Energetic Bio-Activation of Some Organic Molecules and Their Antioxidant Activity in the Pulp of the Moroccan Argan Tree «Argania spinosa L.»
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical and Functional Analysis
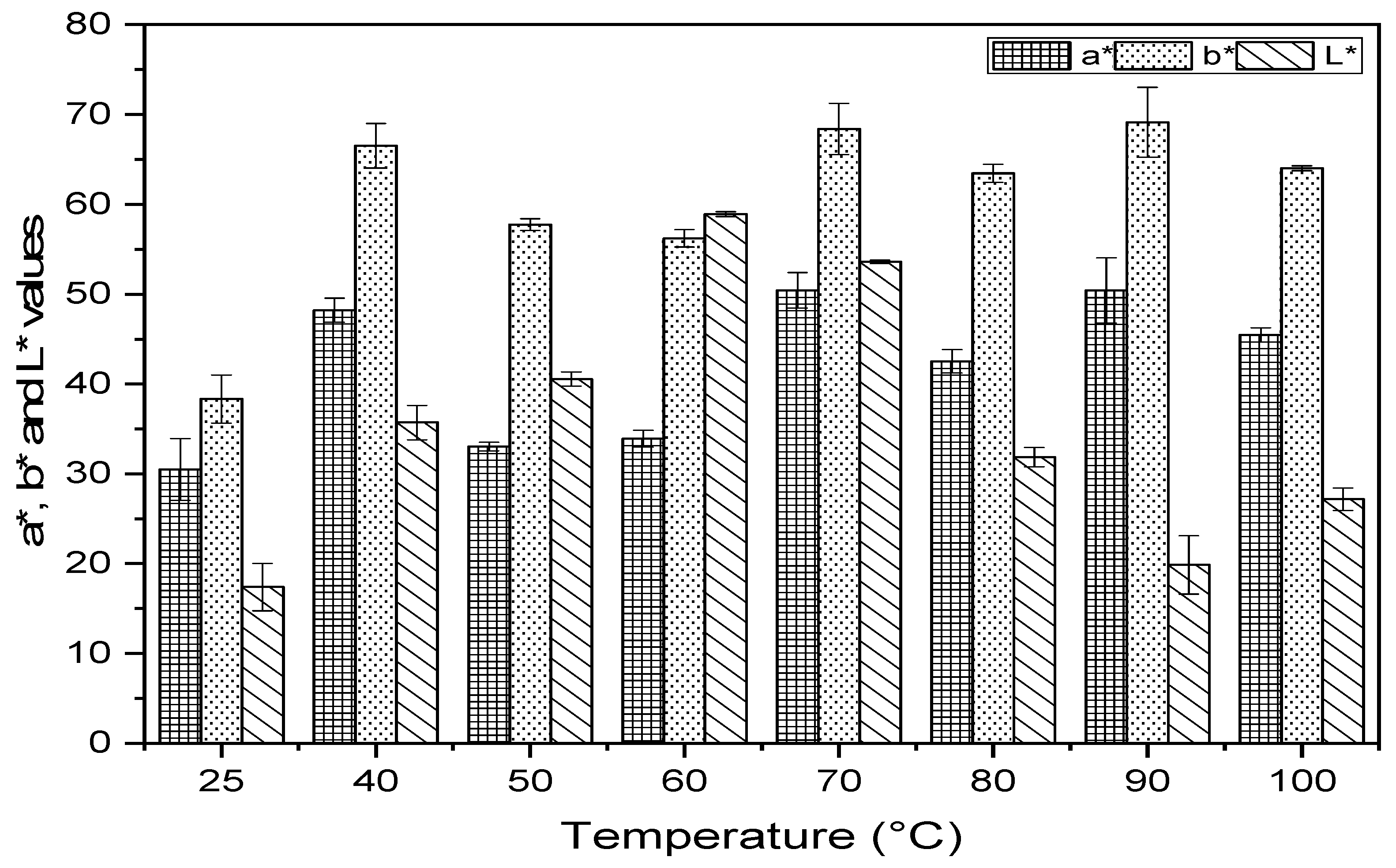
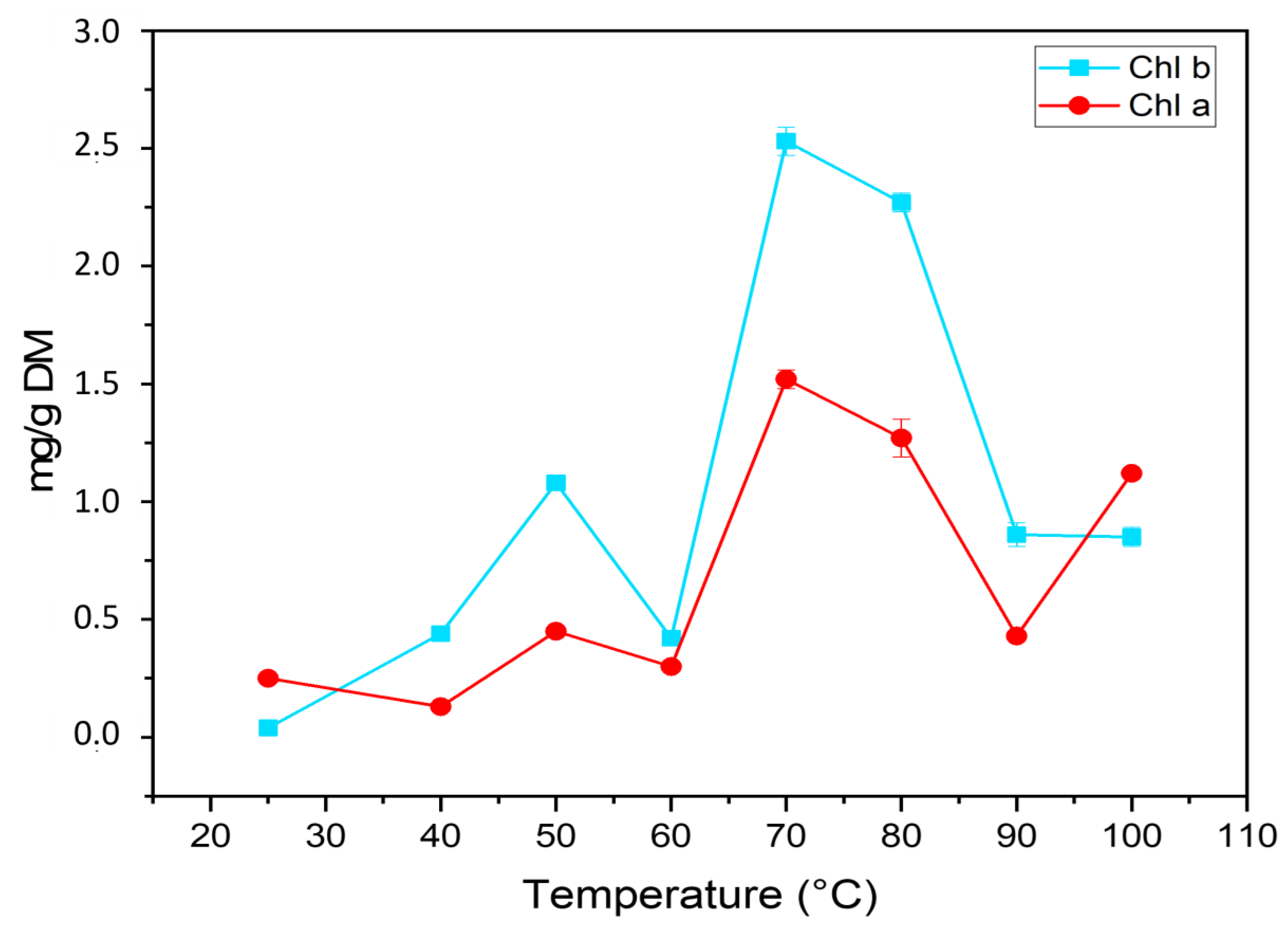
2.2. Determination of Color and Chlorophylls
2.3. Phenolic Analysis
2.3.1. Total Polyphenol Content, Total Flavonoid Content
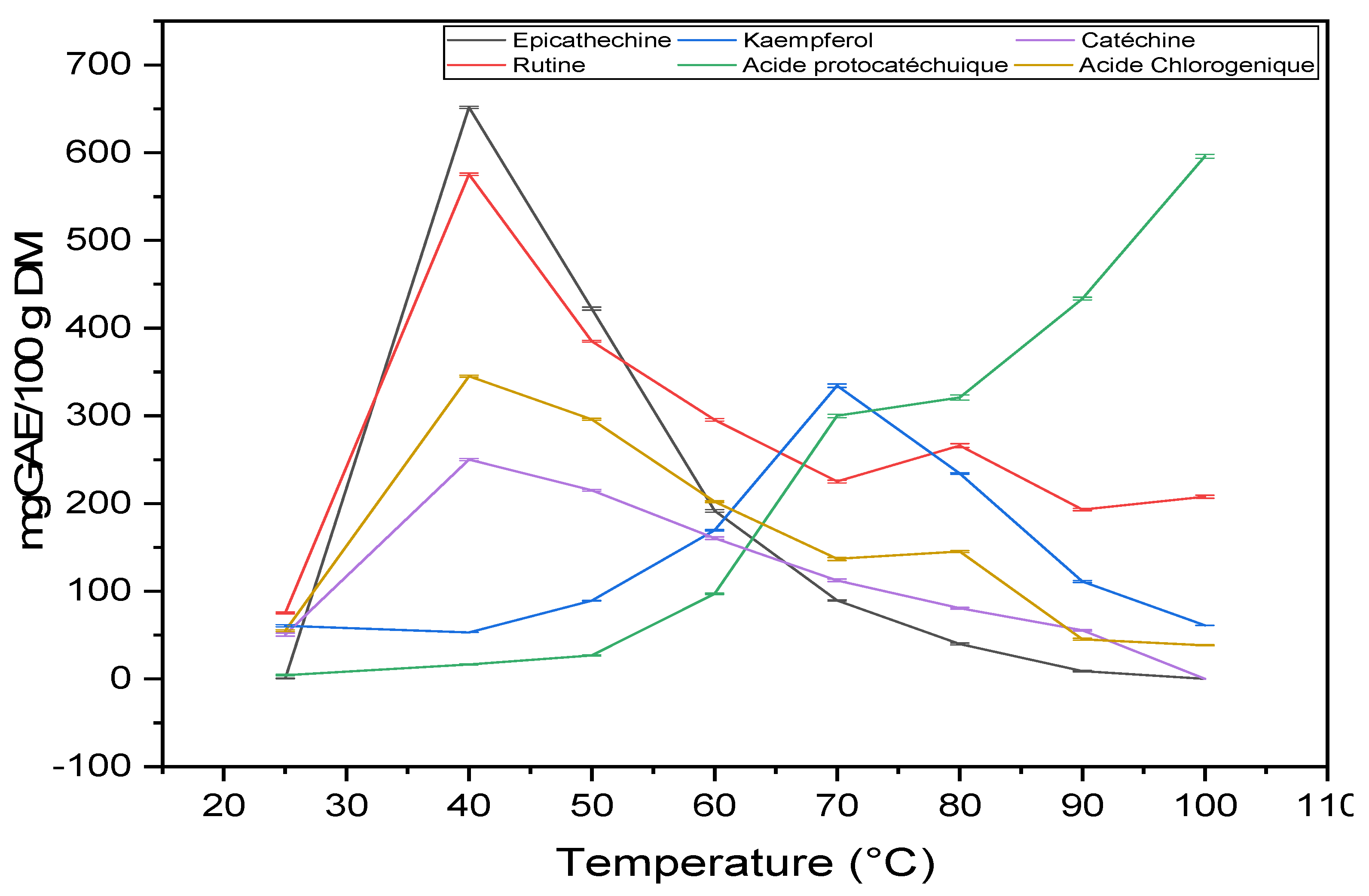
2.3.2. Molecular Analysis by HPLC
2.4. Antioxidant Activity
2.5. GC-MS Analysis
2.6. Principal Component Analysis (PCA)
3. Conclusions
4. Materials and Methods
4.1. Materials and Instruments Used
Plant Material
4.2. Methods
4.2.1. Functional Properties
4.2.2. Physicochemical Analysis
4.2.3. Total Polyphenol Content, Total Flavonoid Content and Antioxidant Activity
4.2.4. Molecular Analysis by HPLC
4.2.5. GC-MS Analysis
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| WAC | Water Absorption Capacity |
| LGC | Least Gelation Concentration |
| SC | Swelling Capacity |
| WSI | Water Solubility Index |
| OAC | Oil Absorption Capacity |
| TPC | Total Phenolic Compounds |
| TFC | Total Flavonoids Compounds |
| PPO | Polyphenoloxidase |
| AAO | Antioxydant Capacity |
| Ash | Ash content |
References
- Guinda, A.; Rada, M.; Delgado, T.; Castellano, J.M. Pentacyclic triterpenic acids from Argania spinosa L. J. Eur. Sci. Technol. Lipids 2010, 113, 231–237. [Google Scholar] [CrossRef]
- Kharbach, M.; Viaene, J.; Yu, H.; Kamal, R.; Marmouzi, I.; Bouklouze, A.; Heyden, Y.V. Secondary-metabolites fingerprinting of Argania spinosa kernels using liquid chromatography-mass spectrometry and chemometrics, for metabolite identification and quantification as well as for geographic classification. J. Chromatogr. A 2022, 1670, 462972. [Google Scholar] [CrossRef] [PubMed]
- Charrouf, Z.; Hilali, M.; Jauregui, O.; Soufiaoui, M.; Guillaume, D. Separation and characterization of phenolic compound in argan fruit pulp using liquid chromatography–negative electrospray ionization tandem mass spectroscopy. Food Chem. 2007, 100, 1398–1401. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Secondary metabolites of Argania spinosa (L.) skeels. Phytochem. Rev. 2002, 1, 345–354. [Google Scholar] [CrossRef]
- Madeleine, Z.; Anna, M. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruit: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, color and texture. Food Chem. 2016, 212, 671–680. [Google Scholar]
- Pokorny, J.; Schmidt, S. The impact of food processing in phytochemicals: The case of antioxidants. In Phytochemical Functional Foods; CRC Press: Boca Raton, FL, USA, 2003; pp. 298–314. [Google Scholar]
- Murcia, M.A.; López-Ayerra, B.; Martínez-Tomé, M.; García-Carmona, F. Effect of industrial processing on amino acid content of broccoli. J. Sci. Food Agric. 2001, 81, 1299–1305. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of of traditional and novel drying techniques and Its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Hamdi, I.; Kooli, S.; Elkhadraoui, A.; Azaizia, Z.; Abdelhamid, F.; Guizani, A. Experimental study and numerical modeling for drying grapes under solar greenhouse. Renew. Energy 2018, 127, 936–946. [Google Scholar] [CrossRef]
- Ouaabou, R.; Nabil, B.; Ouhammou, M.; Idlimam, A.; Lamharrar, A.; Ennahli, S.; Mahrouz, M. Impact of solar drying process on drying kinetics and bioactive profile of Moroccan sweet cherry. Renew. Energy 2020, 151, 908–918. [Google Scholar] [CrossRef]
- Lang, G.H.; Lindemann, I.S.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; Oliveira, M. Effects of drying temperature and longterm storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Shoko, T.; Shai, J.L.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Influence of different types of drying methods on color properties, phenolic metabolites and bioactivities of pumpkin leaves of var. Butternut squash (Cucurbita moschata Duchesne ex Poir. Front. Nutr. 2021, 8, 694649. [Google Scholar] [CrossRef] [PubMed]
- Djebli, A.; Hanini, S.; Badaoui, O.; Haddad, B.; Benhamou, A. Modeling and comparative analysis of solar drying behavior of potatoes. Renew. Energy 2020, 145, 1494–1506. [Google Scholar] [CrossRef]
- Ouhammou, M.; Eladnany, E.M.; Mourjane, A.; Jaouad, A.; Mahrouz, M. Impact of conventional drying an particle size on the physicochemical proprites of the powder of the Pea (Pisum sativum), the Bean (Aquadulce) and the Artichoke (Cymara scolymus) By-products. Food Technol. 2021, 19, 17–27. [Google Scholar]
- Lahsasni, S.; Kouhila, M.; Mahrouz, M.; Idlimam, A.; Jamali, A. Thin layer convective solar drying of prickly pear peel (Opuntia ficus indica). Energy Int. J. 2003, 29, 211–224. [Google Scholar] [CrossRef]
- Oufedjikh, H.; Mahrouz, M.; Amiot, M.J.; Lacroix, M. Effect of gamma irradiation on phenolics compounds and phénylalanine ammonia-lyase activity in relation to peel injury from peel of citrus clementina hort. Ex. tanaka. J. Agric. Food Chem. 2000, 32, 559–565. [Google Scholar] [CrossRef]
- Lahsasni, S.; Kouhila, M.; Mahrouz, M.; Ait Mohamed, L.; Agorram, B. Characteristic drying curve and mathematical modeling of thin-layer solar drying of prickly pear cladode (Opuntia ficus indica). J. Food Process Eng. 2003, 44, 923–936. [Google Scholar] [CrossRef]
- Hsu, S.H. Developing an index for online customer satisfaction: Adaptation of American customer satisfaction index. Expert Syst. Appl. 2008, 34, 3033–3042. [Google Scholar] [CrossRef]
- Simonen, P.P.; Gylling, H.; Miettinen, T.A. The distribution of squalene and non-cholesterol sterols in lipoproteins in type 2 diabetes. Atherosclerosis 2007, 194, 222–229. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water content, water activity, water structure and stability of foods. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Ahmed, J. Effect of particle size and temperature on rheology and creep behavior of barley β-d-glucan concentrate paste. Carbohydr. Polym. 2014, 111, 89–100. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Córdoba-Díaz, D.; Sánchez-Mata, M.C.; Diez-Marques, C.; Goni, I. Effect of boiling on nutritional, antioxidant and physicochemical characteristics of cladodes (Opuntia ficus-indica). LWT—Food Sci. Technol. 2013, 51, 296–302. [Google Scholar] [CrossRef]
- Arslan, D.; Musa Özcan, M. Evaluation of drying methods with respect to drying kinetics, mineral content and colour characteristics of rosemary leaves. Energy Convers. Manag. 2008, 49, 1258–1264. [Google Scholar] [CrossRef]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization and functional properties of chlorophylls, pheophytins and Zn- pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Li, J.; Zhong, L.; Cheng, H.; Ma, Q. Immobilized polyphenol oxidase: Preparation, optimization and oxidation of phenolic compounds. Int. J. Biol. Macromol. 2020, 160, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, L.; Pesquet, E. Phenoloxidases in plants: How structural diversity enables functional specificity. Front. Plant Sci. 2021, 12, 75460. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Razboršek, M.I.; Ivanović, M.; Kolar, M. Validated stability-indicating GC-MS method for characterization of forced degradation products of trans-caffeic acid and trans-ferulic acid. Molecules 2021, 26, 2475. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, B.; Vohra, R.M.; Nandanwar, H.; Sharma, P.; Gupta, K.G.; Sobti, R.C. Rapid degradation of ferulic acid via 4-vinylguaiacol and vanillin by a newly isolated strain of Bacillus coagulans. J. Biotechnol. 2000, 80, 195–202. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; San Cheang, W.; Liu, B.; Zhao, K.; Miao, S.; Rivière, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Bentabet, N.; Boucherit-Otmani, Z.; Boucherit, K. Chemical composition and antioxidant activity of organic extracts of Fredolia aretioides roots from the Bechar region in Algeria. Phytotherapy 2014, 12, 364–371. [Google Scholar] [CrossRef]
- Bursa, E.; Aras, A.; Kılıç, O.; Buldurun, K. Chemical constituent and radical scavenging antioxidant activity of Anthemis kotschyana Boiss. Nat. Prod. Res. 2021, 35, 4794–4797. [Google Scholar] [CrossRef] [PubMed]
- Aras, A.; Bursal, E.; Alan, Y.; Turkan, F.; Alkan, H.; Kilic, O. Polyphenolic content, antioxidant potential and antimicrobial activity of Satureja boissieri. Iran. J. Chem. Chem. Eng. 2018, 37, 209–219. [Google Scholar]
- Santos, F.A.; Frota, J.; Arruda, B.; Melo, T.; Castro Brito, G.; Chaves, M.; Rao, V. Antihyperglycemic and hypolipidemic effects of α,β-amyrin, a triterpenoid mixture from Protium heptaphyllum in the mouse. Lipids Health Dis. 2012, 11, 1–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J. Food Sci. Technol. 2014, 52, 3681–3688. [Google Scholar] [CrossRef] [PubMed]
- Baljeet, S.Y.; Ritika, B.Y.; Roshan, L.Y. Studies on functional properties and incorporation of buckwheat flour for biscuit making. Int. Food Res. J. 2010, 17, 1067–1076. [Google Scholar]
- Adebowale, Y.A.; Adeyemi, I.A.; Oshodi, A.A. Functional and physicochemical properties of flours of six Mucuna species. Afr. J. Biotechnol. 2005, 4, 1461–1468. [Google Scholar]
- Ayadi, M.A.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus indica as a source of dietary fiber: Effect on dough characteristics and cake making. Ind. Crops Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC International, 18th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 2007. [Google Scholar]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Diaz, M.S.; Agüero, A.R.; Guéraud, F.; Nègre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compost. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of polyphenols and vitamin C in plant-derived products. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Nabil, B.; Ouaabou, R.; Ouhammou, M.; Saadouni, L.; Mahrouz, M. Impact of particle size on functional, physicochemical properties and antioxidant activity of cladode powder (Opuntia ficus-indica). J. Food Sci. Technol. 2020, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.; Nagaty, M.; Salman, M.; Bazaid, S. Phytochemical, nutritional and antioxidant properties of two prickly pear (Opuntia ficus indica Mill.) cultivars growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef]
- Boutakiout, A.; Elothmani, D.; Hanine, H. Effects of different harvesting seasons on antioxidant activity and phenolic content of prickly pear cladode juice. J. Saudi Soc. Agric. Sci. 2018, 17, 471–480. [Google Scholar] [CrossRef]
- Puigventós, L.; Navarro, M.; Alechaga, E.; Nunez, O.; Saurina, J.; Hernandez-Cassou, S.; Puignou, L. Determination of polyphenolic profiles by liquid chromatography-electrospray-tandem mass spectrometry for authentication of fruit extracts. Anal. Bioanal. Chem. 2015, 407, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranchant, J. Chromatographie en Phase Gazeuse, 3rd ed.; Masson: Paris, France, 1982. [Google Scholar]



| Temperature (°C) | 25 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|
| Moisture (%) | 14.3 ± 0.25 | 6.73 ± 0.17 | 5.64 ± 0.30 | 5.25 ± 0.31 | 5.00 ± 0.08 | 4.87 ± 0.21 | 3.74 ± 0.30 | 3.14 ± 0.26 |
| Ash (%) | 4.08 ± 0.04 | 4.23 ± 0.06 | 4.30 ± 0.21 | 4.15 ± 0.13 | 4.25 ± 0.10 | 4.16 ± 0.10 | 4.10 ± 0.09 | 4.20 ± 0.06 |
| pH | 4.12 ± 0.05 | 4.12 ± 0.10 | 4.11 ± 0.18 | 4.01 ± 0.13 | 4.16 ± 0.19 | 4.19 ± 0.09 | 4.19 ± 0.18 | 4.09 ± 0.11 |
| Soluble solids (°Brix) | 6.22 ± 0.18 | 6.07 ± 011 | 5.07 ± 0.12 | 3.23 ± 0.12 | 3.87 ± 0.26 | 4.17 ± 0.12 | 2.13 ± 0.12 | 5.33 ± 0.15 |
| Titraible acidity (%citric acid) | 0.34 ± 0.02 | 0.30 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.29 ± 0.01 | 0.19 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 |
| WAC (%) | 12.17 ± 0.75 | 11.17 ± 1.04 | 11.93 ± 1.01 | 11.43 ± 0.75 | 9.21 ± 1.06 | 9.40 ± 0.53 | 10.83 ± 0.76 | 11.41 ± 0.52 |
| OAC (%) | 12.37 ± 1.59 | 11.4 ± 0.46 | 12.1 ± 0.17 | 13.2 ± 0.45 | 12.6 ± 0.46 | 11.9 ± 0.07 | 11.86 ± 0.57 | 10.97 ± 0.50 |
| LGC (%) | 16.00 ± 0.00 | 16.00 ± 0.00 | 18.00 ± 0.00 | 16.00 ± 0.00 | 16.00 ± 0.00 | 18.00 ± 0.00 | 18.00 ± 0.00 | 18.00 ± 0.00 |
| WSI (%) | 54.42 ± 0.49 | 55.33 ± 0.44 | 50.55 ± 0.39 | 51.36 ± 0.47 | 52.63 ± 0.52 | 52.12 ± 0.24 | 47.98 ± 0.40 | 46.39 ± 0.37 |
| Temperature (°C) | RT (min) | 25 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|
| Protocatechuic acid | 6.48 | 4.4 ± 0.9 | 16.6 ± 0.6 | 26.8 ± 0.7 | 96.9 ± 0.9 | 299.9 ± 2.1 | 320. ± 2.85 | 433.6 ± 1.6 | 595.8 ± 2.1 |
| Caffeic acide | 14.19 | ND | ND | ND | ND | ND | ND | ND | ND |
| Ferulic acid | 19.11 | 5.3 ± 0.4 | 24.6 ± 0.1 | 28.16± 0.2 | 33.6 ± 0.1 | ND | ND | 6.3 ± 0.2 | 5.3 ± 0.8 |
| Hesperidin | 20.64 | ND | ND | ND | ND | ND | ND | ND | ND |
| Salicylic acid | 21.76 | ND | ND | ND | ND | ND | ND | ND | ND |
| Vanillic acid | 13.91 | ND | ND | ND | ND | ND | ND | ND | ND |
| Catechin | 10.97 | 50.5 ± 1.6 | 250.1 ± 1.2 | 215.0 ± 1.0 | 160.5 ± 1.5 | 112.5 ± 1.6 | 80.5 ± 0.1 | 55.3 ± 0.9 | ND |
| Chorogenic acid | 12.07 | 54.9 ± 0.9 | 345.3 ± 1.1 | 296.0 ± 1. | 202.1 ± 0.9 | 136.8 ± 1.9 | 145.3 ± 1.1 | 45.3 ± 1.1 | 38.4 ± 0.6 |
| Epicathechin | 13.65 | 0.45 ± 0.3 | 651.6 ± 1.2 | 422.1 ± 1.6 | 191.6 ± 1.5 | 89.5 ± 0.6 | 39.8 ± 0.1 | 8.9 ± 0.8 | ND |
| Vanillin | 15.33 | 5.98 ± 0.5 | ND | ND | ND | ND | 3.1 ± 0.2 | 3.5 ± 0.2 | 2.0 ± 0.1 |
| p-Coumaric acid | 18.54 | ND | ND | ND | ND | ND | ND | ND | ND |
| Sinapic acid | 19.12 | ND | ND | ND | 22.8 ± 0.6 | 33.7 ± 0.4 | 13.9 ± 0.6 | ND | ND |
| Naringin | 20.49 | ND | ND | ND | ND | ND | ND | ND | ND |
| Rutin | 21.69 | 75.3 ± 1.02 | 575.5 ± 1.3 | 385.2 ± 1.0 | 295.5 ± 1.4 | 225.2 ± 1.5 | 266.1 ± 2.1 | 193.1 ± 1.4 | 207.7 ± 1.8 |
| Quercetin | 26.61 | ND | 47.0 ± 0.3 | 36.8 ± 0.3 | 29.0 ± 0.6 | 16.4 ± 0.4 | 6.5 ± 0.9 | 2.4 ± 0.8 | ND |
| Kaempferol | 27.56 | 60.6± 1.1 | 53.0 ± 0.1 | 89.3 ± 0.2 | 169.5 ± 0.7 | 334.4 ± 2.0 | 234.4 ± 1.0 | 111.1 ± 1.2 | 61.1 ± 0.1 |
| Total | 257.6 | 1963.7 | 1548.6 | 1201.6 | 1248.4 | 1110.6 | 859.7 | 910.5 |
| Compound | RT (min) | Mol. Formula | 25 °C | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | 100 °C |
|---|---|---|---|---|---|---|---|---|---|---|
| (e)-3(10)-caren-4-ol (%) | 6.30 | C10H16O | 0.38 ± 0.02 | ND | ND | ND | ND | ND | ND | ND |
| Cis-p-mentha-1(7),8-dien-2-ol (%) | 7.82 | C10H16O | 0.03 ± 0.01 | 0.41 ± 0.02 | 0.02 ± 0.00 | ND | ND | 0.36 ± 0.02 | 0.39 ± 0.04 | 0.36 ± 0.07 |
| Retinal (%) | 11.20 | C20H28O | 4.43 ± 0.03 | ND | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.09 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Lycophyll (%) | 22.77 | C40H56O2 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 |
| Androstatriene, 3-hydroxy-17-oxo (%) | 46.26 | C19H24O2 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | ND | ND | 0.02 ± 0.00 | 0.02 ± 0.01 |
| Carotene, 3,4-didehydro-1,2-dihydro-1-m Ethoxy (%) | 14.54 | C41H58O | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.2 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.001 |
| Olean-12-en-3-ol, acetate (%) | 49.82 | C32H52O2 | ND | ND | ND | 1.55 ± 0.09 | 3.1 ± 0.23 | 4.32 ± 0.56 | 1.16 ± 0.11 | 1.18 ± 0.12 |
| Amyrin (%) | 49.78 | C30H50O | ND | ND | 0.15 ± 0.01 | 0.18 ± 0.03 | 0.2 ± 0.05 | 0.21 ± 0.03 | 0.08 ± 0.01 | 0.04 ± 0.00 |
| 2-Hydroxychalcone (%) | 34.13 | C15H12O2 | ND | ND | 0.03 ± 0.00 | ND | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Ethyl iso-allocholate (%) | 21.88 | C26H44O5 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| Spirost-8-en-11-one, 3-hydroxy (%) | 51.78 | C27H40O4 | 0.03 ± 0.00 | 0.01 ± 0.00 | ND | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.01 | 0.03 ± 0.00 |
| Betulin (%) | 28.91 | C30H50O2 | ND | 0.06 ± 0.01 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.00 |
| Lupeol (%) | 45.97 | C30H50O | ND | ND | ND | 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Octamethyl-docosahydropicene-3,13-diol (%) | 46.13 | C30H52O2 | ND | ND | ND | 0.07 ± 0.01 | 0.01 ± 0.00 | ND | 0.02 ± 0.00 | 0.06 ± 0.01 |
| Betulinaldehyde (%) | 46.28 | C30H48O2 | ND | ND | ND | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.08 ± 0.01 | 0.04 ± 0.00 |
| Carbenoxolone (%) | 46.32 | C34H50O7 | ND | ND | ND | ND | 0.07 ± 0.01 | ND | ND | 0.07 ± 0.01 |
| Astaxanthin (%) | 14.05 | C31H50O3 | ND | ND | 0.04 ± 0.00 | ND | 0.02 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 |
| Urs-12-en-28-oic acid, 3-hydroxy-, methyl ester (%) | 46.24 | C30H50O2 | ND | ND | ND | 0.05 ± 0.01 | 0.03 ± 0.00 | 0.07 ± 0.01 | 0.06 ± 0.00 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourjane, A.; Hanine, H.; El Adnany, E.M.; Ouhammou, M.; Hidar, N.; Nabil, B.; Boumendjel, A.; Bitar, K.; Mahrouz, M. Energetic Bio-Activation of Some Organic Molecules and Their Antioxidant Activity in the Pulp of the Moroccan Argan Tree «Argania spinosa L.». Molecules 2022, 27, 3329. https://doi.org/10.3390/molecules27103329
Mourjane A, Hanine H, El Adnany EM, Ouhammou M, Hidar N, Nabil B, Boumendjel A, Bitar K, Mahrouz M. Energetic Bio-Activation of Some Organic Molecules and Their Antioxidant Activity in the Pulp of the Moroccan Argan Tree «Argania spinosa L.». Molecules. 2022; 27(10):3329. https://doi.org/10.3390/molecules27103329
Chicago/Turabian StyleMourjane, Ayoub, Hafida Hanine, El Mustapha El Adnany, Mourad Ouhammou, Nadia Hidar, Bouchra Nabil, Ahcène Boumendjel, Khalid Bitar, and Mostafa Mahrouz. 2022. "Energetic Bio-Activation of Some Organic Molecules and Their Antioxidant Activity in the Pulp of the Moroccan Argan Tree «Argania spinosa L.»" Molecules 27, no. 10: 3329. https://doi.org/10.3390/molecules27103329
APA StyleMourjane, A., Hanine, H., El Adnany, E. M., Ouhammou, M., Hidar, N., Nabil, B., Boumendjel, A., Bitar, K., & Mahrouz, M. (2022). Energetic Bio-Activation of Some Organic Molecules and Their Antioxidant Activity in the Pulp of the Moroccan Argan Tree «Argania spinosa L.». Molecules, 27(10), 3329. https://doi.org/10.3390/molecules27103329







