Quantification of Aluminum Gallium Arsenide (AlGaAs) Wafer Plasma Using Calibration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS)
Abstract
1. Introduction
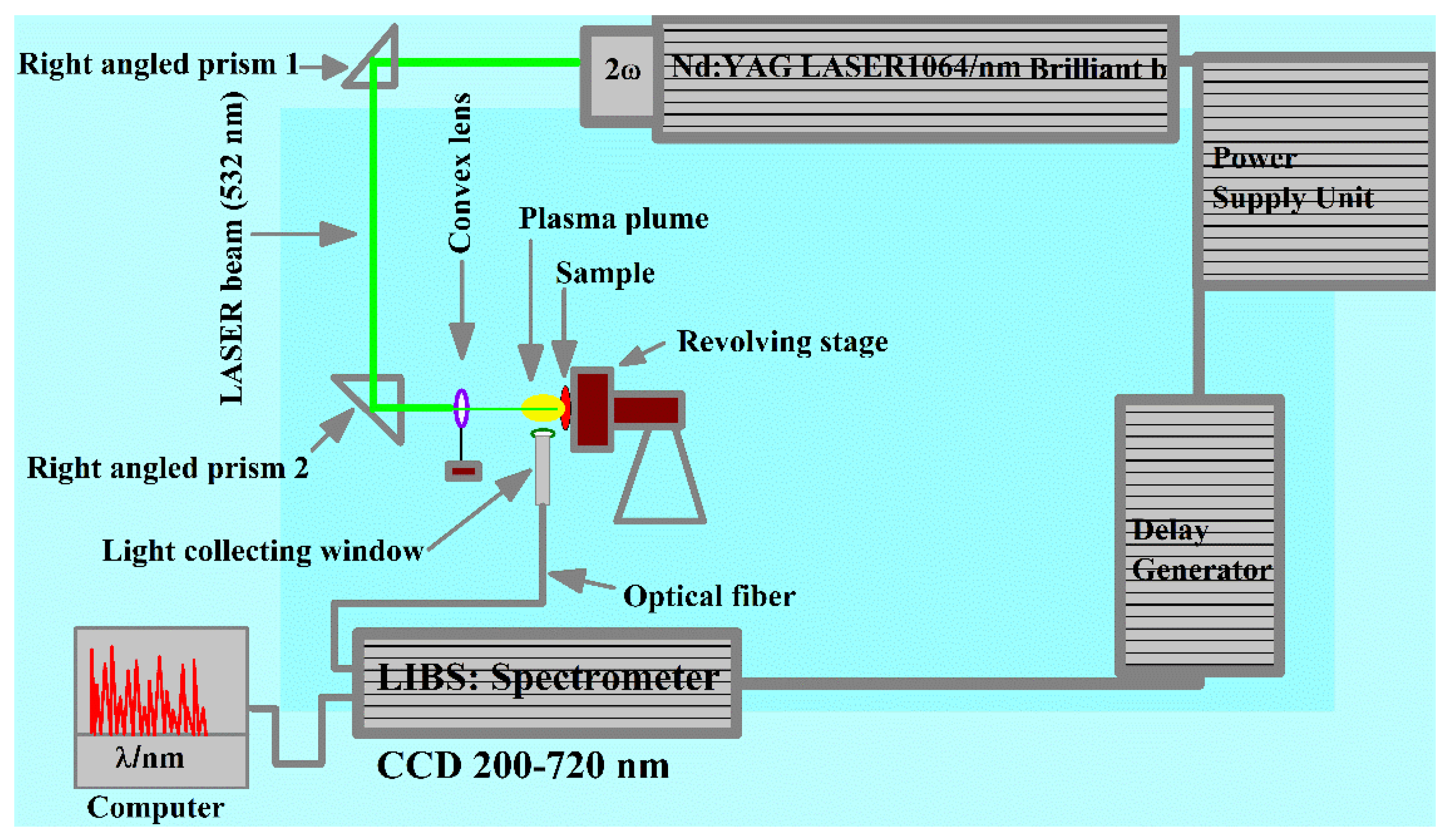
2. Experimental Setup
3. Material
4. Results and Discussions
4.1. LIBS Emission Studies
4.2. Plasma Temperature (Te)
4.3. Plasma Electron Number Density (Ne)
4.4. Local Thermodynamical Equilibrium (LTE)
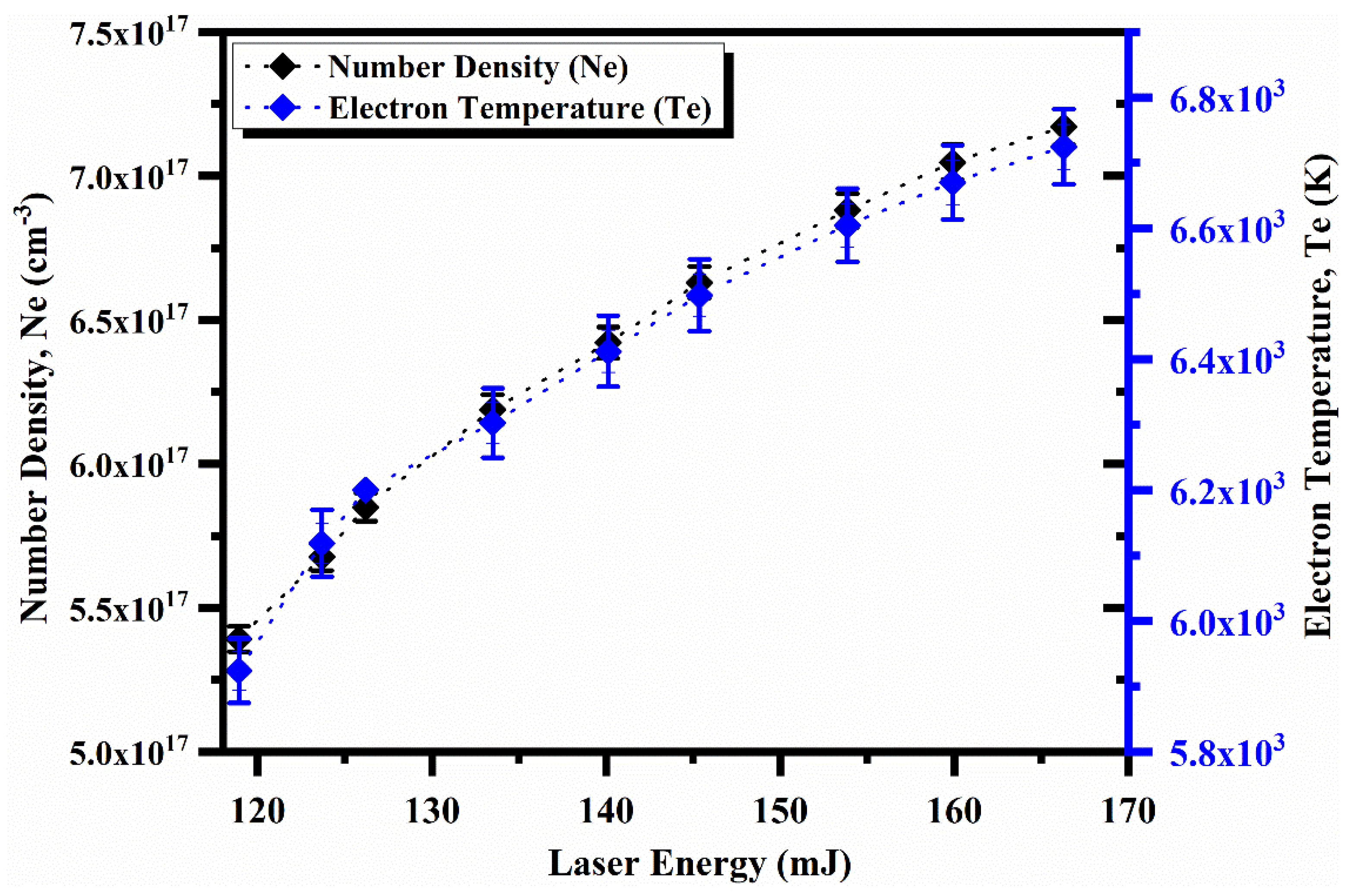
4.5. Laser Irradiance and Spatial Dependence on the Plasma Parameters
5. Chemical Composition by CF-LIBS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karpiuk, U.V.; Al Azzam, K.M.; Abudayeh, Z.H.M.; Kislichenko, V.; Naddaf, A.; Cholak, I.; Yemelianova, O. Qualitative and quantitative content determination of macro-minor elements in Bryonia alba L. roots using flame atomic absorption spectroscopy technique. Adv. Pharm. Bull. 2016, 6, 285. [Google Scholar] [CrossRef] [PubMed]
- Lagalante, A.F. Atomic absorption spectroscopy: A tutorial review. Appl. Spectrosc. Rev. 2004, 34, 173–189. [Google Scholar] [CrossRef]
- Akpinar-Bayizit, A.; Turan, M.A.; Yilmaz-Ersan, L.; Taban, N. Inductively coupled plasma optical-emission spectroscopy determination of major and minor elements in vinegar. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 64–68. [Google Scholar]
- Pröfrock, D.; Prange, A. Inductively coupled plasma-mass spectrometry (ICP-MS) for quantitative analysis in environmental and life sciences: A review of challenges, solutions, and trends. Appl. Spectrosc. 2012, 66, 843–868. [Google Scholar] [CrossRef] [PubMed]
- Sándor, Z.; Tölgyesi, S.; Gresits, I.; Káplán-Juhász, M. Qualitative and quantitative analysis of medieval silver coins by energy dispersive X-ray fluorescence method. J. Radioanal. Nucl. Chem. 2000, 246, 385–389. [Google Scholar] [CrossRef]
- Singh, J.P.; Thakur, S.N. (Eds.) Laser-Induced Breakdown Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Akhtar, M.; Ahmed, N.; Mahmood, S.; Jabbar, A.; Ahmed, R.; Umar, Z.A.; Iqbal, J.; Baig, M.A. Elemental analysis of cement and its components by laser-induced breakdown spectroscopy (LIBS) and laser ablation time of flight mass spectrometry (LA-TOF-MS). Anal. Lett. 2022, 55, 904–916. [Google Scholar] [CrossRef]
- Qasim, M.; Anwar-ul-Haq, M.; Shah, A.; Afgan, M.S.; Haq, S.U.; Khan, R.A.; Baig, M.A. Self-absorption effect in calibration-free laser-induced breakdown spectroscopy: Analysis of mineral profile in Maerua oblongifolia plant. Microchem. J. 2022, 175, 107106. [Google Scholar] [CrossRef]
- Ahmed, N.; Shahida, S.; Kiani, S.M.; Razzaq, M.I.; Hameed, M.U.; Iqbal, S.M.Z.; Abbasi, S.A.; Rafique, M.; Baig, M.A. Analysis of an Iron-Copper Alloy by Calibration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS) and Inductively Coupled Plasma–Mass Spectrometry (ICP-MS). Anal. Lett. 2022, 1–12. [Google Scholar] [CrossRef]
- Iqbal, S.M.Z.; Uddin, Z.; Umar, Z.A.; Ahmed, N.; Ahmed, R.; Baig, M.A. Analysis of Lakhra Coal by Calibration Free Laser-Induced Breakdown Spectroscopy (CF-LIBS) and Comparison of Self-Absorption Correction Procedures. Anal. Lett. 2022, 55, 11–23. [Google Scholar] [CrossRef]
- Iqbal, J.; Asghar, H.; Shah, S.K.H.; Naeem, M.; Abbasi, S.A.; Ali, R. Elemental analysis of sage (herb) using calibration-free laser-induced breakdown spectroscopy. Appl. Opt. 2020, 59, 4927–4932. [Google Scholar] [CrossRef]
- Fabre, C. Advances in Laser-Induced Breakdown Spectroscopy analysis for geology: A critical review. Spectrochim. Acta Part B At. Spectrosc. 2020, 166, 105799. [Google Scholar] [CrossRef]
- Bhatt, C.R.; Jain, J.C.; Goueguel, C.L.; McIntyre, D.L.; Singh, J.P. Determination of rare earth elements in geological samples using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2018, 72, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Noll, R.; Sturm, V.; Aydin, Ü.; Eilers, D.; Gehlen, C.; Höhne, M.; Lamott, A.; Makowe, J.; Vrenegor, J. Laser-induced breakdown spectroscopy—From research to industry, new frontiers for process control. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1159–1166. [Google Scholar] [CrossRef]
- Busser, B.; Moncayo, S.; Coll, J.L.; Sancey, L.; Motto-Ros, V. Elemental imaging using laser-induced breakdown spectroscopy: A new and promising approach for biological and medical applications. Coord. Chem. Rev. 2018, 358, 70–79. [Google Scholar] [CrossRef]
- Villas-Boas, P.R.; Romano, R.A.; de Menezes Franco, M.A.; Ferreira, E.C.; Ferreira, E.J.; Crestana, S.; Milori, D.M.B.P. Laser-induced breakdown spectroscopy to determine soil texture: A fast analytical technique. Geoderma 2016, 263, 195–202. [Google Scholar] [CrossRef]
- Miziolek, A.W.; Palleschi, V.; Schechter, I. (Eds.) Laser Induced Breakdown Spectroscopy; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Yalçın, Ş.; Örer, S.; Turan, R. 2-D analysis of Ge implanted SiO2 surfaces by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1130–1138. [Google Scholar] [CrossRef][Green Version]
- Hu, S.; Ribeiro, E.L.; Davari, S.A.; Tian, M.; Mukherjee, D.; Khomami, B. Hybrid nanocomposites of nanostructured Co3O4 interfaced with reduced/nitrogen-doped graphene oxides for selective improvements in electrocatalytic and/or supercapacitive properties. RSC Adv. 2017, 7, 33166–33176. [Google Scholar] [CrossRef]
- Davari, S.A.; Hu, S.; Ribeiro, E.L.; Mukherjee, D. Rapid elemental composition analysis of intermetallic ternary nanoalloys using calibration-free quantitative Laser Induced Breakdown Spectroscopy (LIBS). MRS Adv. 2017, 2, 3371–3376. [Google Scholar] [CrossRef][Green Version]
- Davari, S.A.; Hu, S.; Mukherjee, D. Calibration-free quantitative analysis of elemental ratios in intermetallic nanoalloys and nanocomposites using Laser Induced Breakdown Spectroscopy (LIBS). Talanta 2017, 164, 330–340. [Google Scholar] [CrossRef]
- Mao, X.; Zeng, X.; Wen, S.B.; Russo, R.E. Time-resolved plasma properties for double pulsed laser-induced breakdown spectroscopy of silicon. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 960–967. [Google Scholar] [CrossRef]
- Milan, M.; Lucena, P.; Cabalin, L.M.; Laserna, J.J. Depth profiling of phosphorus in photonic-grade silicon using laser-induced breakdown spectrometry. Appl. Spectrosc. 1998, 52, 444–448. [Google Scholar] [CrossRef]
- Ji, Z.G.; Xi, J.H.; Mao, Q.N. Determination of oxygen concentration in heavily doped silicon wafer by laser induced breakdown spectroscopy. J. Inorg. Mater. 2010, 25, 893–896. [Google Scholar] [CrossRef]
- Davari, S.A.; Hu, S.; Pamu, R.; Mukherjee, D. Calibration-free quantitative analysis of thin-film oxide layers in semiconductors using Laser Induced Breakdown Spectroscopy (LIBS). J. Anal. At. Spectrom. 2017, 32, 1378–1387. [Google Scholar] [CrossRef]
- Syum, Z.; Woldeghebriel, H. The structure and electronic properties of (GaAs)n and Al/In-doped (GaAs)n (n = 2–20) clusters. Comput. Theor. Chem. 2014, 1048, 7–17. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST Atomic Spectra Database Lines Form. Available online: https://physics.nist.gov/PhysRefData/ASD/lines_form.html (accessed on 2 May 2022). [CrossRef]
- Abbass, Q.; Ahmed, N.; Ahmed, R.; Baig, M.A. A comparative study of calibration free methods for the elemental analysis by laser induced breakdown spectroscopy. Plasma Chem. Plasma Process. 2016, 36, 1287–1299. [Google Scholar] [CrossRef]
- Unnikrishnan, V.K.; Mridul, K.; Nayak, R.; Alti, K.; Kartha, V.B.; Santhosh, C.; Gupta, G.P.; Suri, B.M. Calibration-free laser-induced breakdown spectroscopy for quantitative elemental analysis of materials. Pramana 2012, 79, 299–310. [Google Scholar] [CrossRef]
- McWhirter, R.W.P. Plasma Diagnostic Techniques; Academic Press: New York, NY, USA, 1965. [Google Scholar]
- Borgia, I.; Burgio, L.M.; Corsi, M.; Fantoni, R.; Palleschi, V.; Salvetti, A.; Squarcialupi, M.C.; Tognoni, E. Self-calibrated quantitative elemental analysis by laser-induced plasma spectroscopy: Application to pigment analysis. J. Cult. Herit. 2000, 1, S281–S286. [Google Scholar] [CrossRef]
- Ciucci, A.; Corsi, M.; Palleschi, V.; Rastelli, S.; Salvetti, A.; Tognoni, E. New procedure for quantitative elemental analysis by laser-induced plasma spectroscopy. Appl. Spectrosc. 1999, 53, 960–964. [Google Scholar] [CrossRef]
- Griem, H.R. Principles of Plasma Spectroscopy; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Fayyaz, A.; Liaqat, U.; Adeel Umar, Z.; Ahmed, R.; Aslam Baig, M. Elemental Analysis of Cement by Calibration-Free Laser Induced Breakdown Spectroscopy (CF-LIBS) and Comparison with Laser Ablation–Time-of-Flight–Mass Spectrometry (LA-TOF-MS), Energy Dispersive X-ray Spectrometry (EDX), X-ray Fluorescence Spectroscopy (XRF), and Proton Induced X-ray Emission Spectrometry (PIXE). Anal. Lett. 2019, 52, 1951–1965. [Google Scholar]
- Fujimoto, T.; McWhirter, R.W.P. Validity criteria for local thermodynamic equilibrium in plasma spectroscopy. Phys. Rev. A 1990, 42, 6588. [Google Scholar] [CrossRef]
- Jonkers, J.; De Regt, J.M.; Van der Sijde, B.; Van der Mullen, J.A.M. Spectroscopic techniques for the characterisation of spectrochemical plasma sources. Phys. Scr. 1999, 1999, 146. [Google Scholar] [CrossRef]
- Shakeel, H.; Arshad, S.; Haq, S.U.; Nadeem, A. Electron temperature and density measurements of laser induced germanium plasma. Phys. Plasmas 2016, 23, 053504. [Google Scholar] [CrossRef]
- Umar, Z.A.; Ahmed, N.; Ahmed, R.; Liaqat, U.; Baig, M.A. Elemental composition analysis of granite rocks using LIBS and LA-TOF-MS. Appl. Opt. 2018, 57, 4985–4991. [Google Scholar] [CrossRef] [PubMed]
- Pace, D.D.; Miguel, R.E.; Di Rocco, H.O.; García, F.A.; Pardini, L.; Legnaioli, S.; Lorenzetti, G.; Palleschi, V. Quantitative analysis of metals in waste foundry sands by calibration free-laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2017, 131, 58–65. [Google Scholar] [CrossRef]
- Mal, E.; Junjuri, R.; Gundawar, M.K.; Khare, A. Optimization of temporal window for application of calibration free-laser induced breakdown spectroscopy (CF-LIBS) on copper alloys in air employing a single line. J. Anal. At. Spectrom. 2019, 34, 319–330. [Google Scholar] [CrossRef]






| Species | Sample-1 | Sample-2 | Sample-3 |
|---|---|---|---|
| Aluminium (Al) | 3.0 | 1.00 | 0.69 |
| Gallium (Ga) | 95.0 | 96.05 | 97.32 |
| Arsenide (As) | 2.0 | 2.95 | 1.99 |
| 100 | 100 | 100 |
| Wavelength (nm) | Electron Configuration Transition Upper Level to Lower Level | Upper Level Energy/Ek (cm−1) | Transition Probability (s−1) |
|---|---|---|---|
| Gallium (Ga I) | |||
| Ga I 233.82 | 6d 2D5/2→4p 2P3/2 | 43,580.44 | |
| Ga I 237.12 | 7s 2S1/2→4p 2P1/2 | 42,158.77 | |
| Ga I 241.86 | 7s 2S1/2→4p 2P3/2 | 42,158.77 | |
| Ga I 245.00 | 5d 2D3/2→4p 2P3/2 | 40,802.86 | |
| Ga I 250.01 | 5d 2D5/2→4p 2P3/2 | 40,811.41 | |
| Ga I 265.98 * | 6s 2S1/2→4p 2P1/2 | 37,584.77 | |
| Ga I 271.96 * | 6s 2S1/2→4p 2P3/2 | 37,584.77 | |
| Ga I 287.40 * | 4d 2D3/2→4p 2P1/2 | 34,781.66 | |
| Ga I 294.36 * | 4d 2D5/2→4p 2P3/2 | 34,787.85 | |
| Ga I 403.20 * | 5s 2S1/2→4p 2P1/2 | 24,788.53 | |
| Ga I 417.20 * | 5s 2S1/2→4p 2P3/2 | 24,788.53 | |
| Ga I 639.60 | 6p 2P3/2→5s 2S1/2 | 40,417.62 | |
| Ga I 641.30 | 6p 2P1/2→6p 2S1/2 | 40,376.45 | |
| Arsenide (As I) | |||
| As I 228.81 * | 5s 2P3/2→4p3 2D5/2 | 54,605.30 | |
| As I 234.98 * | 5s 2P1/2→4p3 2D3/2 | 53,135.60 | |
| As I 274.49 * | 5s 2P3/2→4p3 2P1/2 | 54,605.30 | |
| As I 278.02 * | 5s 2P3/2→4p3 2P3/2 | 54,605.30 | |
| As I 286.04 * | 5s 2P1/2→4p3 2P1/2 | 53,135.60 | |
| Aluminium (Al I) | |||
| Al I 308.20 * | 3d 2D3/2→3p 2P1/2 | 32,435.45 | |
| Al I 309.30 * | 3d 2D5/2→3p 2P3/2 | 32,436.79 | |
| Al I 394.40 * | 4s 2S1/2→3p 2P1/2 | 25,347.75 | |
| Al I 396.15 * | 4s 2S1/2→3p 2P3/2 | 25,347.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrebdi, T.A.; Fayyaz, A.; Asghar, H.; Zaman, A.; Asghar, M.; Alkallas, F.H.; Hussain, A.; Iqbal, J.; Khan, W. Quantification of Aluminum Gallium Arsenide (AlGaAs) Wafer Plasma Using Calibration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS). Molecules 2022, 27, 3754. https://doi.org/10.3390/molecules27123754
Alrebdi TA, Fayyaz A, Asghar H, Zaman A, Asghar M, Alkallas FH, Hussain A, Iqbal J, Khan W. Quantification of Aluminum Gallium Arsenide (AlGaAs) Wafer Plasma Using Calibration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS). Molecules. 2022; 27(12):3754. https://doi.org/10.3390/molecules27123754
Chicago/Turabian StyleAlrebdi, Tahani A., Amir Fayyaz, Haroon Asghar, Asif Zaman, Mamoon Asghar, Fatemah H. Alkallas, Atif Hussain, Javed Iqbal, and Wilayat Khan. 2022. "Quantification of Aluminum Gallium Arsenide (AlGaAs) Wafer Plasma Using Calibration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS)" Molecules 27, no. 12: 3754. https://doi.org/10.3390/molecules27123754
APA StyleAlrebdi, T. A., Fayyaz, A., Asghar, H., Zaman, A., Asghar, M., Alkallas, F. H., Hussain, A., Iqbal, J., & Khan, W. (2022). Quantification of Aluminum Gallium Arsenide (AlGaAs) Wafer Plasma Using Calibration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS). Molecules, 27(12), 3754. https://doi.org/10.3390/molecules27123754







