Therapeutic Effect of Renifolin F on Airway Allergy in an Ovalbumin-Induced Asthma Mouse Model In Vivo
Abstract
:1. Introduction
2. Results
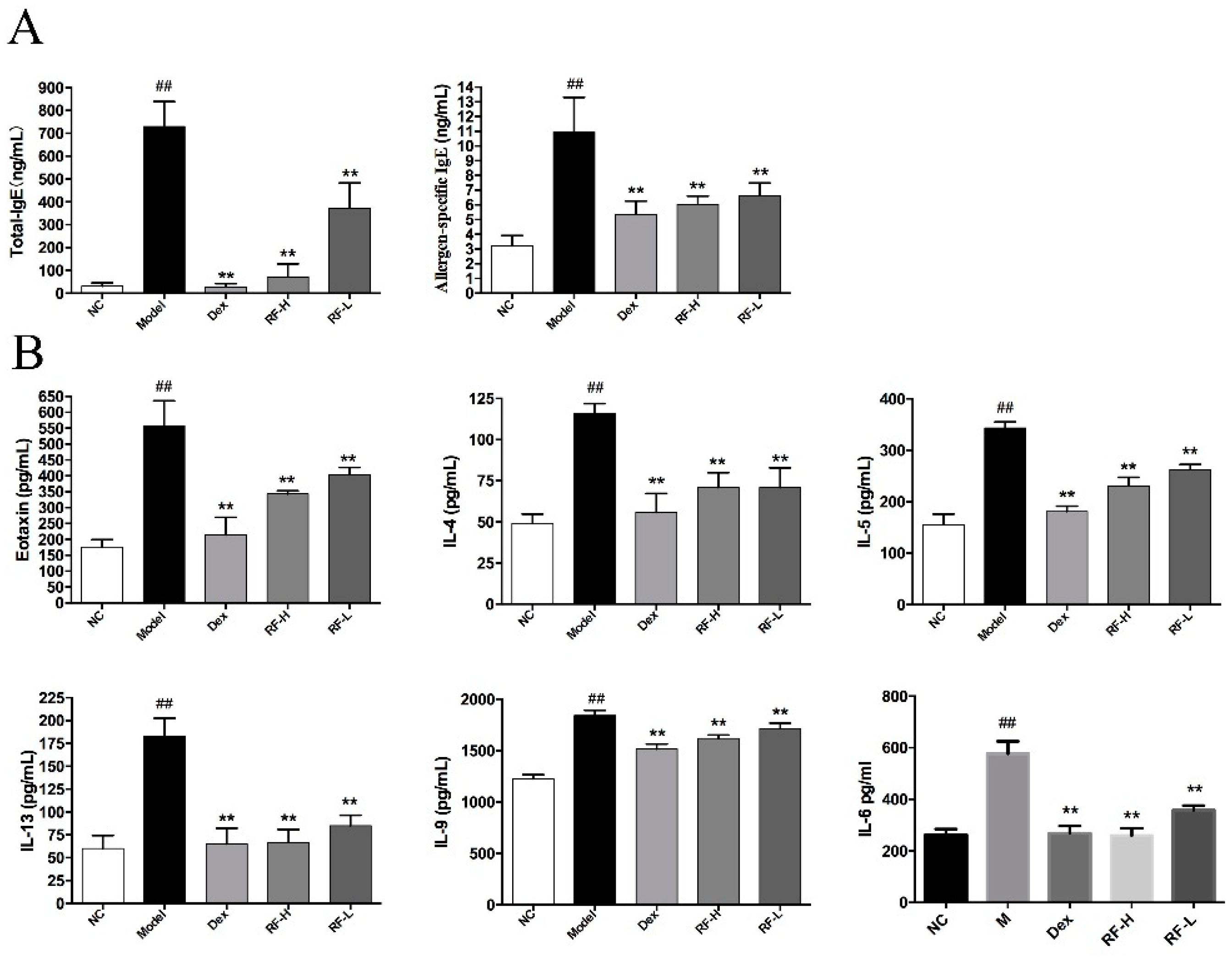
2.1. Effects of Renifolin F on Total Serum IgE and Allergen-Specific IgE Production in Mice Serum
2.2. Effects of Renifolin F on Eotaxin, IL-4, IL-5, IL-13, IL-9 and IL-6 Levels in Mice BALF
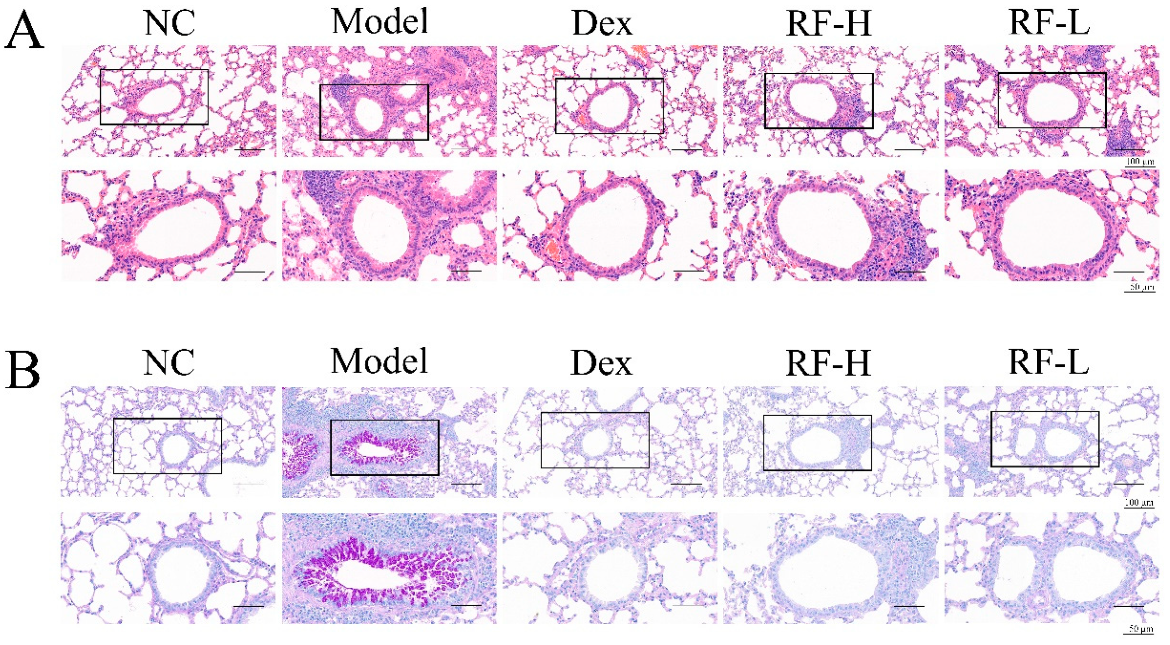
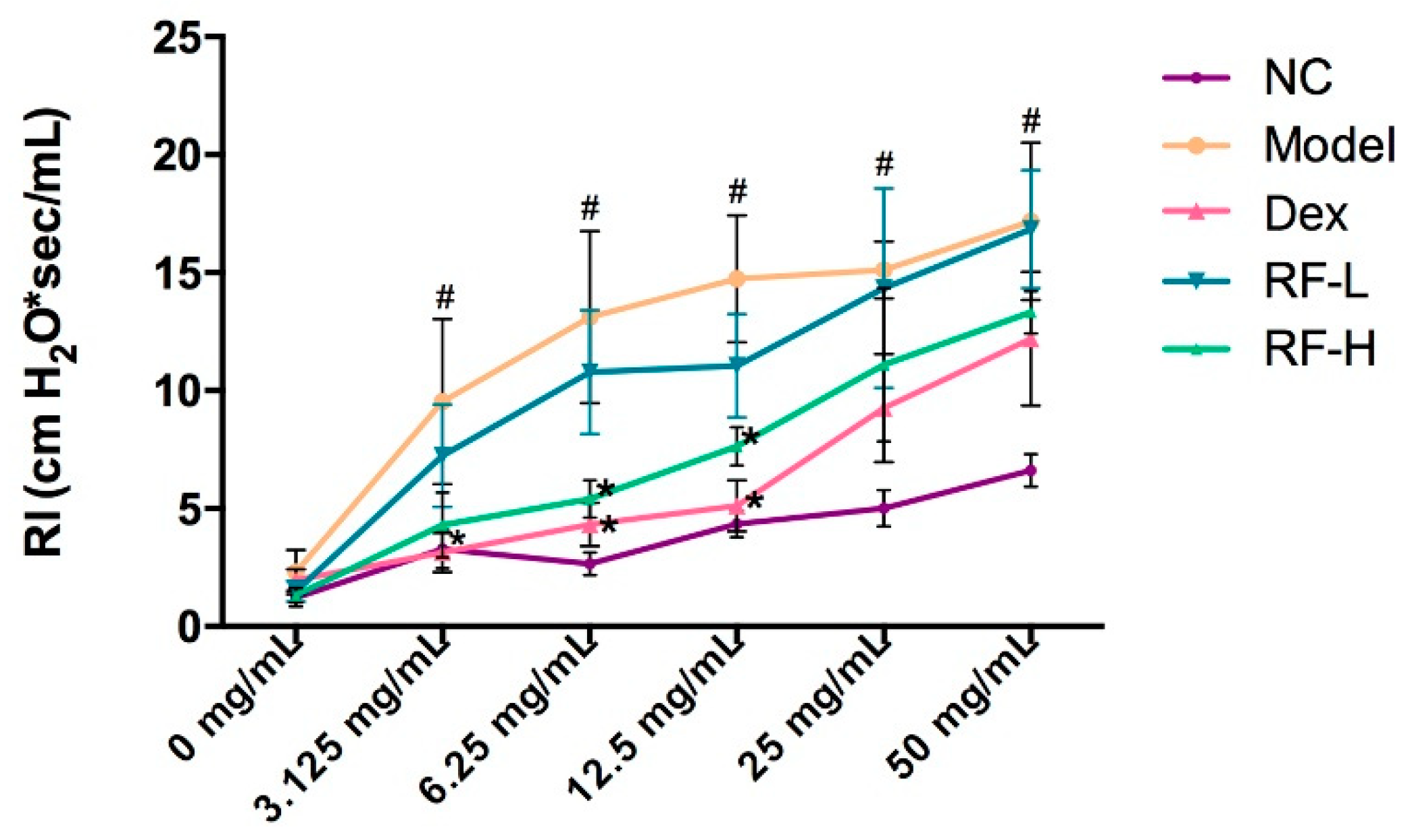
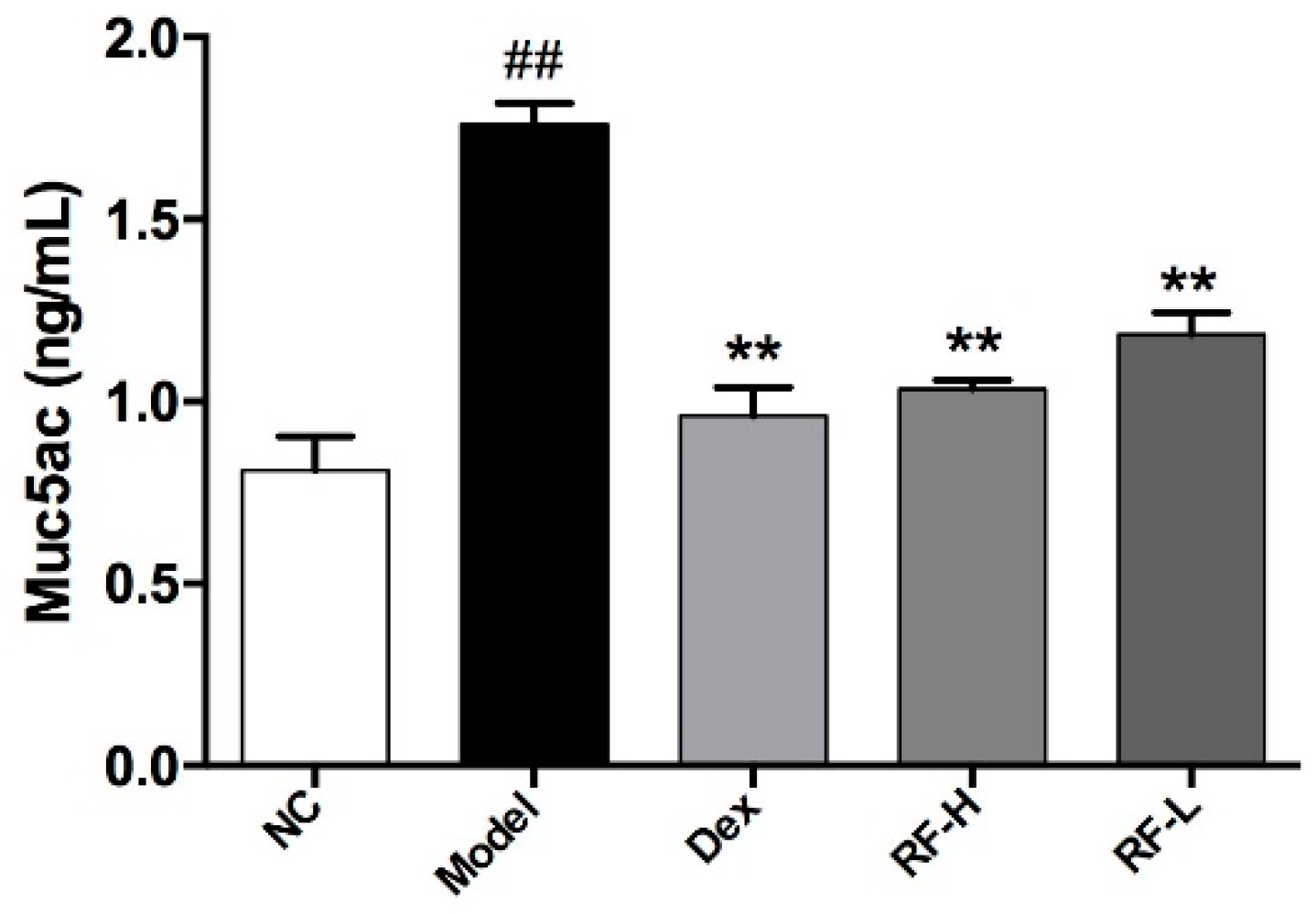
2.3. Effects of Renifolin F on Symptoms and Muc5ac in OVA-Induced Allergic Asthma Mice
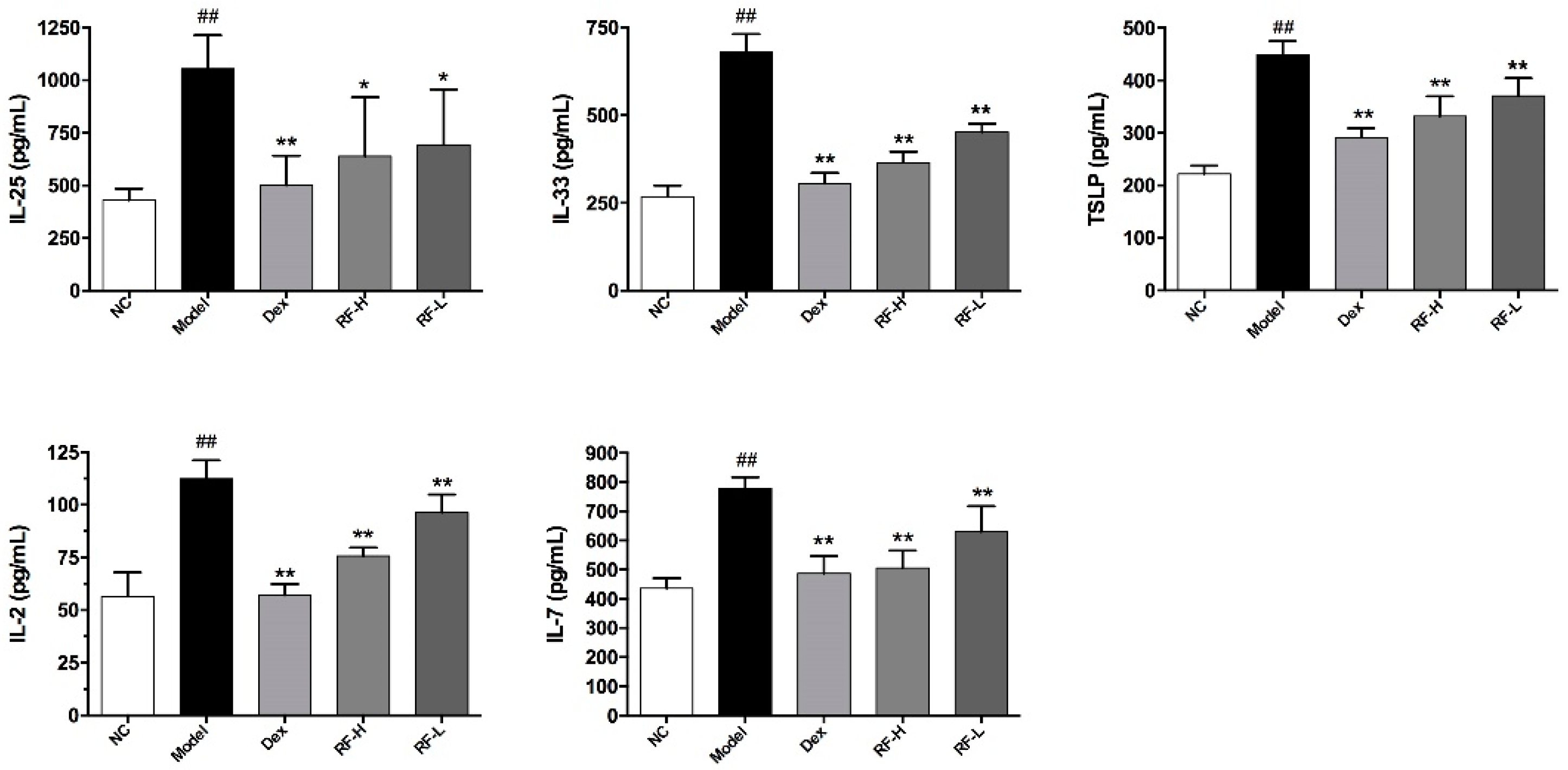
2.4. Effects of Renifolin F on ILC2s Cells and Its Upstream Cytokines in the Lung
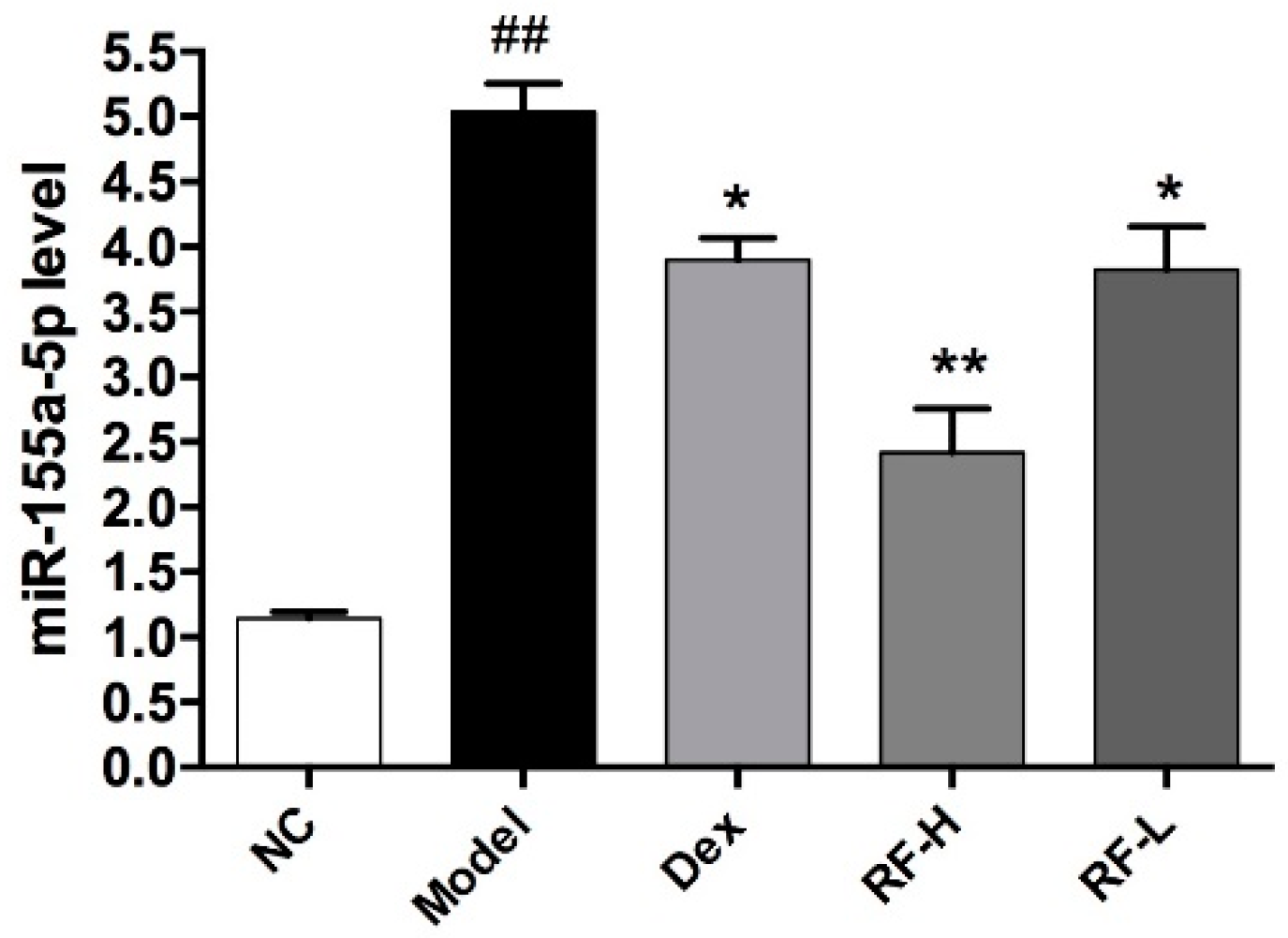
2.5. Effects of Renifolin F on the Expression of microRNA-155 in the Lung of Allergic Asthma Mice
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Renifolin F Analysis
4.2. Mice
4.3. OVA-Induced Allergic Asthma Model and Treatment
4.4. Collection and Management of Serum
4.5. Collection and Management of the Mice Bronchoalveloar Lavage Fluid (BALF)
4.6. Measurement of Cytokine Production Levels by ELISA
4.7. Assessment of Airway Hyperresponsiveness (AHR)
4.8. Histological Analysis of Lung Tissue
4.9. Flow Cytometric Analysis
4.10. RNA Isolation and microRNA-155 Expression
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Farraia, M.; Paciência, I.; Castro Mendes, F.; Cavaleiro Rufo, J.; Shamji, M.; Agache, I.; Moreira, A. Allergen immunotherapy for asthma prevention: A systematic review and meta-analysis of randomized and non-randomized controlled studies. Allergy 2022, 77, 1719–1735. [Google Scholar] [CrossRef]
- Venkitakrishnan, R.; Thomas, P.K.; Bansal, A.; Ghosh, I.; Augustine, d.d.J.; Divya, R.; Cleetus, M. Fluticasone/formoterol compared with other ICS/LABAs in asthma: A systematic review. J. Asthma 2022, 59, 1221–1230. [Google Scholar] [CrossRef]
- Rodríguez-Torres, J.; López-López, L.; Cabrera-Martos, I.; Torres-Sánchez, I.; Prados-Román, E.; Ortíz-Rubio, A.; Valenza, M.C. Symptom severity is associated with signs of central sensitization in patients with asthma. Clin. Respir. J. 2021, 15, 1219–1226. [Google Scholar] [CrossRef]
- Goulden, N.; Cousins, M.; Hart, K.; Jenkins, A.; Willetts, G.; Yendle, L.; Doull, I.; Williams, E.M.; Hoare, Z.; Kotecha, S. Inhaled Corticosteroids Alone and in Combination With Long-Acting β2 Receptor Agonists to Treat Reduced Lung Function in Preterm-Born Children: A Randomized Clinical Trial. JAMA Pediatr. 2022, 176, 133–141. [Google Scholar] [CrossRef]
- Mosnaim, G.; Bizik, B.K.; Wilson, C.; Bensch, G. Efficacy and safety of add-on tiotropium in the management of uncontrolled asthma: A patient case series. J. Asthma 2022, 59, 1231–1236. [Google Scholar] [CrossRef]
- Ying, X.; Su, Z.; Bie, Q.; Zhang, P.; Yang, H.; Wu, Y.; Xu, Y.; Wu, J.; Zhang, M.; Wang, S.; et al. Synergistically increased ILC2 and Th9 cells in lung tissue jointly promote the pathological process of asthma in mice. Mol. Med. Rep. 2016, 13, 5230–5240. [Google Scholar] [CrossRef] [Green Version]
- Van Rijt, L.; Von Richthofen, H.; Van Ree, R. Type 2 innate lymphoid cells: At the cross-roads in allergic asthma. Semin. Immunopathol. 2016, 38, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Kato, A. Group 2 Innate Lymphoid Cells in Airway Diseases. Chest 2019, 156, 141–149. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krabbendam, L.; Bal, S.M.; Spits, H.; Golebski, K. New insights into the function, development, and plasticity of type 2 innate lymphoid cells. Immunol. Rev. 2018, 286, 74–85. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S., Jr. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Cui, X.; Li, W.; Lv, J.; Du, L.; Mi, W.; Li, H.; Chen, Z.; Leng, Q.; Zhou, H.; et al. BLT1 signaling in epithelial cells mediates allergic sensitization via promotion of IL-33 production. Allergy 2019, 74, 495–506. [Google Scholar] [PubMed]
- Ro, M.; Lee, A.J.; Kim, J.H. 5-/12-Lipoxygenase-linked cascade contributes to the IL-33-induced synthesis of IL-13 in mast cells, thus promoting asthma development. Allergy 2018, 73, 350–360. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Cao, J.; Shang, J.H.; Liu, Y.P.; Khan, A.; Wang, H.S.; Qian, Y.; Liu, L.; Ye, M.; Luo, X.D. Airway antiallergic effect and pharmacokinetics of alkaloids from Alstonia scholaris. Phytomedicine 2017, 27, 63–72. [Google Scholar] [CrossRef]
- Lertnimitphun, P.; Zhang, W.H.; Fu, W.W.; Yang, B.C.; Zheng, C.W.; Yuan, M.; Zhou, H.; Zhang, X.; Pei, W.Z.; Lu, Y.; et al. Safranal Alleviated OVA-Induced Asthma Model and Inhibits Mast Cell Activation. Front. Immunol. 2021, 12, 585595. [Google Scholar] [CrossRef]
- Li, Y.P.; Yang, Y.C.; Li, Y.K.; Jiang, Z.Y.; Huang, X.Z.; Wang, W.G.; Gao, X.M.; Hu, Q.F. Five new prenylated chalcones from Desmodium renifolium. Fitoterapia 2014, 95, 214–219. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Ma, X.X.; Tan, W.H.; Zhou, L.B.; Zhuang, X.Y.; Yang, S.D.; Qian, Z.G.; Zhou, Z.H. Two new chalcones from Shuteria sinensis. Nat. Prod. Res. 2015, 29, 1909–1913. [Google Scholar] [CrossRef]
- Peng, C.Z.; Qi, J.J.; Li, X.E. Records of proven prescriptions for the treatment of respiratory diseases by the Wa people in Cangyuan County. Chin. J. Ethnomed. Ethnopharm. 2011, 8, 6. [Google Scholar]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, Mast Cells, Basophils, and Eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Pease, J.E.; Williams, T.J. Eotaxin and Asthma. Curr. Opin. Pharmacol. 2001, 1, 248–253. [Google Scholar] [CrossRef]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, mucus, and goblet cells. Chest 2017, 154, 169–176. [Google Scholar] [CrossRef]
- Johansson, K.; Malmhäll, C.; Ramos-Ramírez, P.; Rådinger, M. MicroRNA-155 is a critical regulator of type 2 innate lymphoid cells and IL-33 signaling in experimental models of allergic airway inflammation. J. Allergy Clin. Immunol. 2017, 39, 1007–1016.e9. [Google Scholar] [CrossRef] [Green Version]
- Hearn, A.P.; Mak, M.S.; Budaj, I.; Qurashi, N.; Snell, O.; Dhariwal, J.; Nanzer, A.M.; Jackson, D.J. The prevalence of mucus plugging in severe eosinophilic asthma and its relationship to clinical efficacy of anti-IL-5R treatment. J. Allergy Clin. Immunol Pract. 2022, 10, 1102–1103. [Google Scholar] [CrossRef]
- Sagara, H.; D’Andrea, P.; Tanase, A.M.; Pethe, A.; Tanaka, Y.; Matsuo, K.; Hosoe, M.; Nakamura, Y. Long-term safety of once-daily indacaterol acetate/glycopyrronium bromide/mometasone furoate high-dose, and indacaterol acetate/mometasone furoate high-dose, in Japanese patients with inadequately controlled asthma: Results from two open-label, 52-week studies. J. Asthma 2022, 30, 1–9. [Google Scholar]
- Park, C.K.; An, T.J.; Kim, J.H.; Rhee, C.K.; Yoon, H.K. Synergistic effect of roflumilast with dexamethasone in a neutrophilic asthma mouse model. Clin. Exp. Pharmacol. Physiol. 2022, 49, 624–632. [Google Scholar] [CrossRef]
- Liu, C.T.; Song, Y.C.; Wu, T.C.; Shiung, K.C.; Chen, I.H.; Chang, T.T.; Liang, S.J.; Yen, H.R. Targeting glycolysis in Th2 cells by pterostilbene attenuates clinical severities in an asthmatic mouse model and IL-4 production in peripheral blood from asthmatic patients. Immunology 2022, 166, 222–237. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Snelgrove, R.J. Type 2 immunity: Expanding our view. Sci. Immunol. 2018, 3, eaat1604. [Google Scholar] [CrossRef] [Green Version]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Kubo, M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev. 2017, 278, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.A.; Broide, D.H. Airway innate lymphoid cells in the induction and regulation of allergy. Allergol. Int. 2019, 68, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, G.; Godbout, K.; Boulay, M.-È.; Boulet, L.P.; Morissette, M.C.; Côté, A. Increase in FeNO Levels Following IL5/IL5R-Targeting Therapies in Severe Asthma: A Case Series. J. Asthma Allergy 2022, 19, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Almas, S.; Fayad, N.; Srivastava, O.; Siddique, M.; Touret, N.; Lacy, P. Cytokine trafficking of IL-9 and IL-13 through TfnRc+ vesicles in activated human eosinophils. J. Leukoc. Biol. 2021, 109, 753–762. [Google Scholar] [CrossRef]
- Koch, S.; Sopel, N.; Finotto, S. Th9 and other IL-9-producing cells in allergic asthma. Semin. Immunopathol. 2017, 39, 55–68. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [Green Version]
- Sugita, K.; Steer, C.A.; Martinez-Gonzalez, I.; Altunbulakli, C.; Morita, H.; Castro-Giner, F.; Kubo, T.; Wawrzyniak, P.; Rückert, B.; Sudo, K.; et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J. Allergy Clin. Immunol. 2018, 141, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Sugita, K.; Altunbulakli, C.; Morita, H.; Sugita, A.; Kubo, T.; Kimura, R.; Goto, H.; Yamamoto, O.; Rückert, B.; Akdis, M.; et al. Human type 2 innate lymphoid cells disrupt skin keratinocyte tight junction barrier by IL-13. Allergy 2019, 74, 2534–2537. [Google Scholar] [CrossRef]
- Gurram, R.K.; Zhu, J.F. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol. Immunol. 2019, 16, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Hellings, P.W.; Steelant, B. Epithelial barriers in allergy and asthma. J. Allergy Clin. Immunol. 2020, 145, 1499–1509. [Google Scholar] [CrossRef]
- Calvén, J.; Ax, E.; Rådinger, M. The Airway Epithelium-A Central Player in Asthma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of immune responses by tuft cells. Nat. Rev. Immunol. 2019, 19, 584–593. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Kabata, H.; Moro, K.; Fukunaga, K.; Suzuki, Y.; Miyata, J.; Masaki, K.; Betsuyaku, T.; Koyasu, S.; Asano, K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat. Commun. 2013, 4, 2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, H.Y.; Liao, S.M.; Chen, F.H.; Yang, Q.T.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804. [Google Scholar] [CrossRef]
- Weidner, J.; Bartel, S.; Kılıç, A.; Zissler, U.M.; Renz, H.; Schwarze, J.; Schmidt-Weber, C.B.; Maes, T.; Rebane, A.; Krauss-Etschmann, S.; et al. Spotlight on microRNAs in allergy and asthma. Allergy 2021, 76, 1661–1678. [Google Scholar] [CrossRef]
- Foster, P.S.; Plank, M.; Collison, A.; Tay, H.L.; Kaiko, G.E.; Li, J.; Johnston, S.L.; Hansbro, P.M.; Kumar, R.K.; Yang, M.; et al. The emerging role of microRNAs in regulating immune and inflammatory responses in the lung. Immunol. Rev. 2013, 253, 198–215. [Google Scholar] [CrossRef]
- Zhou, H.; Li, J.Y.; Gao, P.; Wang, Q.; Zhang, J. miR-155: A Novel Target in Allergic Asthma. Int. J. Mol. Sci. 2016, 17, 1773. [Google Scholar] [CrossRef] [Green Version]
- Fink, M.Y.; Qi, X.; Shirey, K.A.; Fanaroff, R.; Chapoval, S.; Viscardi, R.M.; Vogel, S.N.; Keegan, A.D. Mice Expressing Cosegregating Single Nucleotide Polymorphisms (D298G and N397I) in TLR4 Have Enhanced Responses to House Dust Mite Allergen. J. Immunol. 2022, 208, 2085–2097. [Google Scholar] [CrossRef]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.O.; Byeon, H.W.; Eo, J.C.; Choi, J.Y.; Tanveer, M.; Uyangaa, E.; Kim, K.; Eo, S.K. T cell-intrinsic miR-155 is required for Th2 and Th17-biased responses in acute and chronic airway inflammation by targeting several different transcription factors. Immunology 2022, 11, e13477. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Li, X.; Fu, R.; Hu, M.; Wei, Y.; Hu, X.; Tan, W.; Tong, X.; Huang, F. Therapeutic Effect of Renifolin F on Airway Allergy in an Ovalbumin-Induced Asthma Mouse Model In Vivo. Molecules 2022, 27, 3789. https://doi.org/10.3390/molecules27123789
Yang Z, Li X, Fu R, Hu M, Wei Y, Hu X, Tan W, Tong X, Huang F. Therapeutic Effect of Renifolin F on Airway Allergy in an Ovalbumin-Induced Asthma Mouse Model In Vivo. Molecules. 2022; 27(12):3789. https://doi.org/10.3390/molecules27123789
Chicago/Turabian StyleYang, Zhuya, Xiaohong Li, Rongbing Fu, Min Hu, Yijie Wei, Xuhong Hu, Wenhong Tan, Xiaoyun Tong, and Feng Huang. 2022. "Therapeutic Effect of Renifolin F on Airway Allergy in an Ovalbumin-Induced Asthma Mouse Model In Vivo" Molecules 27, no. 12: 3789. https://doi.org/10.3390/molecules27123789
APA StyleYang, Z., Li, X., Fu, R., Hu, M., Wei, Y., Hu, X., Tan, W., Tong, X., & Huang, F. (2022). Therapeutic Effect of Renifolin F on Airway Allergy in an Ovalbumin-Induced Asthma Mouse Model In Vivo. Molecules, 27(12), 3789. https://doi.org/10.3390/molecules27123789





