Nano-Structured Ridged Micro-Filaments (≥100 µm Diameter) Produced Using a Single Step Strategy for Improved Bone Cell Adhesion and Proliferation in Textile Scaffolds
Abstract
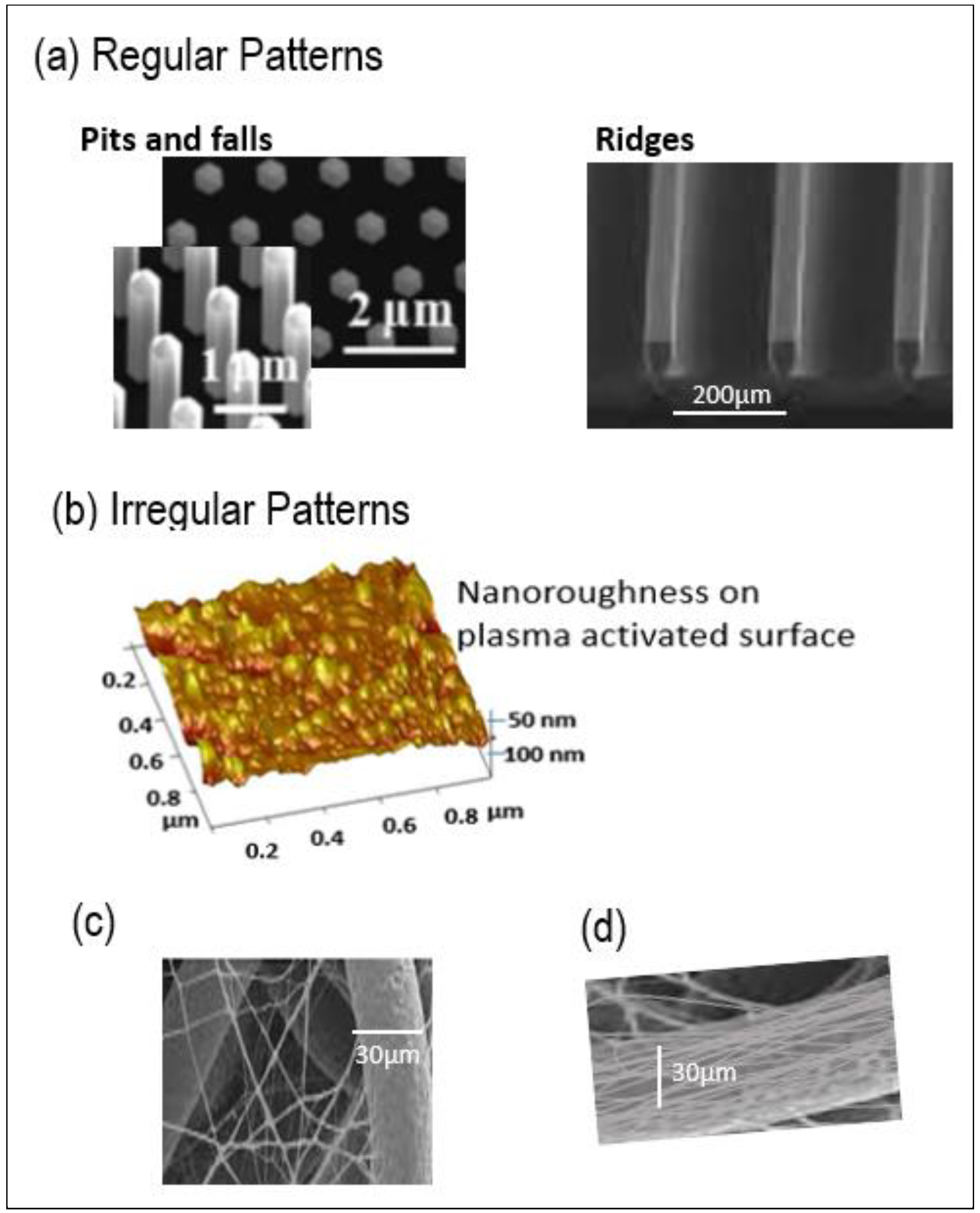
:1. Introduction
2. Materials and Methods
2.1. Materials
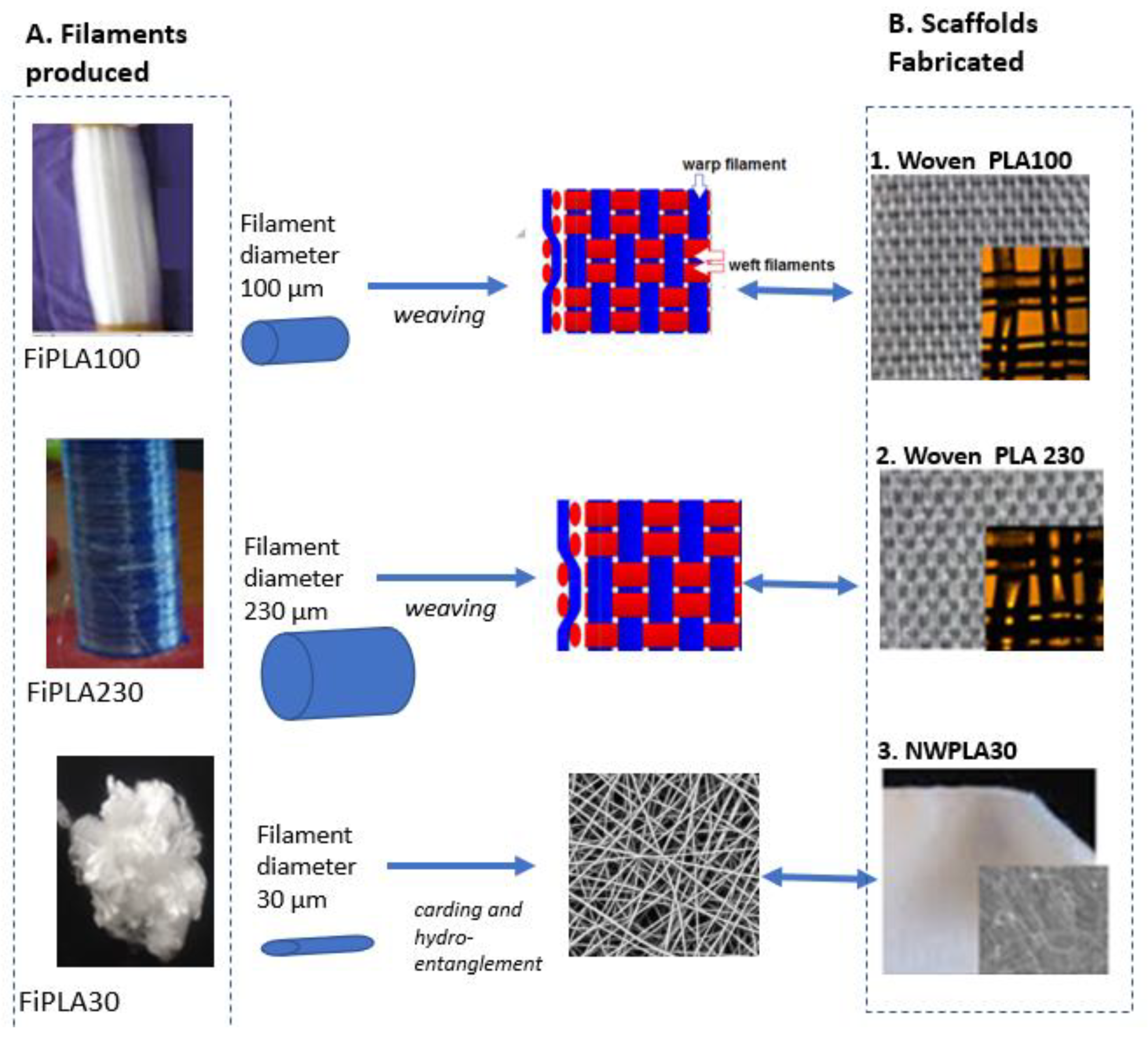
2.2. Spinning of Micro/Micro-Sized Filaments (or Fibers)
2.3. Processing of PLA Micro/Micro-Sized Fibres into Textile Scaffold
2.4. Cleaning of Wovens and Nonwoven to Remove Spin Finish
2.5. Characterization Methods
2.5.1. Fiber Surface Properties
- -
- Atomic Force Microscopy (AFM) analysis
- -
- Water Contact angle
- -
- Tensile strength characteristics of PLA monofilaments
2.5.2. Thermal Characterizations of PLA Monofilaments
2.6. Biological Tests
2.6.1. MG 63 Osteoblastic Cell Proliferation Assays
- (a)
- Seeding of MG 63 cells on the PLA woven, nonwoven and film discs (3 and 6 days of culture)
- (b)
- Seeding of MG 63 cells under the woven PLA discs (up to 21 days of culture)
2.6.2. Immunofluorescence Staining
3. Results and Discussion
3.1. Mechanical and Thermal Properties of Micro-Sized PLA Fibers
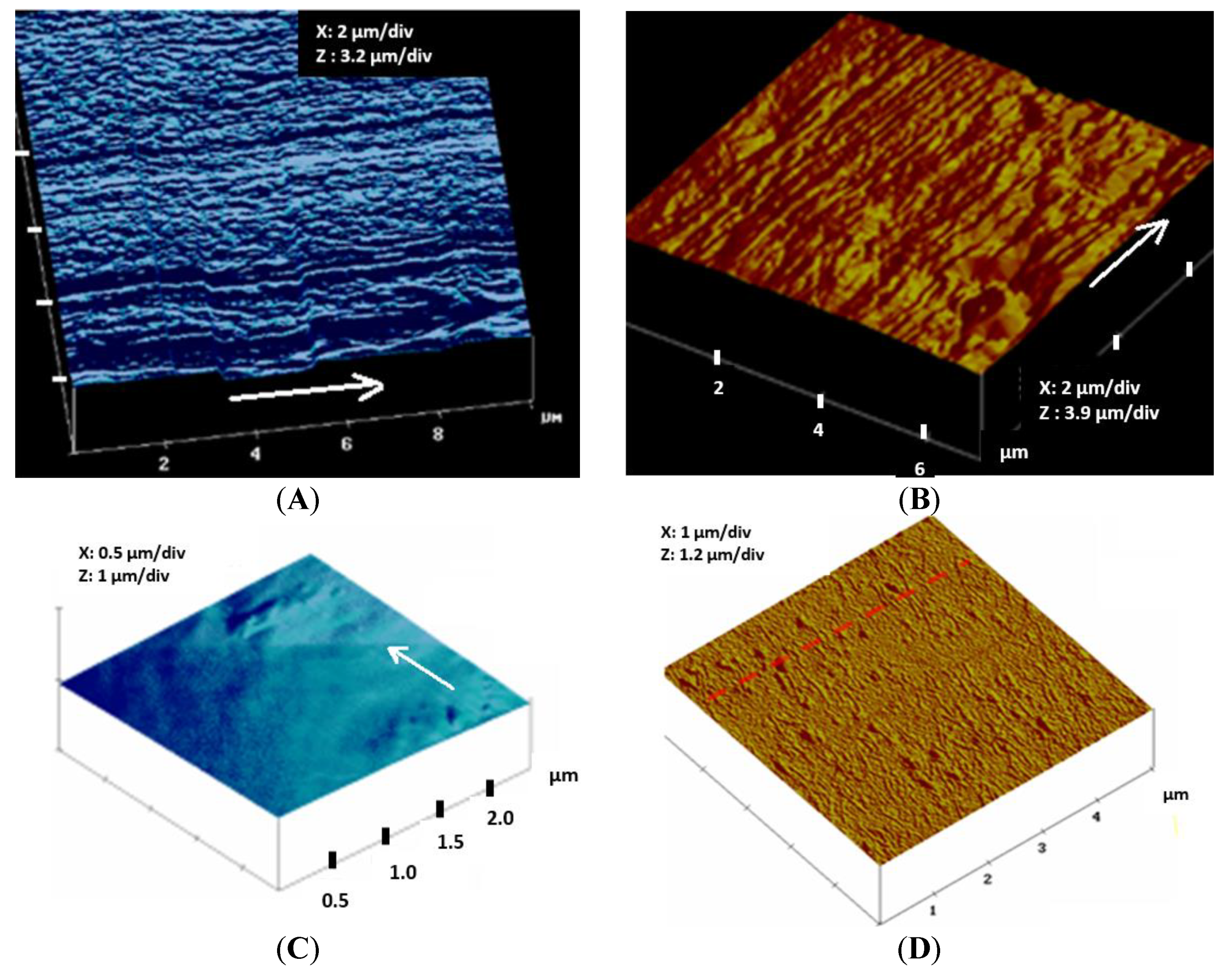
3.2. Topographical Analysis of PLA Filament Surface Analyzed by Atomic Force Microscopy
3.3. Properties of Woven and Nonwoven PLA Scaffolds
3.4. In Vitro Colonization and Osteogenic Expression Induced by the PLLA Scaffolds
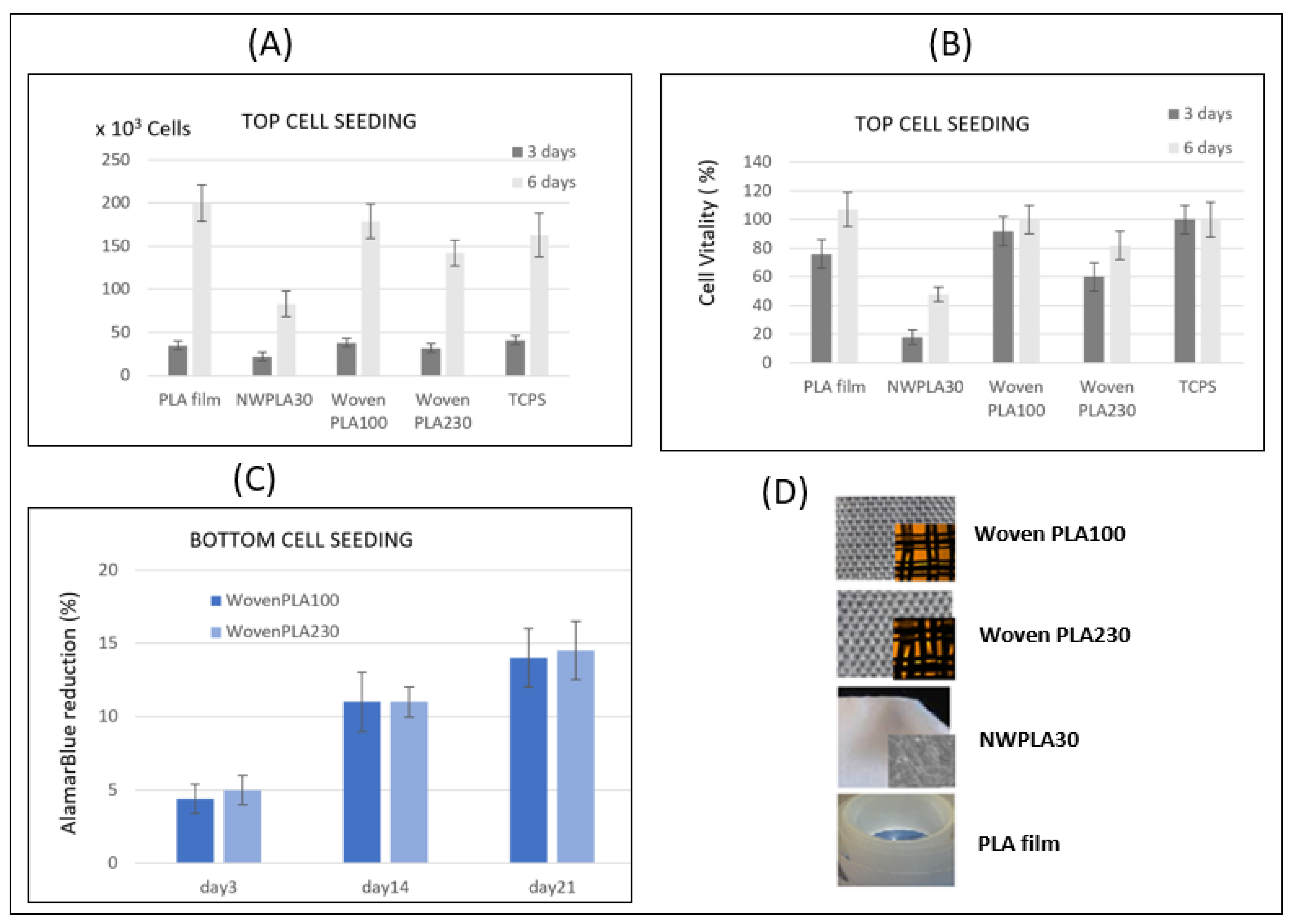
3.4.1. Cell Proliferation Assay
Cell Adhesion and Proliferation after 3 and 6 Days for Cell Seeding over the PLA Discs
Cell Adhesion and Proliferation Assay Using AlamarBlue® up to 21 Days for Cell Seeding under the PLA Wovens
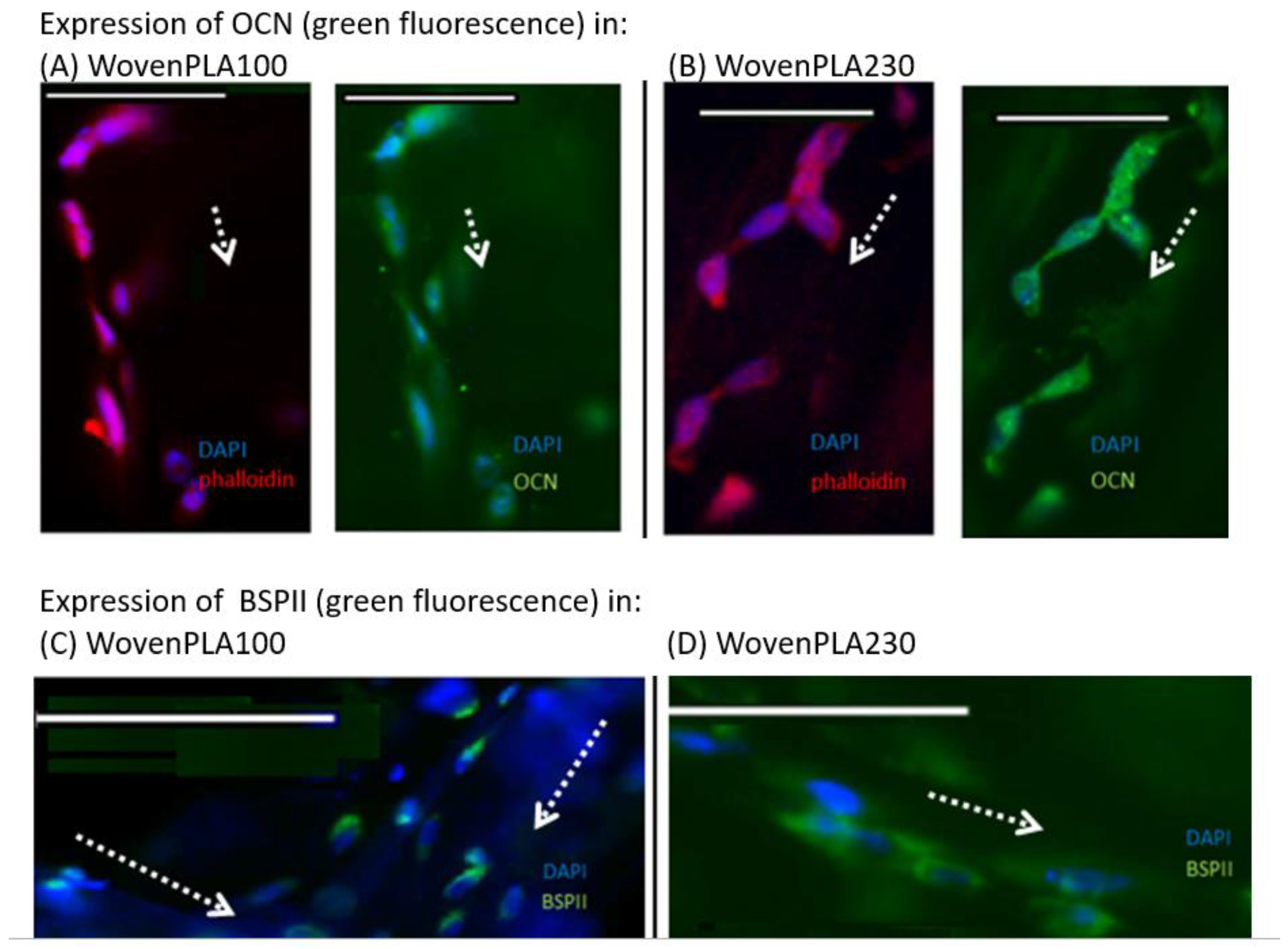
3.4.2. Immunofluorescence Staining In Vitro Osteogenic Expression Induced by the Two Woven PLA Scaffolds
In Vitro Osteogenic Expression Induced Woven PLA Scaffolds
4. Final Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, Y.B.; Zhong, Z.M.; Hou, G.; Jiang, H.; Chen, J.T. Involvement of Oxidative Stress in Age-Related Bone Loss. J. Surg. Res. 2011, 169, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P. The burden of musculoskeletal disease—A global perspective. Clin. Rheumatol. 2006, 25, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Rauh, J.; Milan, F.; Gunther, K.P.; Stiehler, M. Bioreactor systems for bone tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 263–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M.J.; Salmeron-Sanchez, M. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix—A review. Bioact. Mater. 2022, 15, 145–159. [Google Scholar] [CrossRef]
- Vandrovcová, M.; Bacakova, L. Adhesion, Growth and Differentiation of Osteoblasts on Surface-Modified Materials Developed for Bone Implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar] [CrossRef]
- Lerebours, A.; Vigneron, P.; Bouvier, S.; Rassineux, A.; Bigerelle, M.; Egles, C. Additive manufacturing process creates local surface roughness modifications leading to variation in cell adhesion on multifaceted TiAl6V4 samples. Bioprinting 2019, 16, e00054. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Tissue Engineering Applications of Three-Dimensional Bioprinting. Cell Biochem. Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Hu, J. 3-D Fibrous Assemblies: Properties, Applications and Modelling of Three-Dimensional Textile Structures; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Moroni, L.; De Wijn, J.R.; Van Blitterswijk, C.A. Integrating novel technologies to fabricate smart scaffolds. J. Biomater. Sci. Polym. Ed. 2008, 19, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005, 1, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. A 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Price, R.L.; Ellison, K.; Haberstroh, K.M.; Webster, T.J. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J. Biomed. Mater. Res. A 2004, 70, 129–138. [Google Scholar] [CrossRef]
- De Oliveria, P.T.; Nanci, A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials 2004, 25, 403–413. [Google Scholar] [CrossRef]
- Bačáková, L.; Starý, V.; Kofroňová, O.; Lisá, V. Polishing and coating carbon fiber-reinforced carbon composites with a carbon-titanium layer enhances adhesion and growth of osteoblast-like MG63 cells and vascular smooth muscle cells in vitro. J. Biomed. Mater. Res. 2001, 54, 567–578. [Google Scholar] [CrossRef]
- Stary, V.; Glogar, P.; Bacakova, L. A study of surface properties of composite materials and their influence on the biocompatibility. Acta Mont. 2003, 128, 19–36. [Google Scholar]
- Shao, Y.; Fu, J. Integrated Micro/nanoengineered Functional Biomaterials for Cell Mechanics and Mechanobiology: A Materials Perspective. Adv. Mater. 2014, 26, 1494–1533. [Google Scholar] [CrossRef] [Green Version]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Kalbacova, M.; Rezek, B.; Baresova, V.; Wolf-Brandstetter, C.; Kromka, A. Nanoscale topography of nanocrystalline diamonds promotes differentiation of osteoblasts. Acta Biomater. 2009, 5, 3076–3085. [Google Scholar] [CrossRef] [PubMed]
- Lenhert, S.; Meier, M.-B.; Meyer, U.; Chi, L.; Wiesmanna, H.P. Osteoblast alignment, elongation and migration on grooved polystyrene surfaces patterned by Langmuir–Blodgett lithography. Biomaterials 2005, 26, 563–570. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.S.; Ponery, A.; Nur-E-Kamal, A.; Kamal, J.; Meshel, A.S.; Sheets, M.P.; Schindler, M.; Meiners, S. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol. Cell. Biochem. 2007, 301, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Lu, L.; Mikos, A.G. The importance of new processing techniques in tissue engineering. MRS Bull. 1996, 21, 28–32. [Google Scholar] [CrossRef]
- Grayson, W.L.; Ma, T.; Bunnell, B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol. Prog. 2004, 20, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Asthana, A.; Kisaalita, W.S. Biomarkers for simplifying HTS, 3D cell culture platforms for drug discovery: The case for cytokines. Drug Discov. Today 2011, 16, 293–297. [Google Scholar] [CrossRef]
- Ng, R.; Zang, R.; Yang, K.K.; Liu, N.; Yang, S.T. Three-dimensional fibrous scaffolds with microstructures and nanotextures for tissue engineering. RSC Adv. 2012, 2, 10110–10124. [Google Scholar] [CrossRef]
- Ng, R.; Zhang, X.; Liu, N.; Yang, S.T. Modifications of nonwoven polyethylene terephthalate fibrous matrices via NaOH hydrolysis: Effects on pore size, fiber diameter, cell seeding and proliferation. Process Biochem. 2009, 44, 992–998. [Google Scholar] [CrossRef]
- Kim, H.K.; Yoon, T.R.; Kim, S.H.; Park, S.A.; Shin, J.W. In vitro and animal study of novel nano-hydroxyapatite/poly(e-caprolactone) composite scaffolds fabricated by layer manufacturing process. Tissue Eng. Part A 2009, 15, 977–989. [Google Scholar]
- Zawadzak, E.; Bil, M.; Ryszkowska, J.; Nazhat, S.N.; Cho, J.; Bretcanu, O.; Roether, J.A.; Boccaccini, A.R. Polyurethane foams electrophoretically coated with carbon nanotubes for tissue engineering scaffolds. Biomed. Mater. 2009, 4, 015008–015016. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol–Gel Science: The Physics and Chemistry of Sol–Gel Processing; Academic Press: San Diego, CA, USA, 1990; p. 907. [Google Scholar]
- Thorvaldsson, A.; Stenhamre, H.; Gatenholm, P.; Walkenstron, P. Electrospinning of Highly Porous Scaffolds for Cartilage Regeneration. Biomacromolecules 2008, 9, 1044–1049. [Google Scholar] [CrossRef]
- Tuzlakoglu, K.; Bolgen, N.; Salgado, A.J.; Gomes, M.E.; Piskin, E.; Reis, R.L. Nano-and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J. Mater. Sci. Mater. Med. 2005, 16, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houdali, A.; Behary, N.; Hornez, J.C.; Vroman, P.; Campagne, C.; Chai, F. A Composite Porous Scaffold by immobilising HA (hydroxyapatite) microparticles on PLA nonwoven using layer by layer deposition technique. Text. Res. J. 2017, 87, 2028–2038. [Google Scholar] [CrossRef]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-based smart hybrid materials: A physico-chemical perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef]
- Leenslag, J.W.; Gogolewki, S.; Pennings, A.J. Resorbable Materials of Poly(L-lactide). V. Influence of Secondary Structure on the Mechanical Properties and Hydrolyzability of Poly(L-lactide) Fibers Produced by a Dry-Spinning Method. J. Appl. Polym. Sci. 1984, 29, 2829–2842. [Google Scholar] [CrossRef]
- Leenslag, L.; Pegoretti, A.; Fenner, R.; Incardona, S.D.; Migliaresi, C. Biodegradable fibres of poly ( L-lactic acid ) produced by melt spinning. Polymer 1997, 38, 79–85. [Google Scholar]
- Petrie, C.J.; Denn, M.M. Instabilities in polymer processing. AIChE J. 1976, 22, 209–236. [Google Scholar] [CrossRef]
- Denn, M.M. Extrusion instabilities and wall slip. Annu. Rev. Fluid Mech. 2001, 33, 265–287. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.; Rothstein, J.P. Control of the sharkskin instability in the extrusion of polymer melts using induced temperature gradients. Rheol. Acta 2004, 44, 160–173. [Google Scholar] [CrossRef]
- Cicero, J.A.; Dorgan, J.R.; Janzen, J.; Garrett, J.; Runt, J.; Lin, J.S. Supramolecular morphology of two-step, melt-spun poly (lactic acid) fibers. J. Appl. Polym. Sci. 2002, 86, 2828–2838. [Google Scholar] [CrossRef]
- Prevorsek, D.C.; Kwon, Y.D.; Sharma, R.K. Structure and properties of Nylon 6 and PET fibres: The effects of crystallite dimensions. J. Mater. Sci. 1977, 12, 2310. [Google Scholar] [CrossRef]
- Solarski, S.; Ferreira, M.; Devaux, E. Thermal and mechanical characteristics of polylactide filaments drawn at different temperatures. J. Text. Inst. 2007, 98, 227–236. [Google Scholar] [CrossRef]
- Behary, N.; Ghenaim, A.; El Achari, A.; Caze, C. Tribological Analysis of Glass Fibers Using Atomic Force Microscopy/Lateral Force Microscopy. J. Appl. Polym. Sci. 2000, 75, 1013–1025. [Google Scholar] [CrossRef]
- Takke, V.; Behary, N.; Perwuelz, A.; Campagne, C. Studies on the atmospheric air–plasma treatment of PET (polyethylene terephtalate) woven fabrics: Effect of process parameters and of aging. J. Appl. Polym. Sci. 2009, 114, 348–357. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Niederauer, G.G.; Athanasiou, K.A. Fabrication and Characterization of PLA-PGA Orthopedic Implants. Tissue Eng. 1995, 1, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Cai, Q.; Zhang, S.; Yang, X.; Deng, X. The effect of poly (L-lactic acid) nanofiber orientation on osteogenic responses of human osteoblast-like MG63 cells. J. Mech. Behav. Biomed. Mater. 2011, 4, 600–609. [Google Scholar] [CrossRef]
- Paletta, J.; Erffmeier, K.; Theisen, C.; Hussain, D.; Wendorff, J.H.; Greiner, A.; Fuchs-Winkelmann, S.; Schofer, M.D. Influence of Poly-(L-Lactic Acid) Nanofiber Functionalization on Maximum Load, Young’s Modulus, and Strain of Nanofiber Scaffolds Before and After Cultivation of Osteoblasts: An In Vitro Study. Sci. World J. 2009, 9, 1382–1393. [Google Scholar] [CrossRef] [Green Version]


| Textile Scaffold | Fiber Diameter (μm) | Water Contact Angle (°) | Areal Density (g/m2) | Thickness (μm) | Porosity (%) | Average Pore Size and Morphology (μm) |
|---|---|---|---|---|---|---|
| Woven PLA100 | 100 ± 5 | 83 ± 2° | 144 ± 5 | 590 ± 5 | 80 ± 2 | Rectangular pore 100 × 100 µm |
| Woven PLA230 | 230 ± 10 | 76.0 ± 3° | 273 ± 5 | 790 ± 5 | 72 ± 4 | Rectangular pore 50 × 100 µm |
| Nonwoven NWPLA30 | 30 ± 3 | 130 ± 5° | 265 ± 4 | 1000 ± 10 | 90 ± 3 | undefined pore morphology |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behary, N.; Eap, S.; Cayla, A.; Chai, F.; Benkirane-Jessel, N.; Campagne, C. Nano-Structured Ridged Micro-Filaments (≥100 µm Diameter) Produced Using a Single Step Strategy for Improved Bone Cell Adhesion and Proliferation in Textile Scaffolds. Molecules 2022, 27, 3790. https://doi.org/10.3390/molecules27123790
Behary N, Eap S, Cayla A, Chai F, Benkirane-Jessel N, Campagne C. Nano-Structured Ridged Micro-Filaments (≥100 µm Diameter) Produced Using a Single Step Strategy for Improved Bone Cell Adhesion and Proliferation in Textile Scaffolds. Molecules. 2022; 27(12):3790. https://doi.org/10.3390/molecules27123790
Chicago/Turabian StyleBehary, Nemeshwaree, Sandy Eap, Aurélie Cayla, Feng Chai, Nadia Benkirane-Jessel, and Christine Campagne. 2022. "Nano-Structured Ridged Micro-Filaments (≥100 µm Diameter) Produced Using a Single Step Strategy for Improved Bone Cell Adhesion and Proliferation in Textile Scaffolds" Molecules 27, no. 12: 3790. https://doi.org/10.3390/molecules27123790
APA StyleBehary, N., Eap, S., Cayla, A., Chai, F., Benkirane-Jessel, N., & Campagne, C. (2022). Nano-Structured Ridged Micro-Filaments (≥100 µm Diameter) Produced Using a Single Step Strategy for Improved Bone Cell Adhesion and Proliferation in Textile Scaffolds. Molecules, 27(12), 3790. https://doi.org/10.3390/molecules27123790









