Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Delignification and Yield of the Hemicelluloses
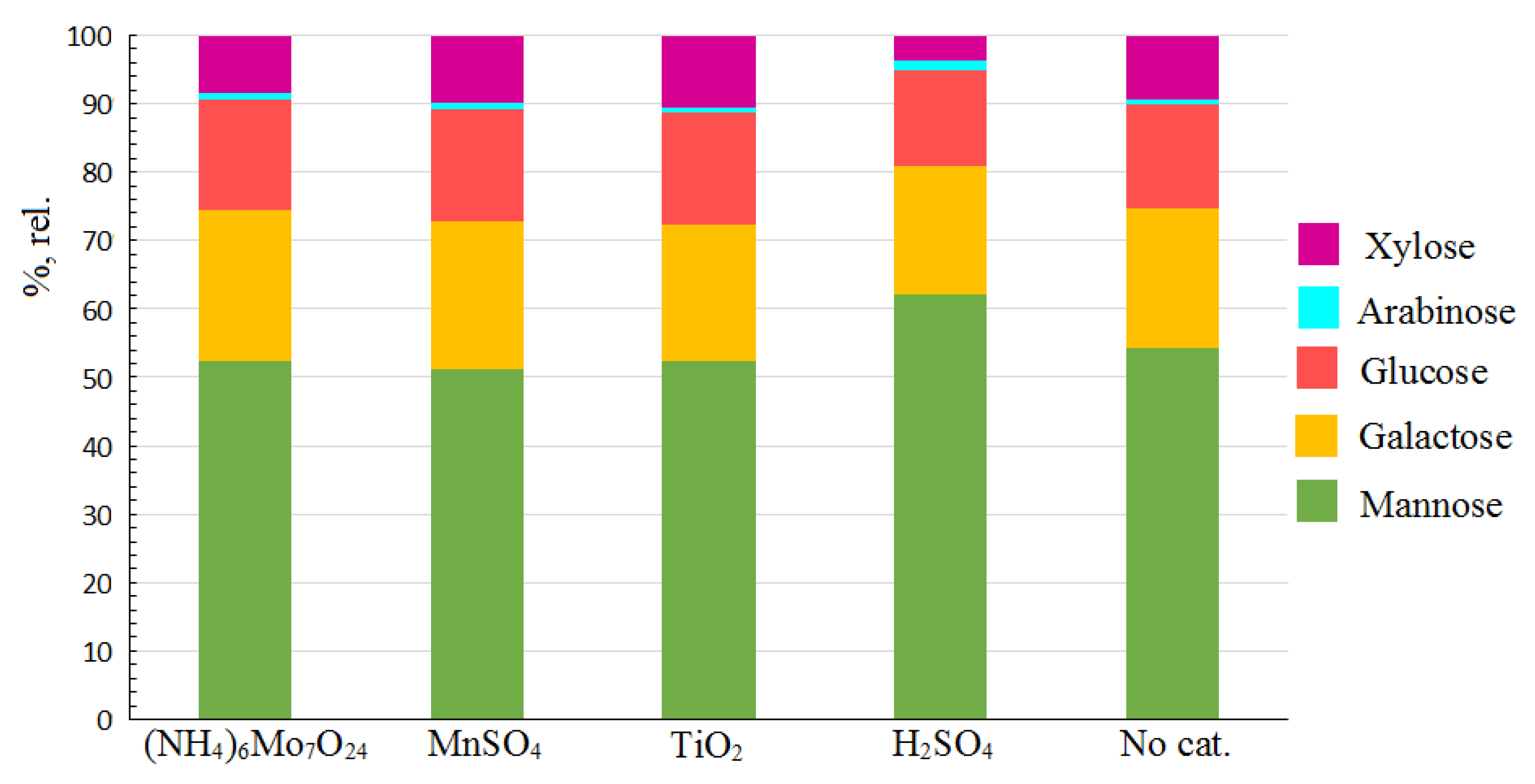
2.2. Monosaccharide Composition of the Hemicelluloses
2.3. Gel Permeation Chromatography Study of the Hemicelluloses
2.4. Fourier-Transform Infra-Red Spectroscopy Study of the Hemicelluloses
2.5. Thermogravimetric Analysis of the Hemicelluloses
2.6. Elemental Analysis of the Hemicelluloses
2.7. Antioxidant Activity of the Hemicelluloses
3. Materials and Methods
3.1. Materials
3.2. Spruce Wood Delignification and Extraction of the Hemicelluloses
3.3. Monosaccharide Composition of the Hemicelluloses
3.4. Gel Permeation Chromatography
3.5. Fourier-Transform Infra-Red Spectroscopy Study
3.6. Thermogravimetric Analysis
3.7. Elemental Analysis
3.8. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chudina, A.I.; Malyar, Y.N.; Sudakova, I.G.; Kazachenko, A.S.; Skripnikov, A.M.; Borovkova, V.S.; Kondrasenko, A.A.; Mazurova, E.V.; Fetisova, O.Y.; Ivanov, I.P. Physicochemical characteristics of polysaccharides from catalytic and noncatalytic acetic acid-peroxide delignification of larch wood. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Willför, S.; Sundberg, K.; Tenkanen, M.; Holmbom, B. Spruce-derived mannans—A potential raw material for hydrocolloids and novel advanced natural materials. Carbohydr. Polym. 2008, 72, 197–210. [Google Scholar] [CrossRef]
- Várnai, A.; Siika-aho, M.; Viikari, L. Restriction of the enzymatic hydrolysis of steam-pretreated spruce by lignin and hemicellulose. Enzym. Microb. Technol. 2010, 46, 185–193. [Google Scholar] [CrossRef]
- Sun, X.-F.; Wang, H.; Jing, Z.; Mohanathas, R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr. Polym. 2013, 92, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Deloule, V.; Boisset, C.; Hannani, D.; Suau, A.; Gouellec, A.; Chroboczek, J.; Botté, C.; Yamaryo-Botté, Y.; Chirat, C.; Toussaint, B. Prebiotic role of softwood hemicellulose in healthy mice model. J. Funct. Foods 2020, 64, 103688. [Google Scholar] [CrossRef]
- Mendes, F.R.S.; Bastos, M.S.R.; Mendes, L.G.; Silva, A.R.A.; Sousa, F.D.; Monteiro-Moreira, A.C.O.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocoll. 2017, 70, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Pranovich, A.; Uppstu, P.; Wang, X.; Kronlund, D.; Hemming, J.; Öblom, H.; Moritz, N.; Preis, M.; Sandler, N.; et al. Novel biorenewable composite of wood polysaccharide and polylactic acid for three dimensional printing. Carbohydr. Polym. 2018, 187, 51–58. [Google Scholar] [CrossRef]
- Gautam, D.; Kumari, S.; Ram, B.; Chauhan, G.S.; Chauhan, K. A new hemicellulose-based adsorbent for malachite green. J. Environ. Chem. Eng. 2018, 6, 3889–3897. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Kirjoranta, S.; Xu, C.; Hemming, J.; Pranovich, A.; Bhattarai, M.; Peltonen, L.; Kilpeläinen, P.; Maina, N.; Tenkanen, M.; et al. Environmentally-compatible alkyd paints stabilized by wood hemicelluloses. Ind. Crop. Prod. 2019, 133, 212–220. [Google Scholar] [CrossRef]
- Krogell, J.; Korotkova, E.; Eränen, K.; Pranovich, A.; Salmi, T.; Murzin, D.; Willför, S. Intensification of hemicellulose hot-water extraction from spruce wood in a batch extractor—Effects of wood particle size. Bioresour. Technol. 2013, 143, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Pranovich, A.; Holmbom, B. Effects of pH control with phthalate buffers on hot-water extraction of hemicelluloses from spruce wood. Bioresour. Technol. 2011, 102, 10518–10523. [Google Scholar] [CrossRef]
- Gallina, G.; Cabeza, Á.; Grénman, H.; Biasi, P.; García-Serna, J.; Salmi, T. Hemicellulose extraction by hot pressurized water pretreatment at 160 °C for 10 different woods: Yield and molecular weight. J. Supercrit. Fluids 2018, 133, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Vasco, C.; Zhang, X. Alkaline hydrogen peroxide pretreatment of softwood: Hemicellulose degradation pathways. Bioresour. Technol. 2013, 150, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Chadni, M.; Bals OZiegler-Devin, I.; Brosse, N.; Grimi, N. Microwave-assisted extraction of high-molecular-weight hemicelluloses from spruce wood. Comptes Rendus Chim. 2019, 22, 574–584. [Google Scholar] [CrossRef]
- Oh, M.H.; Yoon, K.Y. Comparison of the Biological Activity of Crude Polysaccharide Fractions Obtained from Cedrela sinensis Using Different Extraction Methods. Pol. J. Food Nutr. Sci. 2018, 68, 327–334. [Google Scholar] [CrossRef]
- Chadni, M.; Grimi, N.; Bals, O.; Ziegler-Devin, I.; Brosse, N. Steam explosion process for the selective extraction of hemicelluloses polymers from spruce sawdust. Ind. Crop. Prod. 2019, 141, 111–757. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Fetisova, O.Y.; Chudina, A.I.; Sudakova, I.G.; Antonov, A.V.; Borovkova, V.S.; Kuznetsova, S.A. Isolation and sulfation of galactoglucomannan from larch wood (Larix sibirica). Wood Sci. Technol. 2021, 55, 1091–1107. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Chesnokov, N.V.; Sudakova, I.G.; Garyntseva, N.V.; Kuznetsova, S.A.; Malyar, Y.u.N.; Yakovlev, V.A.; Djakovitch, L. Green catalytic processing of native and organosolv lignins. Catal. Today 2018, 309, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, B.N.; Sudakova, I.G.; Yatsenkova, O.V.; Garyntseva, N.V.; Rataboul, F.; Djakovitch, L. Optimizing Single-Stage Processes of Microcrystalline Cellulose Production via the Peroxide Delignification of Wood in the Presence of a Titania Catalyst. Catal. Ind. 2018, 10, 360–367. [Google Scholar] [CrossRef]
- Bragatto, J.; Segato, F.; Squina, F.M. Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Ind. Crop. Prod. 2013, 51, 123–129. [Google Scholar] [CrossRef]
- Wen, P.; Zhang, T.; Wang, J.; Lian, Z.; Zhang, J. Production of xylooligosaccharides and monosaccharides from poplar by a two-step acetic acid and peroxide/acetic acid pretreatment. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vila, C.; Santos, V.; Parajó, J.C. Simulation of an Organosolv Pulping Process: Generalized Material Balances and Design Calculations. Ind. Eng. Chem. Res. 2003, 42, 349–356. [Google Scholar] [CrossRef]
- Suchy, M.; Argyropoulos, D.S. Catalysis and activation of oxygen and peroxide delignification of chemical pulps: A review. Tappi J. 2002, 1, 1–18. [Google Scholar]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic Oxidation of Biorefinery Lignin to Value-added Chemicals to Support Sustainable Biofuel Production. ChemSusChem. 2014, 8, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Zhang, F.; Wang, Z.-J.; Shen, M.-Y.; Nie, S.-P.; Xie, M.-Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Wang, C.-L.; Wang, Y.-R.; Li, Z.-J.; Zhang, Y.-N. The polysaccharide isolated from Pleurotus nebrodensis (PN-S) shows immune-stimulating activity in RAW264.7 macrophages. Chin. J. Nat. Med. 2015, 13, 355–360. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, C.; Qiu, J.; Wang, L.; Bao, J.; Wang, K.; Zhang, Y.; Chen, M.; Wan, J.; Su, H.; et al. Purification, structural characterization and anticancer activity of the novel polysaccharides from Rhynchosia minima root. Carbohydr. Polym. 2015, 132, 67–71. [Google Scholar] [CrossRef]
- Fyhrquist, P.; Virjamo, V.; Hiltunen, E.; Julkunen-Tiitto, R. Epidihydropinidine, the main piperidine alkaloid compound of Norway spruce (Picea abies) shows promising antibacterial and anti-Candida activity. Fitoterapia 2017, 117, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Normand, M.L.; Mélida, H.; Holmbom, B.; Michaelsen, T.E.; Inngjerdingen, M.; Bulone, V.; Paulsen, B.S.; Ek, M. Hot-water extracts from the inner bark of Norway spruce with immunomodulating activities. Carbohydr. Polym. 2014, 101, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Bittkau, K.S.; Alban, S. Size distribution and chain conformation of six different fucoidans using size-exclusion chromatography with multiple detection. J. Chromatogr. A 2020, 1612, 460658. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Obernberger, I. Application of a detailed biomass pyrolysis kinetic scheme to hardwood and softwood torrefaction. Fuel 2016, 167, 158–167. [Google Scholar] [CrossRef]
- Hu, T.T.; Liu, D.; Chen YWu, J.; Wang, S.S. Antioxidant activity of sulfatedpolysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef]
- Dumore, N.S.; Mukhopadhyay, M. Antioxidant properties of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reduction. J. Mol. Struct. 2020, 1205, 127637. [Google Scholar] [CrossRef]
- Hara, K.; Someya, T.; Sano, K.; Sagane, Y.; Watanabe, T.; Wijesekara, R.G.S. Antioxidant activities of traditional plants in Sri Lanka by DPPH free radical-scavenging assay. Data Brief. 2018, 17, 870–875. [Google Scholar] [CrossRef]
- Fadda, A.; Serra, M.; Molinu, M.G.; Azara, E.; Barberis, A.; Sanna, D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. J. Food Compos. Anal. 2014, 35, 112–119. [Google Scholar] [CrossRef]
- Ji, X.; Liu, F.; Ullah, N.; Wang, M. Isolation, purification, and antioxidant activities of polysaccharides from Ziziphus Jujuba cv. Muzao. Int. J. Food Prop. 2018, 21, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Abdelhalim, A.O.E.; Meshcheriakov, A.A.; Maistrenko, D.N.; Molchanov, O.E.; Ageev, S.V.; Ivanov, D.A.; Iamalov, N.R.; Luttsev, M.D.; Vasina, L.V.; Sharoyko, V.V.; et al. Graphene oxide enriched with oxygen-containing groups: On the way to an increase of antioxidant activity and biocompatibility. Colloids Surf. B Biointerfaces 2021, 112232. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M. Characterization and antioxidant activities of polysaccharides extracted from flageolet bean pods waste. Curr. Res. Green Sustain. Chem. 2021, 4, 100154. [Google Scholar] [CrossRef]
- Yi, J.; Li, X.; Wang, S.; Wu, T.; Liu, P. Steam explosion pretreatment of Achyranthis bidentatae radix: Modified polysaccharide and its antioxidant activities. Food Chem. 2021, 375, 131746. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Enma, S.R.; Moure, C.A.; Juan, H.D.; Parajó, C. Characterization, refining and antioxidant activity of saccharides derived from hemicelluloses of wood and rice husks. Food Chem. 2013, 141, 495–502. [Google Scholar] [CrossRef]
- Bogdanović, J.; Mitrović, P.A.; Spasojević, I. A comparative study of antioxidative activities of cell-wall polysaccharides. Carbohydr. Res. 2011, 346, 2255–2259. [Google Scholar]
- Ebringerová, A.; Hromádková, Z.; Hříbalová, V.; Xu, C.; Holmbom, B.; Sundberg, A.; Willför, S. Norway spruce galactoglucomannans exhibiting immunomodulating and radical-scavenging activities. Int. J. Biol. Macromol. 2008, 42, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, E.; Alén, R. Analytical Methods of Wood Chemistry, Pulping, and Papermaking; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Xua, Y.; Songa, S.; Weia, Y.; Wangb, F.; Zhaoa, M.; Guoa, J.; Zhanga, J. Sulfated modification of the polysaccharide from Sphallerocarpusgracilis and its antioxidant activities. Int. J. Biol. Macromol. 2016, 87, 180–190. [Google Scholar] [CrossRef] [PubMed]





| Samples 1 | Hemicellulose Yield (wt%) 2 | |||
|---|---|---|---|---|
| 90 (°C) | 100 (°C) | |||
| 3 (h) | 4 (h) | 3 (h) | 4 (h) | |
| HCno cat 3 | 8.6 | 10.1 | 10.4 | 11.7 |
| HCMo 4 | 7.9 | 9.2 | 10.1 | 10.9 |
| HCMn 5 | 5.9 | 8.2 | 10.7 | 9.05 |
| HCTi 6 | 5.8 | 8.0 | 8.4 | 7.0 |
| 7 | 0.9 | 1.1 | 2.3 | 1.5 |
| HC Sample | Catalyst | T (°C) | t (h) | Mw (g/mol) | PDI | K | α |
|---|---|---|---|---|---|---|---|
| HCMo 90-4 | (NH4)6Mo7O24 | 90 | 4 | 17,367 | 3.354 | 442.43 | 0.45 |
| HCMo 100-3 | (NH4)6Mo7O24 | 100 | 3 | 16,797 | 1.883 | 37.48 | 0.63 |
| HCMo 100-4 | (NH4)6Mo7O24 | 100 | 4 | 12,471 | 2.11 | 69.17 | 0.63 |
| HCMn 90-4 | MnSO4 | 90 | 4 | 18,963 | 2.022 | 56.36 | 0.61 |
| HCMn 100-3 | MnSO4 | 100 | 3 | 20,382 | 2.053 | 64.85 | 0.58 |
| HCMn 100-4 | MnSO4 | 100 | 4 | 19,061 | 2.198 | 272.03 | 0.42 |
| HCTi 90-4 | TiO2 | 90 | 4 | 42,793 | 3.255 | 472.17 | 0.33 |
| HCTi 100-3 | TiO2 | 100 | 3 | 29,962 | 2.612 | 128.00 | 0.46 |
| HCTi 100-4 | TiO2 | 100 | 4 | 35,363 | 2.646 | 273.17 | 0.35 |
| HCno cat 90-4 | w/o cat | 90 | 4 | 47,645 | 2.145 | 25.41 | 0.59 |
| HC no cat 100-4 | w/o cat | 100 | 4 | 40,885 | 2.917 | 148.32 | 0.41 |
| 100-4 | H2SO4 | 100 | 2 | 12,845 | 1.815 | 1.57 | 1.01 |
| Sample | C (%) | H (%) | O 1 (%) |
|---|---|---|---|
| HCMo | 40.96 | 5.96 | 53.08 |
| HCMn | 39.74 | 5.92 | 54.34 |
| HCTi | 42.10 | 6.08 | 51.82 |
| 36.44 | 5.77 | 57.78 | |
| HCno cat | 42.85 | 6.14 | 51.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266. https://doi.org/10.3390/molecules27010266
Borovkova VS, Malyar YN, Sudakova IG, Chudina AI, Skripnikov AM, Fetisova OY, Kazachenko AS, Miroshnikova AV, Zimonin DV, Ionin VA, et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules. 2022; 27(1):266. https://doi.org/10.3390/molecules27010266
Chicago/Turabian StyleBorovkova, Valentina S., Yuriy N. Malyar, Irina G. Sudakova, Anna I. Chudina, Andrey M. Skripnikov, Olga Yu. Fetisova, Alexander S. Kazachenko, Angelina V. Miroshnikova, Dmitriy V. Zimonin, Vladislav A. Ionin, and et al. 2022. "Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification" Molecules 27, no. 1: 266. https://doi.org/10.3390/molecules27010266







