4,5-Bis(arylethynyl)-1,2,3-triazoles—A New Class of Fluorescent Labels: Synthesis and Applications
Abstract
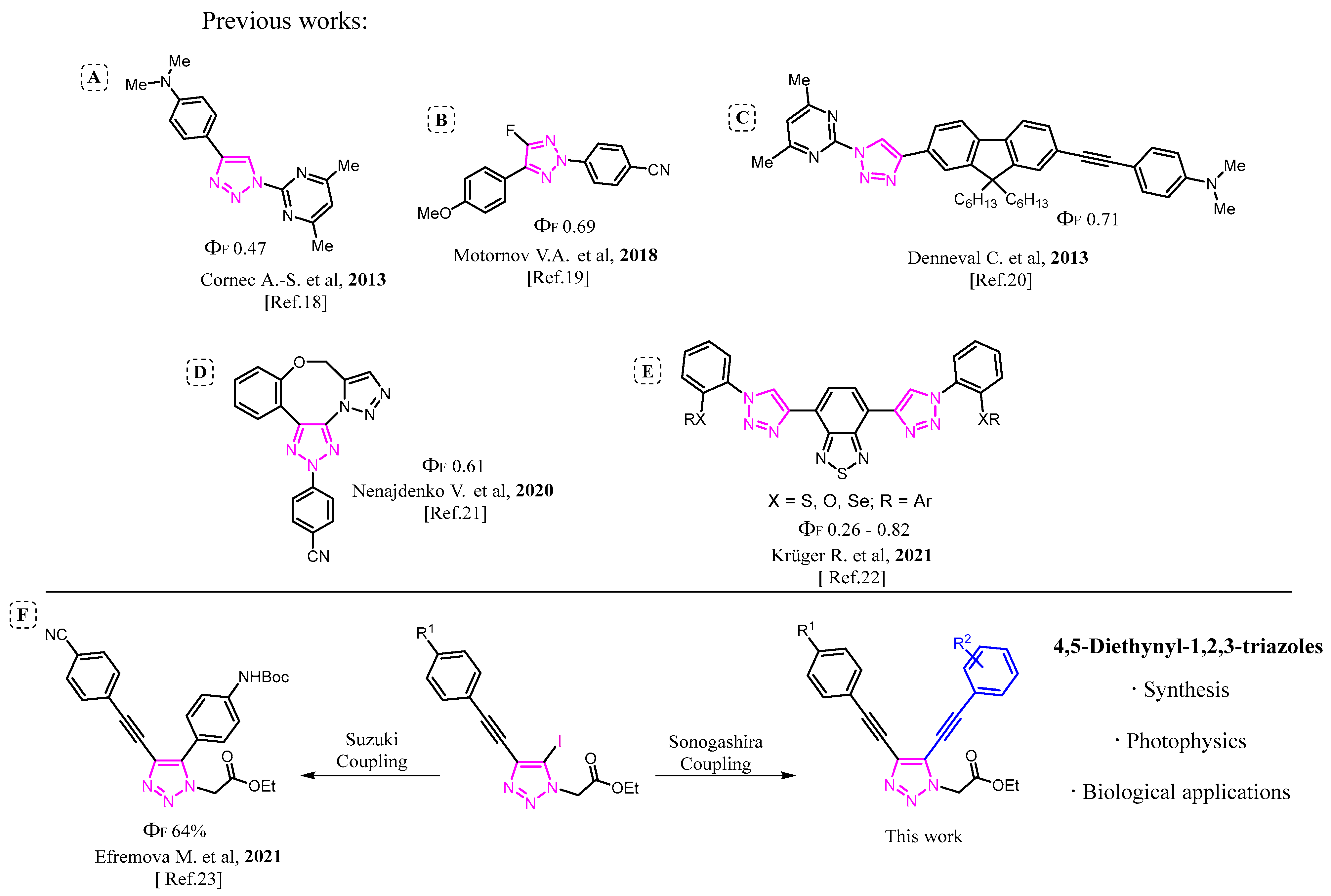
:1. Introduction
2. Results and Discussion
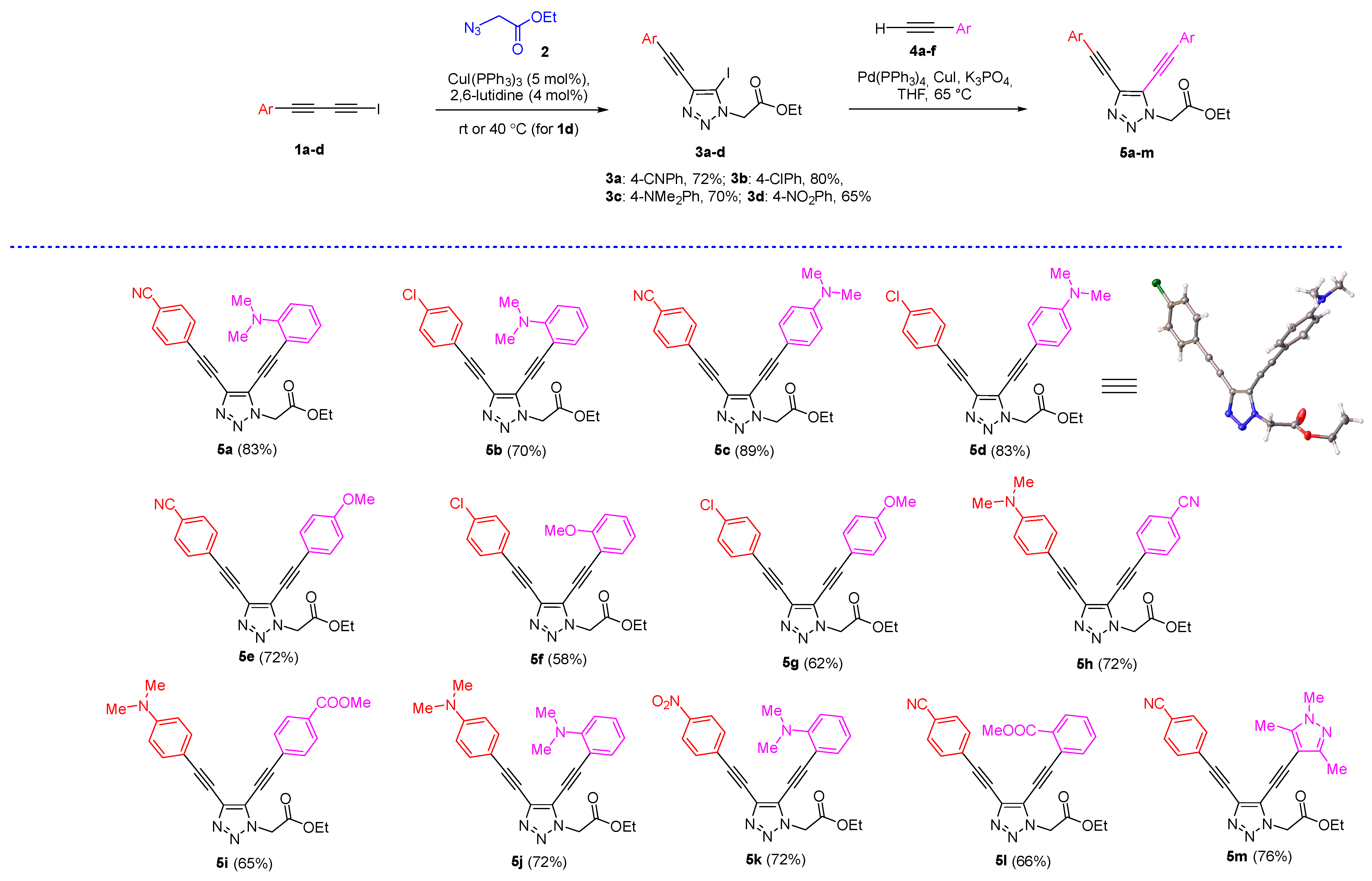
2.1. Synthesis of 4,5-Bis(arylethynyl)triazoles
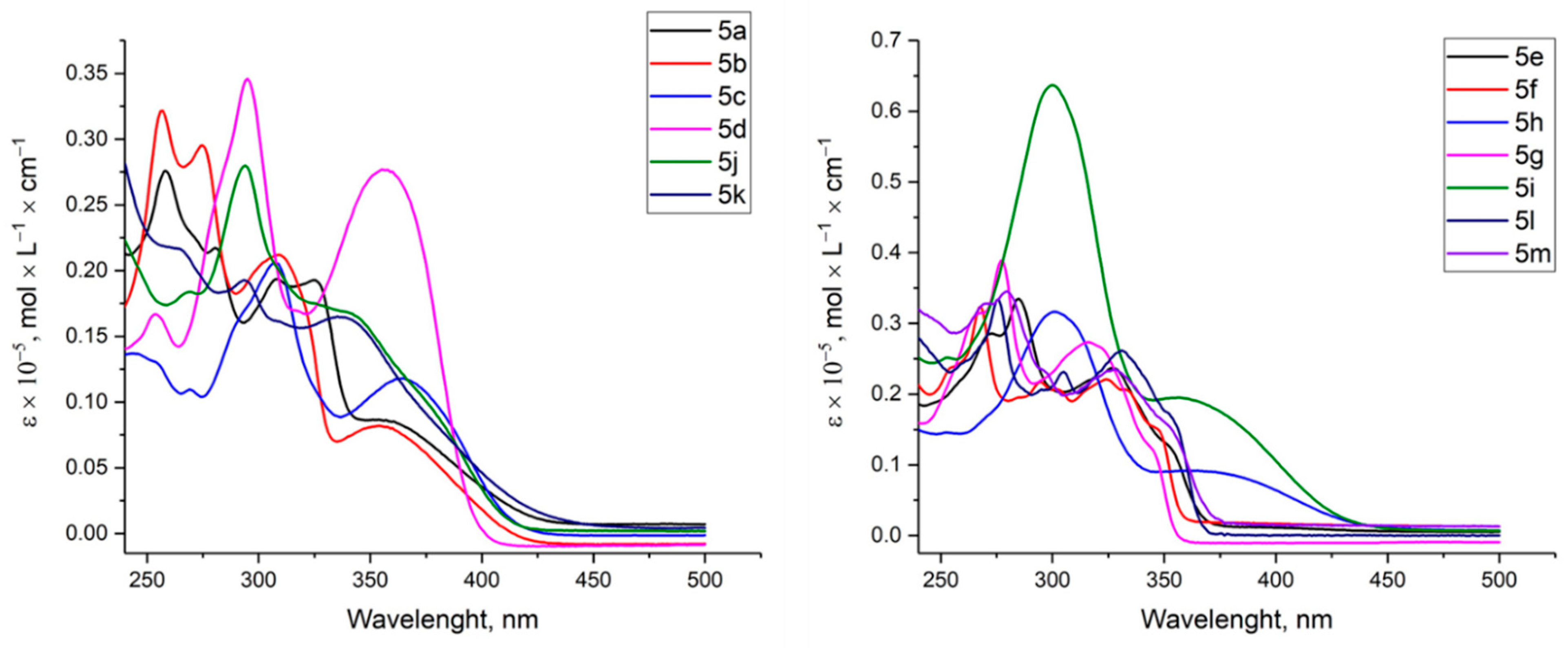
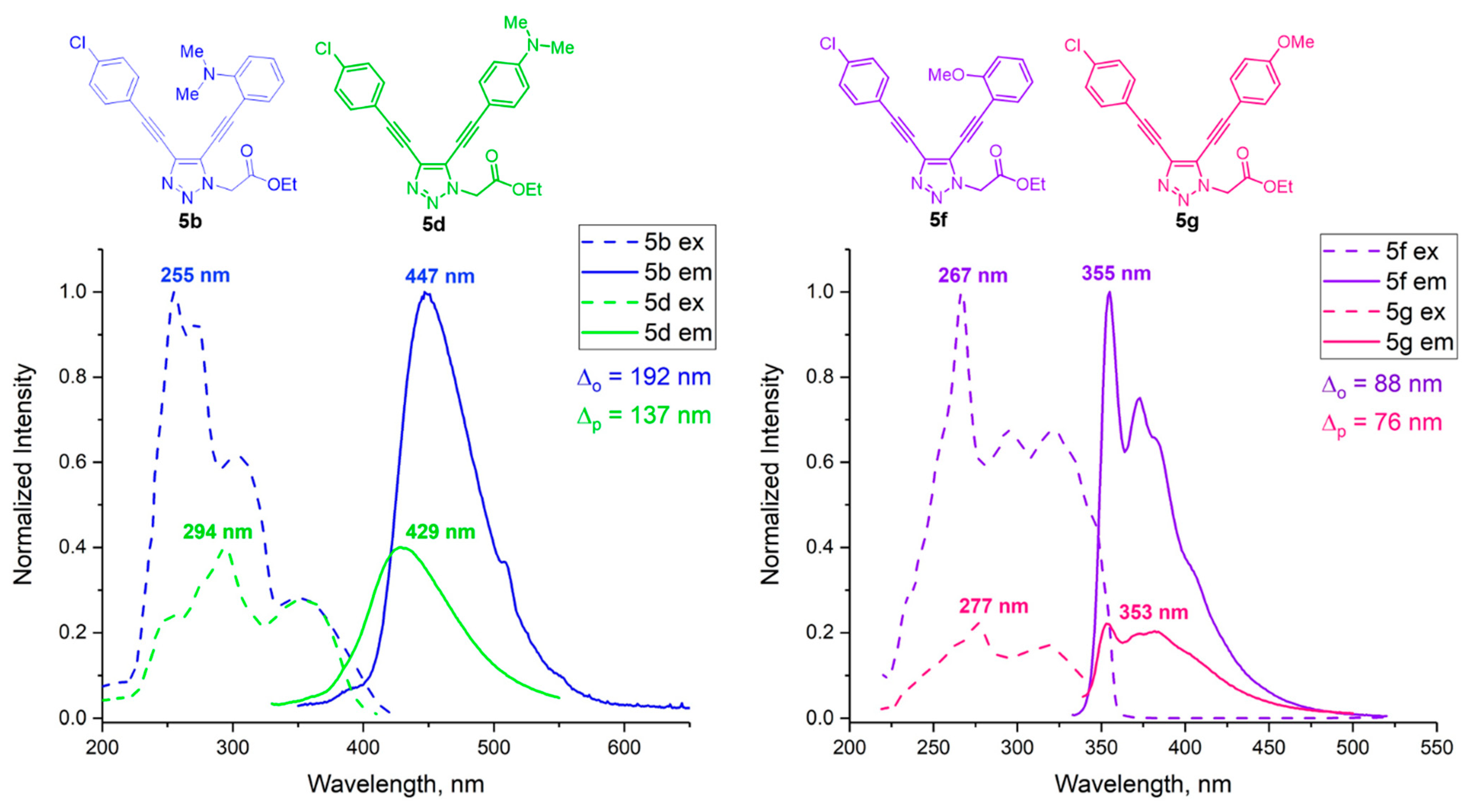
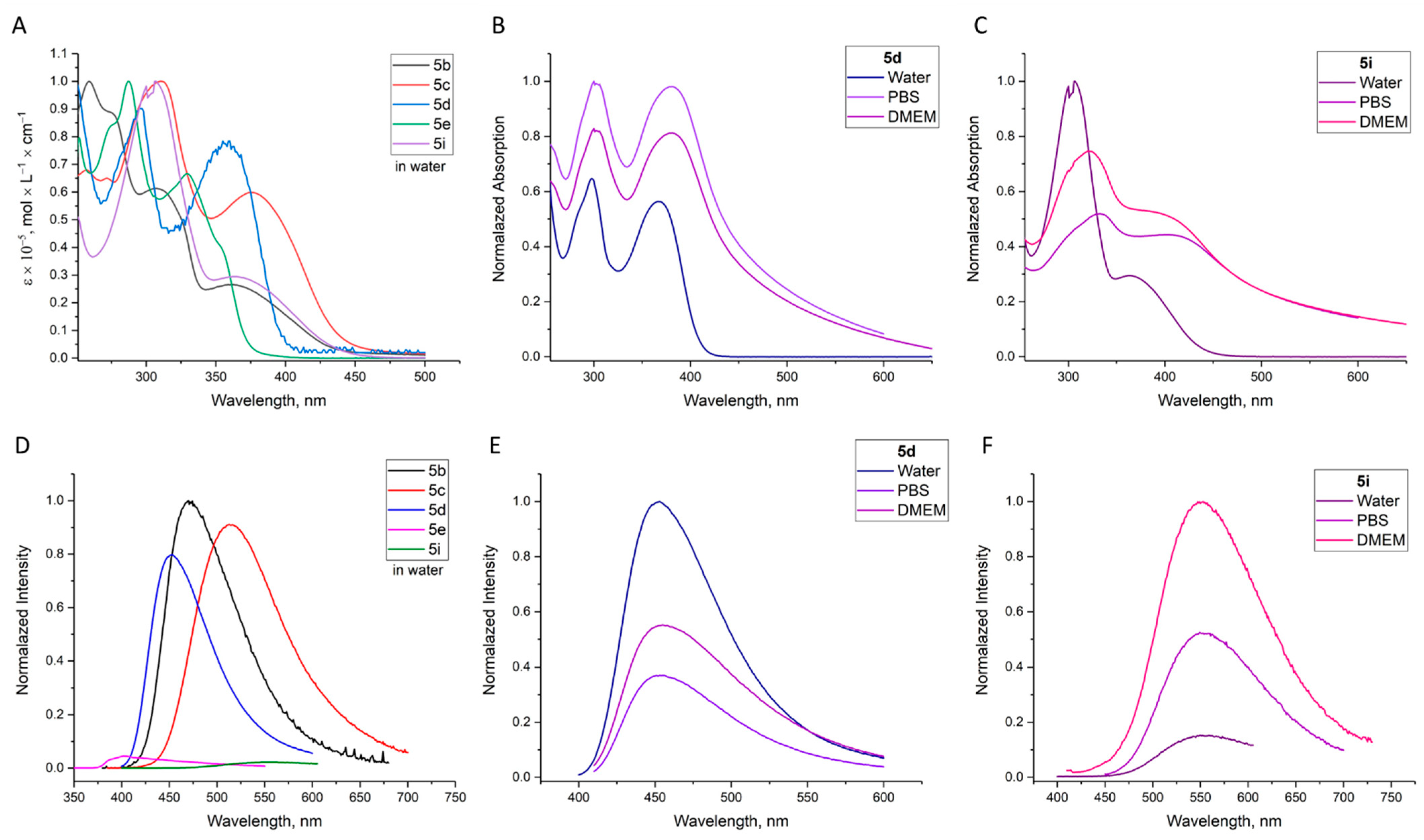
2.2. Investigation of Optical Properties
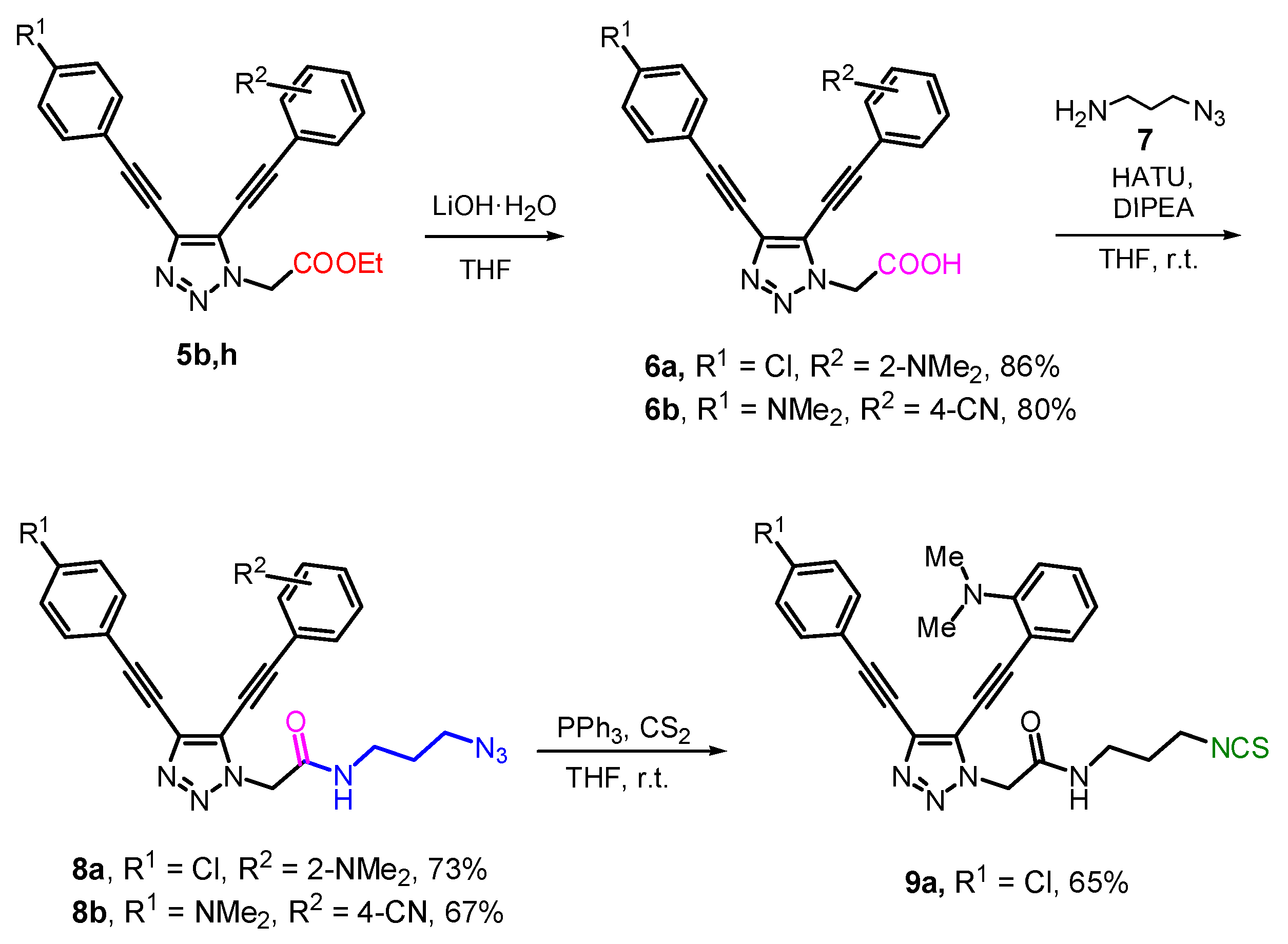
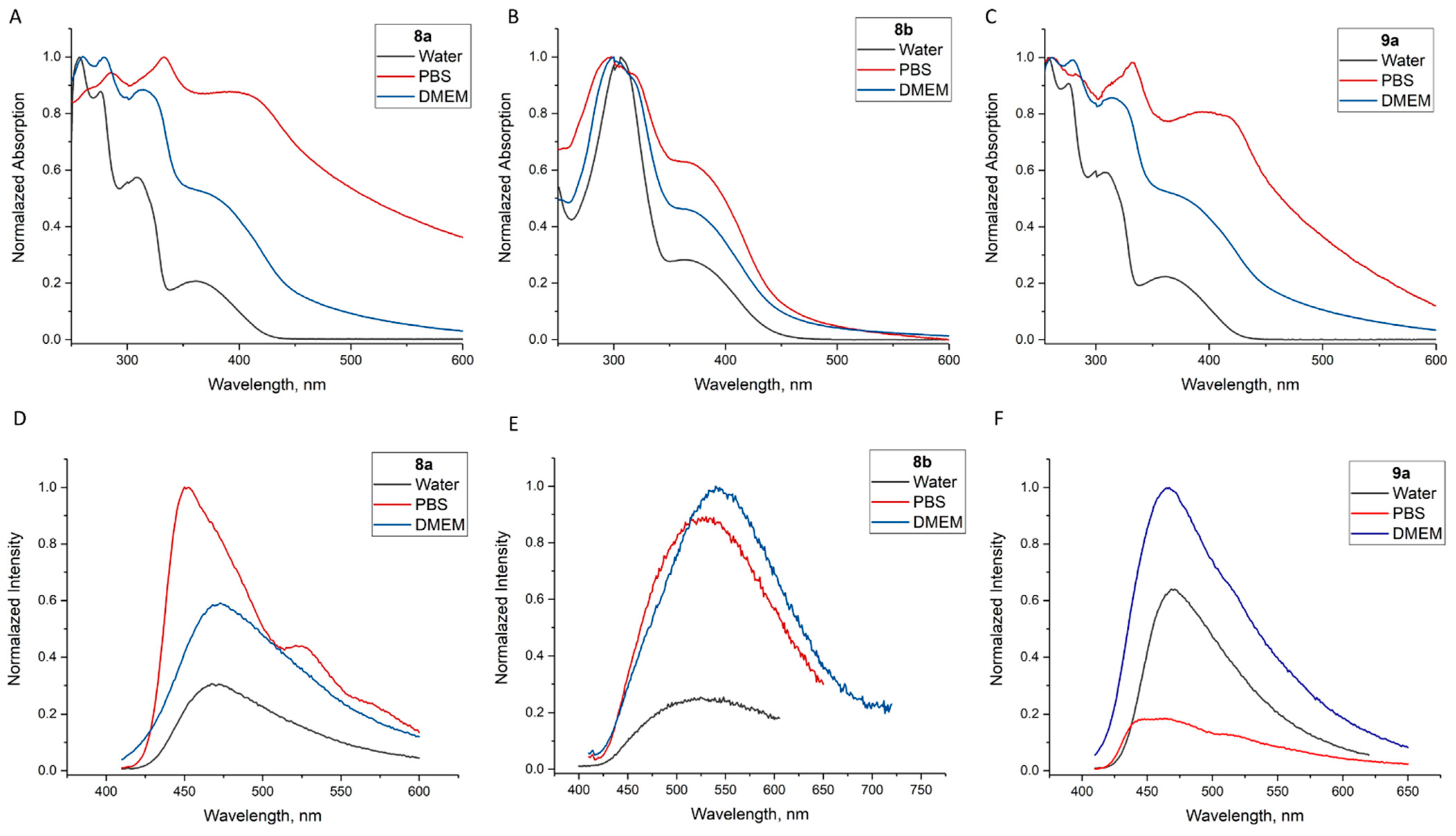
2.3. Modification of 5b,h by ”Clickable“ Groups, Study of Their Optical Properties, and Application as Fluorescent Dyes
2.4. The Study of 4,5-diarylethynyl-1H-1,2,3-triazole Cytotoxycity
3. Materials and Methods
3.1. General Information
3.2. Synthetic Methods and Analytic Data of Compounds
3.2.1. General Procure for the CuAAC
3.2.2. General Procure for the Sonogashira Cross-Coupling
3.2.3. General Procure for the Synthesis of Triazoloacids 7
3.2.4. General Procure for the Synthesis of Amidoazides 8
3.3. The Absolute Fluorescence Quantum Yield Measurements
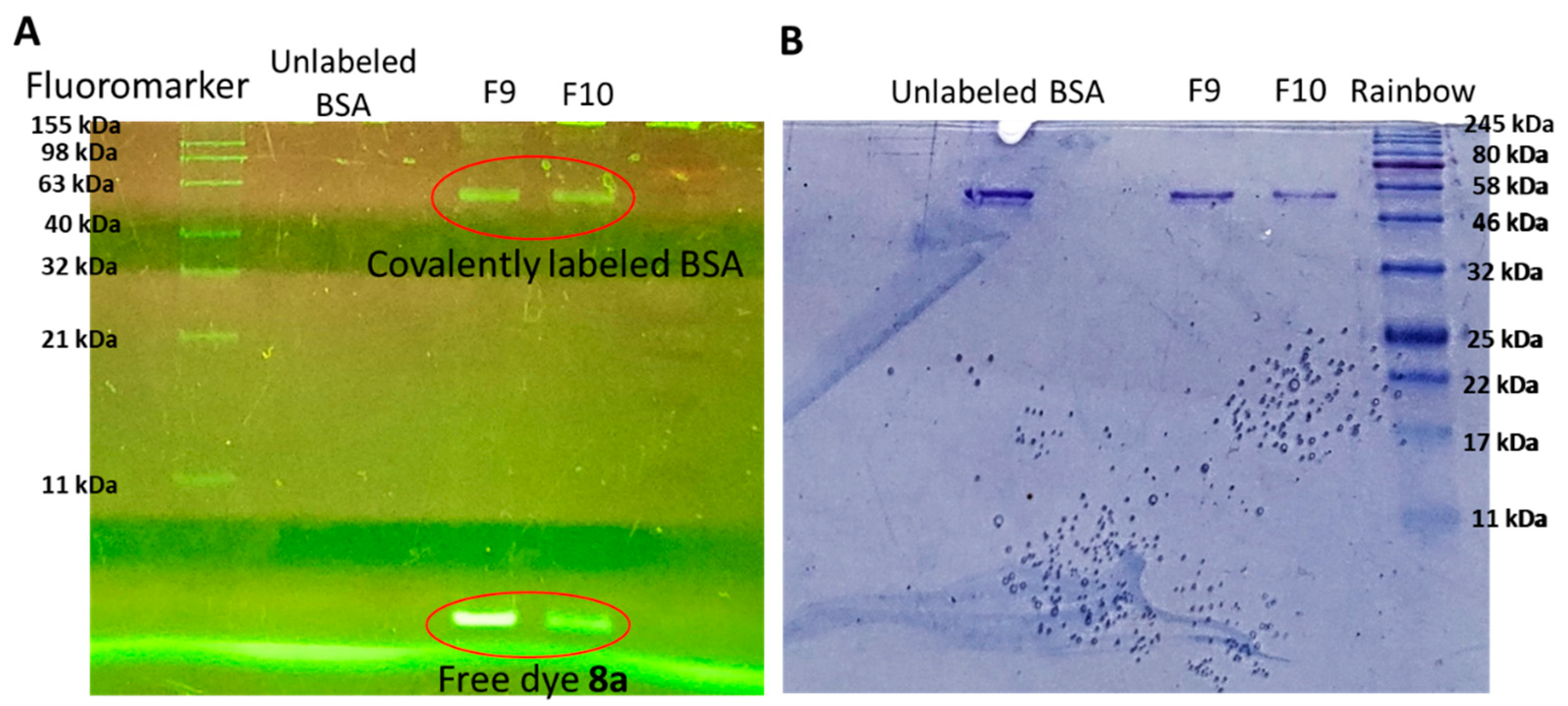
3.4. Protein Labeling
3.5. Confocal Laser Scanning Microscopy (CLSM)
3.6. Cell Culture Cultivation and Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, H.W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.B.; Tan, W. Recent Progresses in Small-Molecule Enzymatic Fluorescent Probes for Cancer Imaging. Chem. Soc. Rev. 2018, 47, 7140–7180. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Tian, H.; Zang, Y.; Sedgwick, A.C.; Li, J.; Sessler, J.L.; He, X.P.; James, T.D. Small-Molecule Fluorescence-Based Probes for Interrogating Major Organ Diseases. Chem. Soc. Rev. 2021, 50, 9391–9429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Huang, H.; Kang, X.; Yang, L.; Xi, Z.; Sun, H.; Pluth, M.D.; Yi, L. NBD-Based Synthetic Probes for Sensing Small Molecules and Proteins: Design, Sensing Mechanisms and Biological Applications. Chem. Soc. Rev. 2021, 50, 7436–7495. [Google Scholar] [CrossRef]
- Tian, Z.; Tian, X.; Feng, L.; Tian, Y.; Huo, X.; Zhang, B.; Deng, S.; Ma, X.; Cui, J. A Highly Sensitive and Selective Two-Photon Fluorescent Probe for Glutathione S-Transferase Detection and Imaging in Living Cells and Tissues. J. Mater. Chem. B 2019, 7, 4983–4989. [Google Scholar] [CrossRef]
- Hanif, M.; Rafiq, M.; Yousuf, M.; Kotwica-Mojzych, K.; Saleem, M.; Mojzych, M. Organic Small Molecular Receptors as Fluorimetric/Bioimaging Probe for Extracellular/Intracellular Zinc Sensation. Bioorg. Chem. 2020, 94, 103398. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-B.; Liu, H.-W.; Fu, T.; Wang, R.; Zhang, X.-B.; Tan, W. Recent Progress in Small-Molecule Near-IR Probes for Bioimaging. Trends Chem. 2019, 1, 224–234. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, S.; Yang, N.D.; Zhang, C.; Wu, Q.; Yu, C. Recent Development of Small-Molecule Organic Fluorophores for Multifunctional Bioimaging in the Second near-Infrared Window. J. Lumin. 2020, 225, 117338. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, D. Identification of the Donor-Substitution Effect of Tetraphenylethylene AIEgen: Synthesis, Photophysical Property Analysis, and Bioimaging Applications. Dye. Pigment. 2022, 199, 110098. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [Green Version]
- Colas, K.; Doloczki, S.; Posada Urrutia, M.; Dyrager, C. Prevalent Bioimaging Scaffolds: Synthesis, Photophysical Properties and Applications. Eur. J. Org. Chem. 2021, 2021, 2133–2144. [Google Scholar] [CrossRef]
- Ha, Y.; Choi, H.K. Recent Conjugation Strategies of Small Organic Fluorophores and Ligands for Cancer-Specific Bioimaging. Chem. Biol. Interact. 2016, 248, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Lavis, L.D. Development of Photostable Fluorophores for Molecular Imaging. Curr. Opin. Chem. Biol. 2017, 39, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Brunel, D.; Dumur, F. Recent Advances in Organic Dyes and Fluorophores Comprising a 1,2,3-Triazole Moiety. New J. Chem. 2020, 44, 3546–3561. [Google Scholar] [CrossRef]
- Kalra, P.; Kaur, R.; Singh, G.; Singh, H.; Singh, G.; Pawan; Kaur, G.; Singh, J. Metals as “Click” Catalysts for Alkyne-Azide Cycloaddition Reactions: An Overview. J. Organomet. Chem. 2021, 944, 121846. [Google Scholar] [CrossRef]
- Saini, P.; Sonika; Singh, G.; Kaur, G.; Singh, J.; Singh, H. Robust and Versatile Cu(I) Metal Frameworks as Potential Catalysts for Azide-Alkyne Cycloaddition Reactions: Review. Mol. Catal. 2021, 504, 111432. [Google Scholar] [CrossRef]
- Meisner, Q.J.; Accardo, J.V.; Hu, G.; Clark, R.J.; Jiang, D.-E.; Zhu, L. Fluorescence of Hydroxyphenyl-Substituted “Click” Triazoles. J. Phys. Chem. A 2018, 122, 2956–2973. [Google Scholar] [CrossRef]
- Cserép, G.B.; Herner, A.; Kele, P. Bioorthogonal Fluorescent Labels: A Review on Combined Forces. Methods Appl. Fluoresc. 2015, 3, 042001. [Google Scholar] [CrossRef]
- Cornec, A.S.; Baudequin, C.; Fiol-Petit, C.; Plé, N.; Dupas, G.; Ramondenc, Y. One “Click” to Access Push-Triazole-Pull Fluorophores Incorporating a Pyrimidine Moiety: Structure-Photophysical Properties Relationships. Eur. J. Org. Chem. 2013, 2013, 1908–1915. [Google Scholar] [CrossRef]
- Motornov, V.A.; Tabolin, A.A.; Novikov, R.A.; Shepel, N.E.; Nenajdenko, V.G.; Ioffe, S.L. Synthesis of 2,5-Diaryl-4-Halo-1,2,3-Triazoles and Comparative Study of Their Fluorescent Properties. Tetrahedron 2018, 74, 3897–3903. [Google Scholar] [CrossRef]
- Denneval, C.; Moldovan, O.; Baudequin, C.; Achelle, S.; Baldeck, P.; Plé, N.; Darabantu, M.; Ramondenc, Y. Synthesis and Photophysical Properties of Push-Pull Structures Incorporating Diazines as Attracting Part with a Fluorene Core. Eur. J. Org. Chem. 2013, 2013, 5591–5602. [Google Scholar] [CrossRef]
- Tsyrenova, B.; Nenajdenko, V. Synthesis and Spectral Study of a New Family of 2,5-Diaryltriazoles Having Restricted Rotation of the 5-Aryl Substituent. Molecules 2020, 25, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, R.; Larroza, A.; Fronza, M.G.; Tisoco, I.; Savegnago, L.; Reis, J.S.; Back, D.F.; Iglesias, B.A.; Alves, D. Bis-Triazolylchalcogenium-Functionalized Benzothiadiazole Derivatives as Light-up Sensors for DNA and BSA. J. Org. Chem. 2021, 86, 17866–17883. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.M.; Govdi, A.I.; Frolova, V.V.; Rumyantsev, A.M.; Balova, I.A. Design and Synthesis of New 5-Aryl-4-Arylethynyl-1H-1,2,3-Triazoles with Valuable Photophysical and Biological Properties. Molecules 2021, 26, 2801. [Google Scholar] [CrossRef]
- Govdi, A.I.; Danilkina, N.A.; Ponomarev, A.V.; Balova, I.A. 1-Iodobuta-1,3-Diynes in Copper-Catalyzed Azide-Alkyne Cycloaddition: A One-Step Route to 4-Ethynyl-5-Iodo-1,2,3-Triazoles. J. Org. Chem. 2019, 84, 1925–1940. [Google Scholar] [CrossRef]
- Zhu, Y.; Guang, S.; Su, X.; Xu, H.; Xu, D. Dependence of the Intramolecular Charge Transfer on Molecular Structure in Triazole Bridge-Linked Optical Materials. Dye. Pigment. 2013, 97, 175–183. [Google Scholar] [CrossRef]
- An, W.F. Fluorescence-Based Assays. Methods Mol. Biol. 2009, 486, 97–107. [Google Scholar] [CrossRef]
- Toseland, C.P. Fluorescent Labeling and Modification of Proteins. J. Chem. Biol. 2013, 6, 85–95. [Google Scholar] [CrossRef]
- Banks, P.R.; Paquette, D.M. Comparison of Three Common Amine Reactive Fluorescent Probes Used for Conjugation to Biomolecules by Capillary Zone Electrophoresis. Bioconjug. Chem. 1995, 6, 447–458. [Google Scholar] [CrossRef]
- Barbero, N.; Barolo, C.; Viscardi, G. Bovine Serum Albumin Bioconjugation with FITC. World J. Chem. Educ. 2016, 4, 80–85. [Google Scholar] [CrossRef]
- Dirks, A.J.; Van Berkel, S.S.; Hatzakis, N.S.; Opsteen, J.A.; Van Delft, F.L.; Cornelissen, J.J.L.M.; Rowan, A.E.; Van Hest, J.C.M.; Rutjes, F.P.J.T.; Nolte, R.J.M. Preparation of Biohybrid Amphiphiles via the Copper Catalysed Huisgen [3 + 2] Dipolar Cycloaddition Reaction. Chem. Commun. 2005, 33, 4172–4174. [Google Scholar] [CrossRef]
- Presolski, S.I.; Hong, V.P.; Finn, M.G. Copper-Catalyzed Azide–Alkyne Click Chemistry for Bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Al-Oqail, M.M.; Farshori, N.N.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Saquib, Q.; Siddiqui, M.A.; Al-Khedhairy, A.A. Oxidative Stress Mediated Cytotoxicity, Cell Cycle Arrest, and Apoptosis Induced by Rosa Damascena in Human Cervical Cancer HeLa Cells. Oxid. Med. Cell. Longev. 2021, 2021, 6695634. [Google Scholar] [CrossRef] [PubMed]
- Jeyarani, S.; Vinita, N.M.; Puja, P.; Senthamilselvi, S.; Devan, U.; Velangani, A.J.; Biruntha, M.; Pugazhendhi, A.; Kumar, P. Biomimetic Gold Nanoparticles for Its Cytotoxicity and Biocompatibility Evidenced by Fluorescence-Based Assays in Cancer (MDA-MB-231) and Non-Cancerous (HEK-293) Cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111715. [Google Scholar] [CrossRef] [PubMed]
- Maini, L.; Braga, D.; Mazzeo, P.P.; Ventura, B. Polymorph and Isomer Conversion of Complexes Based on CuI and PPh 3 Easily Observed via Luminescence. Dalt. Trans. 2012, 41, 531–539. [Google Scholar] [CrossRef]
- Shi, Y.; Pierce, J.G. Synthesis of the 5,6-Dihydroxymorpholin-3-One Fragment of Monanchocidin A. Org. Lett. 2015, 17, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Azarakhsh, M.; Rumyantsev, A.M.; Lebedeva, M.A.; Lutova, L.A. Cytokinin Biosynthesis Genes Expressed during Nodule Organogenesis Are Directly Regulated by the KNOX3 Protein in Medicago Truncatula. PLoS ONE 2020, 15, e0232352. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis for the Separation of Proteins in the Range from 1 to 100 KDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Arndt, C.; Koristka, S.; Feldmann, A.; Bergmann, R.; Bachmann, M. Coomassie Brilliant Blue Staining of Polyacrylamide Gels. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2018; Volume 1853, pp. 27–30. ISBN 9781493987450. [Google Scholar]
- Langdon, S.P. Cancer Cell Culture; Humana Press: Totowa, NJ, USA, 2003; Volume 88, ISBN 1-59259-406-9. [Google Scholar]





| Compound | λabs, nm | ε | λex, nm | λem, nm | ΦF,%, THF | ΦF,%, H2O | τTΓΦ, ns | τH2O, ns | Δν, cm−1 | Δν, nm |
|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 258 | 27.570 | 258 | 492 | 60 | 27 | 7.7 | 4.8 | 18.434 | 234 |
| 5b | 255 | 27.570 | 255 | 447 | 60 | 23 | 5.4 | 4.6 | 16.844 | 192 |
| 5c | 307 | 20.610 | 306 | 514 | 47 | 22 | 5.6 | 3.1 | 13.224 | 208 |
| 5d | 295 | 40.050 | 294 | 429 | 19 | n.d. | 1.3 | n.d. | 10.703 | 135 |
| 5e | 270 | 34.690 | 285 | 395 | 40 | n.d. | 2.6 | n.d. | 9771 | 110 |
| 5f | 252 | 42.140 | 267 | 355 | 56 | n.d. | 1.5 | n.d. | 9284 | 88 |
| 5g | 277 | 36.420 | 277 | 353 | 7 | n.d. | 0.5 | n.d. | 7772 | 76 |
| 5h | 300 | 32.480 | 302 | 609 | 5 | 2 | 1.6 | 4.0, 14.0 | 16.692 | 307 |
| 5i | 306 | 49.870 | 299 | 588 | 5 | n.d. | 0.2, 1.8 | n.d. | 16.438 | 289 |
| 5j | 295 | 27.850 | 292 | 457 | 36 | n.d. | 4.2 | n.d. | 12.365 | 165 |
| 5k | 265 | 18.030 | 272 | 398 | n.d. | n.d. | n.d. | n.d. | 11.639 | 126 |
| 5l | 276 | 33.334 | 274 | 385 | 16 | n.d. | 0.7 | n.d. | 10.522 | 111 |
| 5m | 270 | 29.570 | 279 | 407 | 19 | n.d. | 1.3 | n.d. | 11.272 | 128 |
| Compound | λabs, nm | ε | λex, nm | λem, nm | ΦF,%, THF | ΦF,%, H2O | τTΓΦ, ns | τH2O, ns | Δν, cm−1 | Δν, nm |
|---|---|---|---|---|---|---|---|---|---|---|
 | 256 | 45.210 | 330 | 532 | 33 | 10 | 6.0 | 3.9 | 11.541 | 202 |
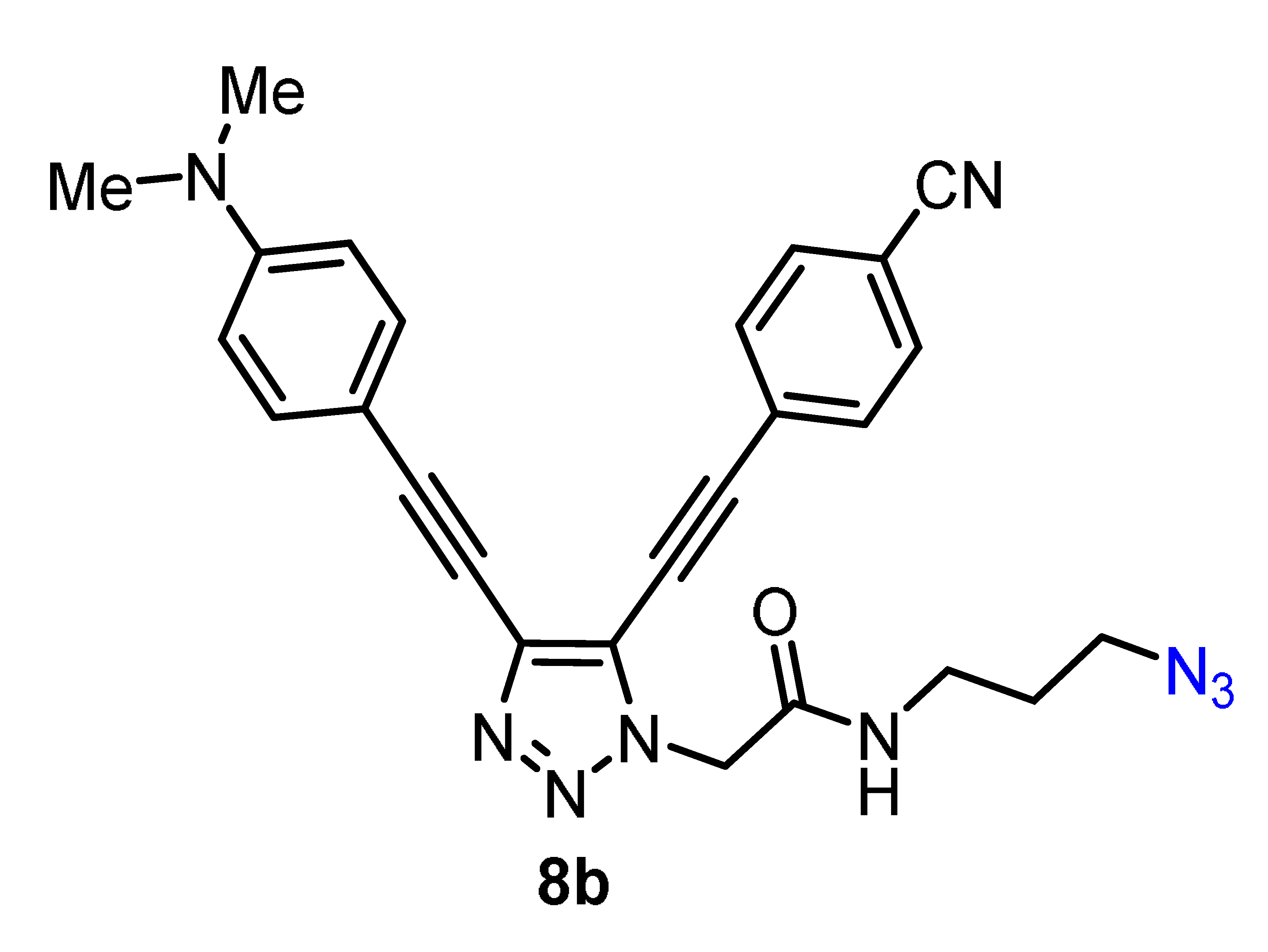 | 299 | 23.124 | 290 | 556 | 7.9 | 2.7 | n.d. | 2.1, 8.5 | 16.497 | 266 |
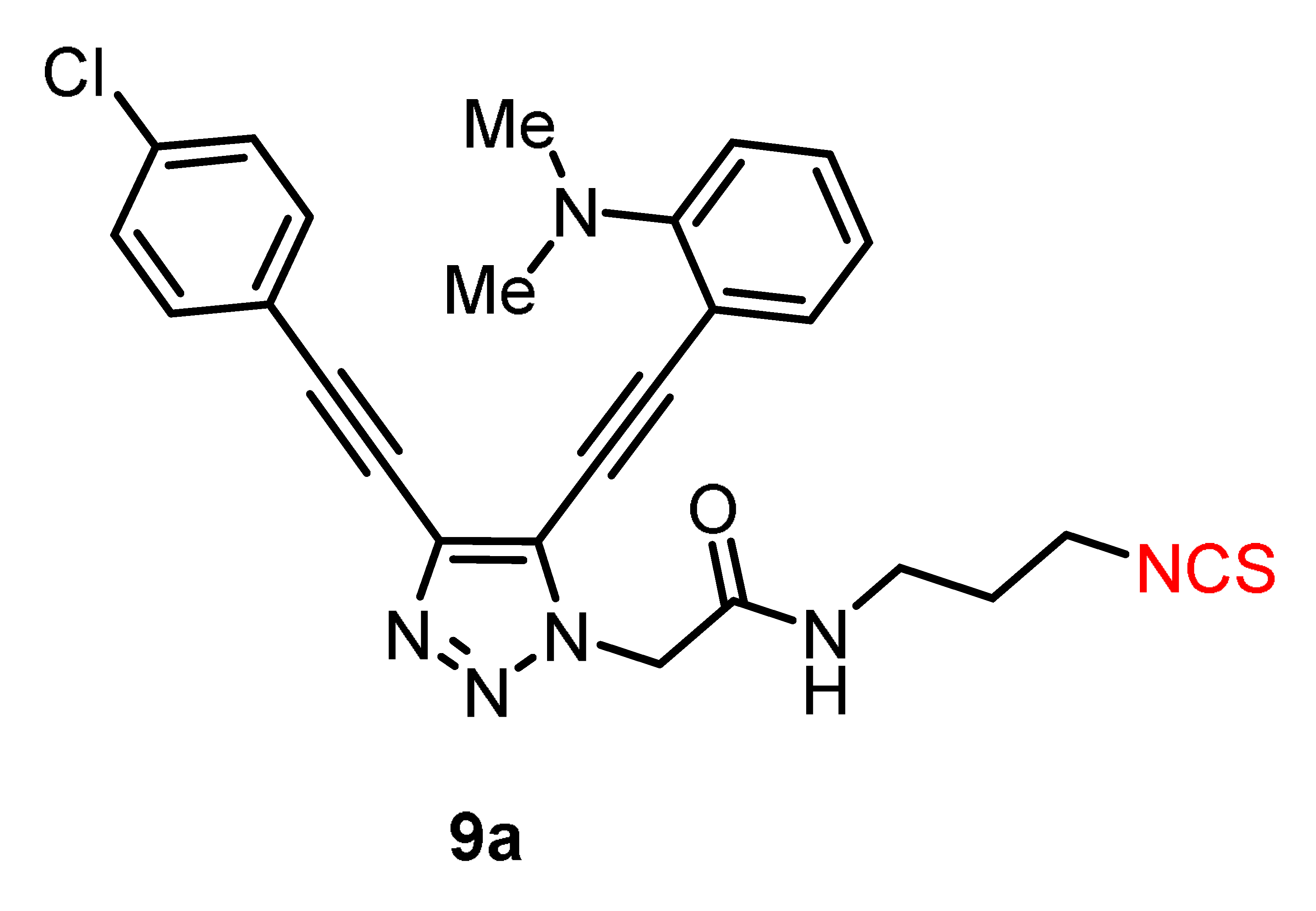 | 255 | 30.043 | 330 | 448 | 26 | 26 | 6.0 | 1.1 | 7981 | 118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govdi, A.I.; Tokareva, P.V.; Rumyantsev, A.M.; Panov, M.S.; Stellmacher, J.; Alexiev, U.; Danilkina, N.A.; Balova, I.A. 4,5-Bis(arylethynyl)-1,2,3-triazoles—A New Class of Fluorescent Labels: Synthesis and Applications. Molecules 2022, 27, 3191. https://doi.org/10.3390/molecules27103191
Govdi AI, Tokareva PV, Rumyantsev AM, Panov MS, Stellmacher J, Alexiev U, Danilkina NA, Balova IA. 4,5-Bis(arylethynyl)-1,2,3-triazoles—A New Class of Fluorescent Labels: Synthesis and Applications. Molecules. 2022; 27(10):3191. https://doi.org/10.3390/molecules27103191
Chicago/Turabian StyleGovdi, Anastasia I., Polina V. Tokareva, Andrey M. Rumyantsev, Maxim S. Panov, Johannes Stellmacher, Ulrike Alexiev, Natalia A. Danilkina, and Irina A. Balova. 2022. "4,5-Bis(arylethynyl)-1,2,3-triazoles—A New Class of Fluorescent Labels: Synthesis and Applications" Molecules 27, no. 10: 3191. https://doi.org/10.3390/molecules27103191
APA StyleGovdi, A. I., Tokareva, P. V., Rumyantsev, A. M., Panov, M. S., Stellmacher, J., Alexiev, U., Danilkina, N. A., & Balova, I. A. (2022). 4,5-Bis(arylethynyl)-1,2,3-triazoles—A New Class of Fluorescent Labels: Synthesis and Applications. Molecules, 27(10), 3191. https://doi.org/10.3390/molecules27103191







