Fock-Slater transition-state method. Inorg. Chem. 1979, 18, 1558–1565. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.A.; Larionov, V.A.; Smolyakov, A.F.; Godovikova, M.I.; Titova, E.M.; Maleev, V.I.; Shubina, E.S. Interaction of a trinuclear copper(I) pyrazolate with alkynes and carbon-carbon triple bond activation. Chem. Commun. 2019, 55, 290–293. [Google Scholar] [CrossRef]
- Huisgen, R.; Szeimies, G.; Moebius, L. 1,3-Dipolar cycloadditions. XXXII. Kinetics of the addition of organic azides to carbon-carbon multiple bonds. Chem. Ber. 1967, 100, 2494–2507. [Google Scholar] [CrossRef]
- Trofimenko, S. Recent advances in poly(pyrazolyl)borate (scorpionate) chemistry. Chem. Rev. 1993, 93, 943–980. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Lovely, C.J. Carbonyl and Olefin Adducts of Coinage Metals Supported by Poly(pyrazolyl)borate and Poly(pyrazolyl)alkane Ligands and Silver Mediated Atom Transfer Reactions. Chem. Rev. 2008, 108, 3223–3238. [Google Scholar] [CrossRef]
- Wang, T.-H.; Wu, F.-L.; Chiang, G.-R.; He, S.-T.; Lo, Y.-H. Preparation of ruthenium azido complex containing a Tp ligand and ruthenium-catalyzed cycloaddition of organic azides with alkynes in organic and aqueous media: Experimental and computational studies. J. Organomet. Chem. 2014, 774, 57–60. [Google Scholar] [CrossRef]
- Munoz-Molina, J.M.; Belderrain, T.R.; Perez, P.J. Trispyrazolylborate coinage metals complexes: Structural features and catalytic transformations. Coord. Chem. Rev. 2019, 390, 171–189. [Google Scholar] [CrossRef]
- Cano, I.; Nicasio, M.C.; Perez, P.J. Copper(I) complexes as catalysts for the synthesis of N-sulfonyl-1,2,3-triazoles from N-sulfonylazides and alkynes. Org. Biomol. Chem. 2010, 8, 536–538. [Google Scholar] [CrossRef]
- Bahsis, L.; Ben El Ayouchia, H.; Anane, H.; Ramirez de Arellano, C.; Bentama, A.; El Hadrami, E.M.; Julve, M.; Domingo, L.R.; Stiriba, S.-E. Clicking azides and alkynes with poly(pyrazolyl)borate-copper(I) catalysts: An experimental and computational study. Catalysts 2019, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Larionov, V.A.; Stashneva, A.R.; Titov, A.A.; Lisov, A.A.; Medvedev, M.G.; Smol’yakov, A.F.; Tsedilin, A.M.; Shubina, E.S.; Maleev, V.I. Mechanistic study in azide-alkyne cycloaddition (CuAAC) catalyzed by bifunctional trinuclear copper(I) pyrazolate complex: Shift in rate-determining step. J. Catal. 2020, 390, 37–45. [Google Scholar] [CrossRef]
- Noonikara-Poyil, A.; Munoz-Castro, A.; Boretskyi, A.; Mykhailiuk, P.K.; Dias, H.V.R. When SF5 outplays CF3: Effects of pentafluorosulfanyl decorated scorpionates on copper. Chem. Sci. 2021, 12, 14618–14623. [Google Scholar] [CrossRef]
- Noonikara-Poyil, A.; Cui, H.; Yakovenko, A.A.; Stephens, P.W.; Lin, R.-B.; Wang, B.; Chen, B.; Dias, H.V.R. A Molecular Compound for Highly Selective Purification of Ethylene. Angew. Chem. Int. Ed. 2021, 133, 27390–27394. [Google Scholar] [CrossRef]
- Thomas, J.; John, J.; Parekh, N.; Dehaen, W. A Metal-Free Three-Component Reaction for the Regioselective Synthesis of 1,4,5-Trisubstituted 1,2,3-Triazoles. Angew. Chem. 2014, 126, 10319–10323. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
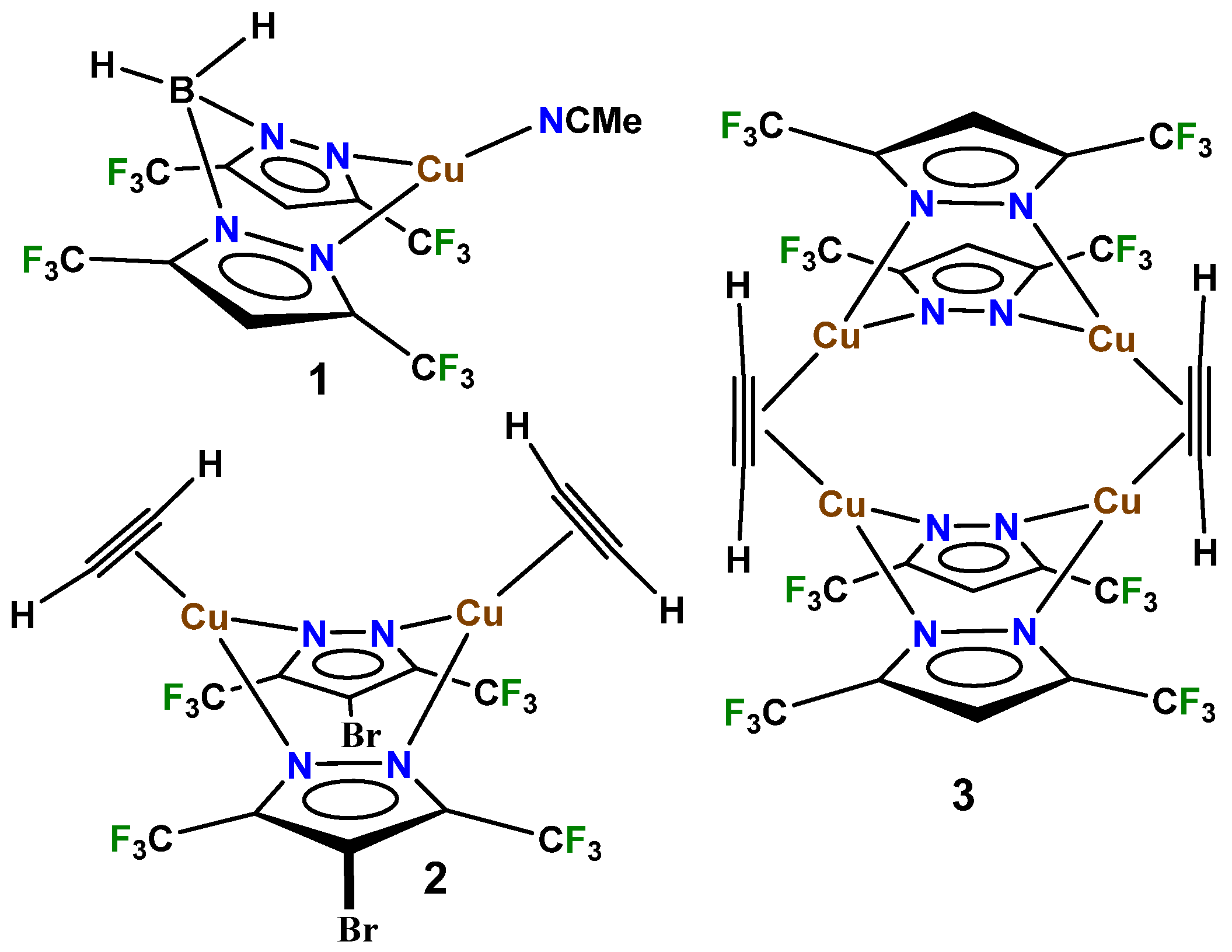
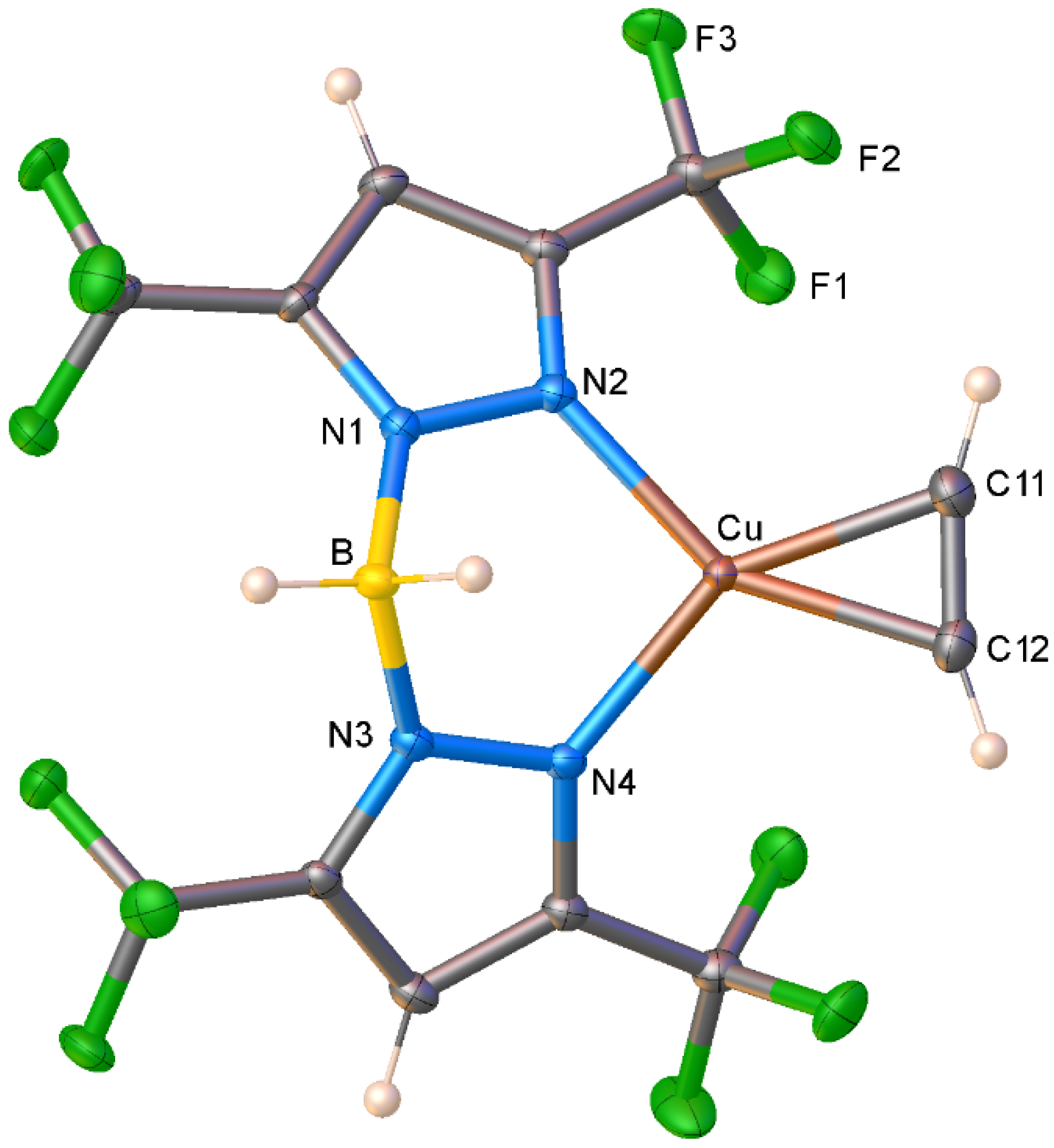

| Complex\ Parameter | 4 | 2 | [Cu{NH(Py)2} (HC≡CH)]BF4 | [Cu(phen) (HC≡CH)]ClO4 |
|---|---|---|---|---|
| Temp. | 100 | 100 | 173 | 283–303 |
| Cu-C | 1.972(3) 1.973(3) | 1.966(3) 1.974(3) | 1.971(3) 1.971(3) | 1.930(5) 1.961(5) |
| C≡C | 1.225(5) | 1.227(4) | 1.188(11) | 1.190(7) |
| Cu-N | 1.981(3) 1.981(3) | 1.9697(18) 1.9742(18) | 1.968(3) 1.968(3) | 1.979(4) 1.978(4) |
| C-Cu-C | 36.17(14) | 36.29(11) | 35.1(3) | 35.6(2) |
| N-Cu-N | 96.63(10) | 98.94(8) | 96.8(2) | 84.9(2) |
| ῡ(C≡C) | 1819 | 1811 | 1795 | 1800 |
| 1H | 4.40 | 4.75 | 5.21 | - |
| 13C | 80.2 | - | - | - |
| ref | This work | [47] | [57] | [56] |
| Mononuclear, Bis(pyrazolyl)Borate Copper Complexes, L = [H2B(3,5-(CF3)2Pz)2]; L’ = [H2B(4-Br-3,5-(CF3)2Pz)2] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LCu(HC≡CH) 4 | LCu(EtC≡CEt) 6 | LCu(PhC≡CH) 8 | LCu(HC≡CSiMe3) 5 | L’Cu(HC≡CH) | ||||||
| ΔEPauli | 121.8 | 131.0 | 124.8 | 123.6 | 119.9 | |||||
| ΔEElstat | −100.4 | 59.5% | −112.6 | 61.7% | −102.3 | 58.8% | −103.7 | 59.0% | −99.1 | 59.5% |
| ΔEorb | −62.2 | 36.9% | −59.9 | 32.8% | −61.8 | 35.5% | −60.3 | 34.3% | −61.5 | 36.9% |
| ΔEDisp | −6.0 | 3.6% | −10.0 | 5.5% | −10.0 | 5.7% | −11.7 | 6.7% | −6.0 | 3.6% |
| ΔEint | −46.8 | −51.5 | −49.4 | −52.1 | −46.7 | |||||
| π→Cu | −35.6 | 57.2% | −31.0 | 51.8% | −33.8 | 54.6% | −31.5 | 52.2% | −34.8 | 56.5% |
| σ←Cu | −16.3 | 26.3% | −17.5 | 29.2% | −16.6 | 26.9% | −16.7 | 27.7% | −16.5 | 26.8% |
| vC≡C, Calc. (Exp.) | 1817 (1819) | 2056 (2064) | 1954 (1927) | 1870 (1870) | 1818 | |||||
| Dinuclear, Copper Pyrazolate Complexes | ||||||||||
| Cu2(μ-[3,5-(CF3)2Pz])2(HC≡CH)2 10 | Cu2(μ-[3,5-(CF3)2Pz])2(EtC≡CEt)2 7 | Cu2(μ-[3,5-(CF3)2Pz])2(PhC≡CH)2 9 | Cu2(μ-[4-Br-3,5-(CF3)2Pz])2(HC≡CH)2 2 | |||||||
| ΔEPauli | 128.0 | 143.2 | 142.0 | 124.6 | ||||||
| ΔEElstat | −102.7 | 59.8% | −118.2 | 61.3% | −109.8 | 57.8% | −99.9 | 59.6% | ||
| ΔEorb | −62.8 | 36.5% | −62.2 | 32.3% | −65.6 | 34.5% | −61.5 | 36.7% | ||
| ΔEDisp | −6.3 | 3.7% | −12.2 | 6.3% | −14.5 | 7.6% | −6.1 | 3.7% | ||
| ΔEint | −43.8 | −49.4 | −47.8 | −42.9 | ||||||
| π→Cu | −35.8 | 57.0% | −32.3 | 52.0% | −35.7 | 54.5% | −34.8 | 56.6% | ||
| σ←Cu | −16.7 | 26.5% | −17.9 | 28.7% | −17.0 | 25.9% | −16.6 | 27.1% | ||
| vC≡C, Calc. (Exp.) | 1814 | 2055 (2033, 2066) | 1950 | 1813 (1811) | ||||||
 | ||||
|---|---|---|---|---|
| Entry | Alkyne | Yield (%) [H2B(3,5-(CF3)2Pz)2]Cu(NCMe) catalyst | Yield (%) {μ-[3,5-(CF3)2Pz]Cu}3 Catalyst Method III | |
| Method I | Method II | |||
| 1 | HC≡CH | - | 80 | 99 |
| 2 | n-PrC≡CH | 99 | 85 | 99 |
| 3 | n-BuC≡CH | 99 | 89 | 99 |
| 4 | n-C8H17C≡CH | 99 | 91 | 99 |
| 5 | PhC≡CH | 99 | 84 | 99 |
| 6 | Me3SiC≡CH | 99 | 56 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noonikara-Poyil, A.; Muñoz-Castro, A.; Dias, H.V.R. Terminal and Internal Alkyne Complexes and Azide-Alkyne Cycloaddition Chemistry of Copper(I) Supported by a Fluorinated Bis(pyrazolyl)borate. Molecules 2022, 27, 16. https://doi.org/10.3390/molecules27010016
Noonikara-Poyil A, Muñoz-Castro A, Dias HVR. Terminal and Internal Alkyne Complexes and Azide-Alkyne Cycloaddition Chemistry of Copper(I) Supported by a Fluorinated Bis(pyrazolyl)borate. Molecules. 2022; 27(1):16. https://doi.org/10.3390/molecules27010016
Chicago/Turabian StyleNoonikara-Poyil, Anurag, Alvaro Muñoz-Castro, and H. V. Rasika Dias. 2022. "Terminal and Internal Alkyne Complexes and Azide-Alkyne Cycloaddition Chemistry of Copper(I) Supported by a Fluorinated Bis(pyrazolyl)borate" Molecules 27, no. 1: 16. https://doi.org/10.3390/molecules27010016
APA StyleNoonikara-Poyil, A., Muñoz-Castro, A., & Dias, H. V. R. (2022). Terminal and Internal Alkyne Complexes and Azide-Alkyne Cycloaddition Chemistry of Copper(I) Supported by a Fluorinated Bis(pyrazolyl)borate. Molecules, 27(1), 16. https://doi.org/10.3390/molecules27010016