Quantitative Determination of Four Potential Genotoxic Impurities in the Active Pharmaceutical Ingredients in TSD-1 Using UPLC-MS/MS
Abstract
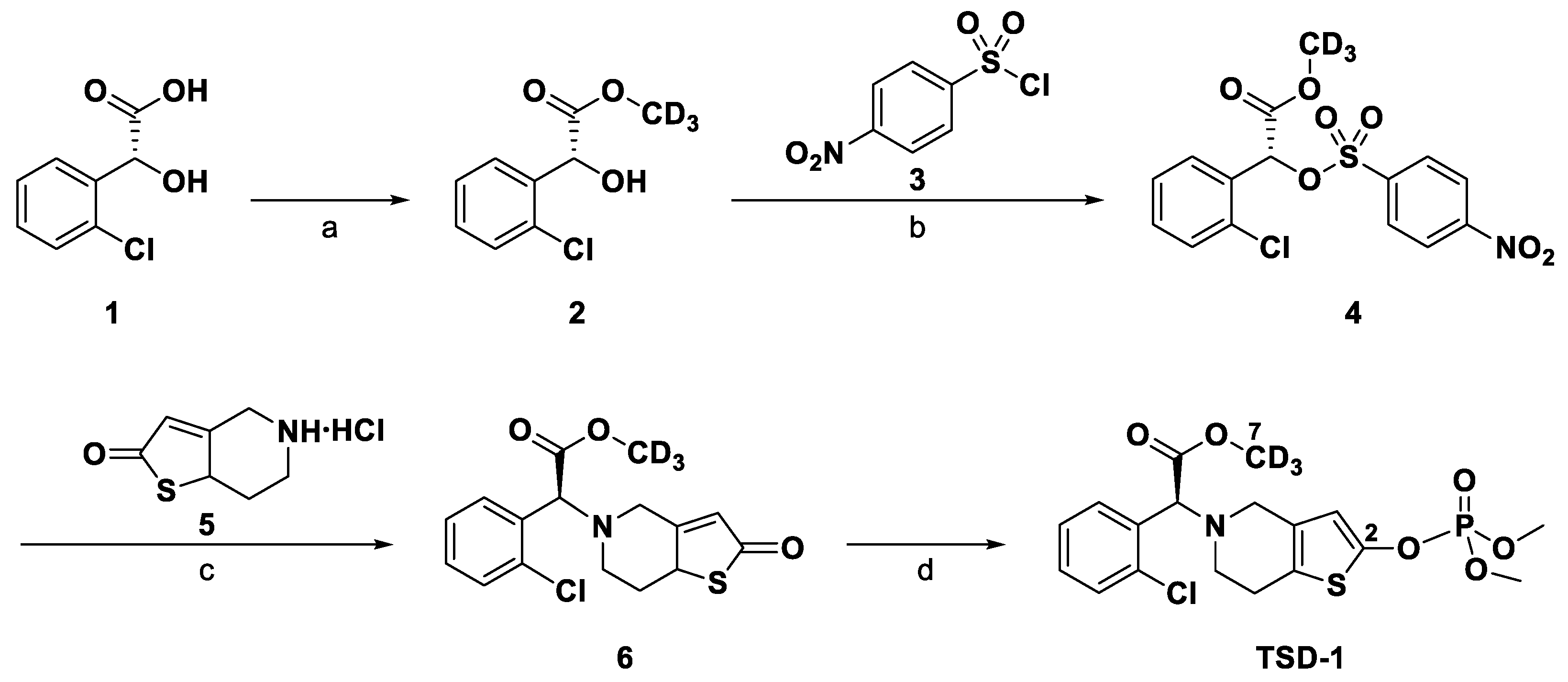
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. Optimization of Sample Preparation
2.1.2. Selection of Chromatographic Column and Separation
2.1.3. Optimization of Mass Spectrometric Parameters
2.2. Method Validation
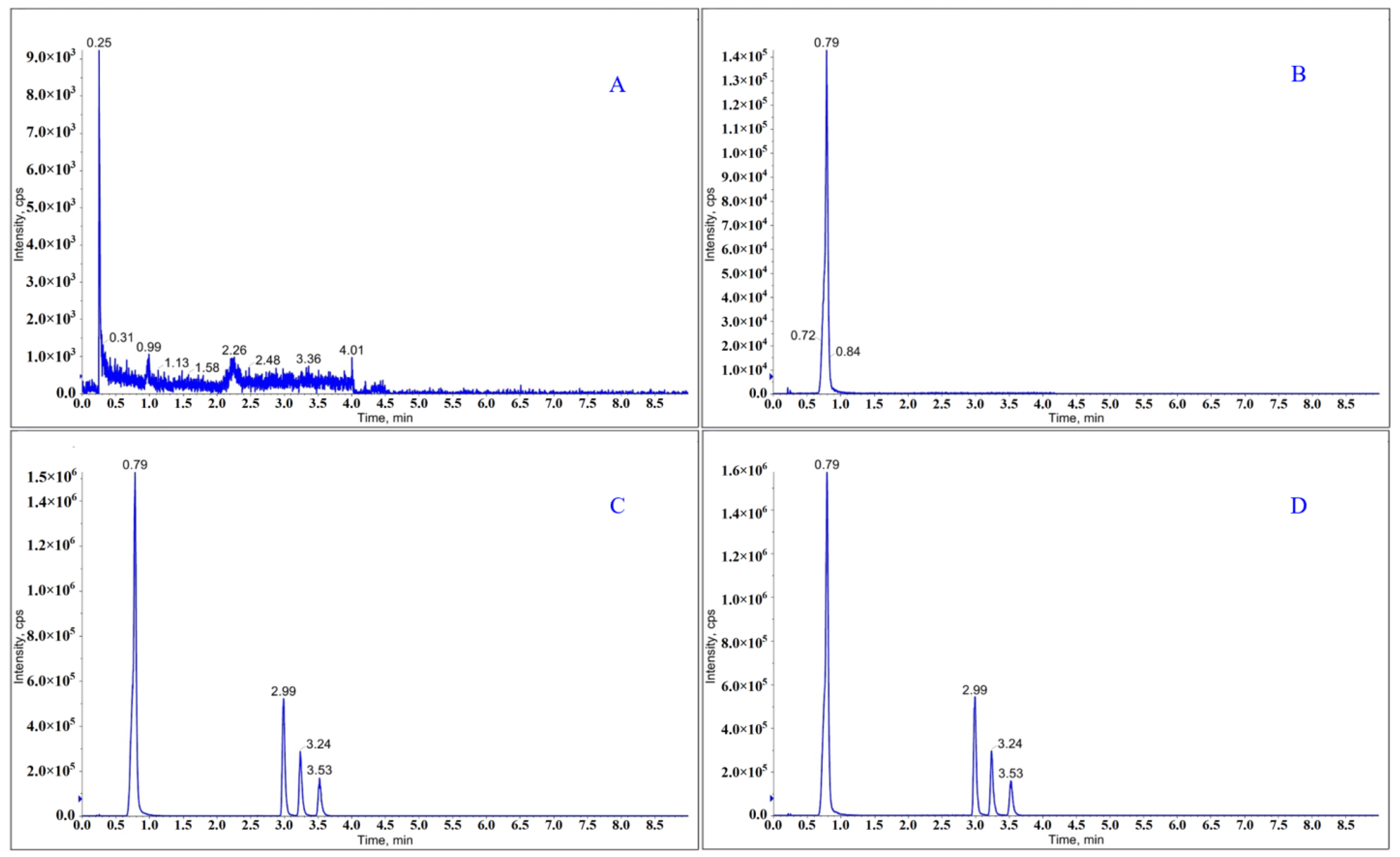
2.2.1. Specificity
2.2.2. LOD, LOQ, and Linearity
2.2.3. Accuracy
2.2.4. Repeatability and Intermediate Precision
2.2.5. Solution Stability
2.2.6. Robustness
2.3. Analysis of TSD-1 API Batches
3. Materials and Methods
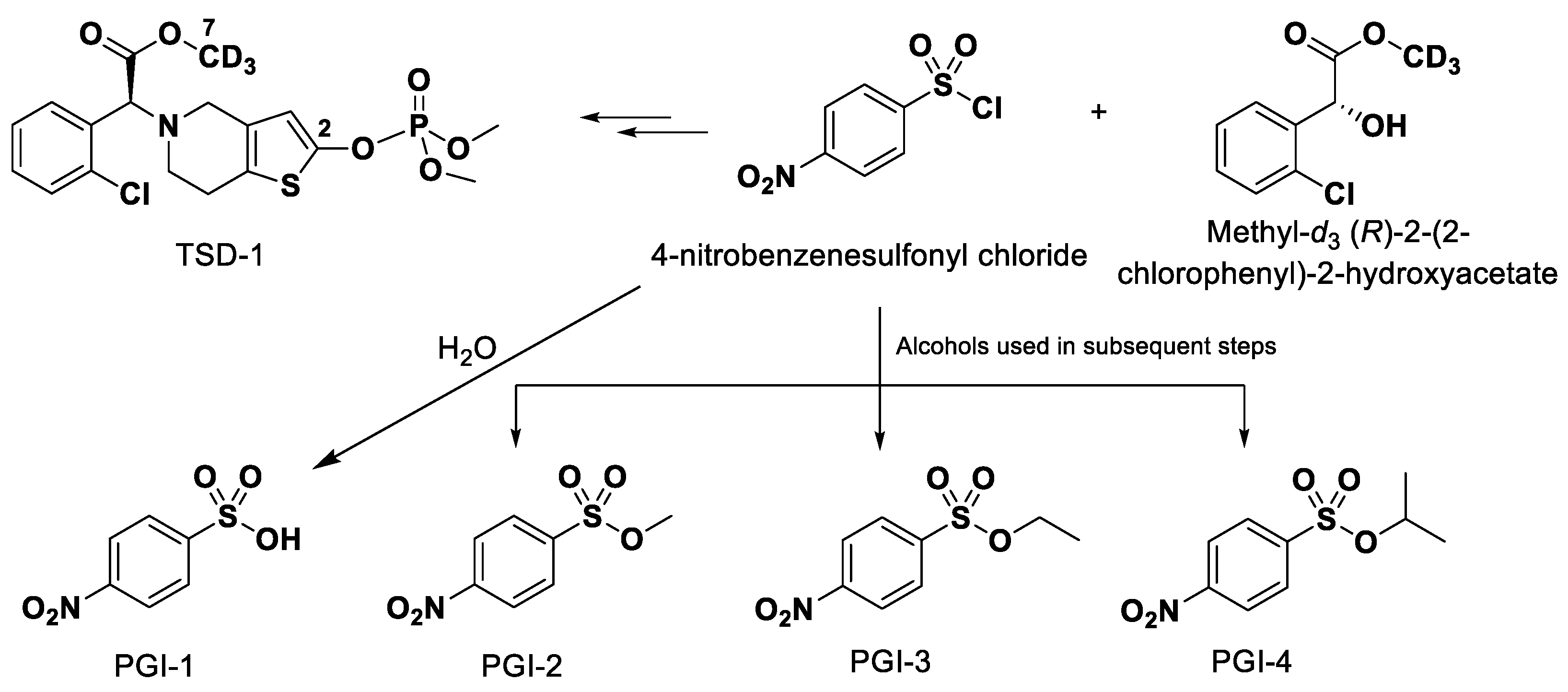
3.1. Chemicals and Reagents
3.2. Solutions for Method Validation
3.3. Chromatographic and Mass Spectrometric Conditions
3.4. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akkaif, M.A.; Daud, N.A.A.; Sha’aban, A.; Ng, M.L.; Abdul Kader, M.A.S.; Noor, D.A.M.; Ibrahim, B. The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules 2021, 26, 1987. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, R.; Nabilah, R.A.; Rasyid, A.; Harris, S.; Harahap, A.R.; Herqutanto, H.; Louisa, M.; Listyaningsih, E.; Rambe, A.S.; Loho, T. Clopidogrel Resistance Among Ischemic Stroke Patients and Its Risk Factors in Indonesia. Acta Med. Acad. 2022, 51, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Jafrin, S.; Naznin, N.E.; Reza, M.S.; Aziz, M.A.; Islam, M.S. Risk of stroke in CYP2C19 LoF polymorphism carrier coronary artery disease patients undergoing clopidogrel therapy: An ethnicity-based updated meta-analysis. Eur. J. Intern. Med. 2021, 90, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Honig, A.; Sacagiu, T.; Filioglo, A.; Simaan, N.; Kalish, Y.; Gomori, J.M.; Horev, A.; Leker, R.R.; Cohen, J.E. Clopidogrel underactivity is a common in patients with acute symptomatic severe carotid stenosis. J. Neurol. Sci. 2021, 425, 117450. [Google Scholar] [CrossRef] [PubMed]
- Song, B.L.; Wan, M.; Tang, D.; Sun, C.; Zhu, Y.B.; Linda, N.; Fan, H.W.; Zou, J.J. Effects of CYP2C19 Genetic Polymorphisms on the Pharmacokinetic and Pharmacodynamic Properties of Clopidogrel and Its Active Metabolite in Healthy Chinese Subjects. Clin. Ther. 2018, 40, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Tian, X.; Cai, W. Predicting the Effects of CYP2C19 and Carboxylesterases on Vicagrel, a Novel P2Y12 Antagonist, by Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling Approach. Front. Pharm. 2020, 11, 591854. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, X.; Yang, Z.; Wang, H.; Meng, X.; Su, C.; Liu, M.; Fawcett, J.P.; Yang, Y.; Gu, J. Significant Improvement of Metabolic Characteristics and Bioactivities of Clopidogrel and Analogs by Selective Deuteration. Molecules 2016, 21, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sabti, B.; Harbali, J. Development and validation of an analytical method for quantitative determination of three potentially genotoxic impurities in vildagliptin drug material using HPLC-MS. J. Sep. Sci. 2021, 44, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Srivastava, V.; Kumar, R.; Dangi, V.; Hiriyanna, S.G.; Kumar, A.; Kumar, P. Determination of five potential genotoxic impurities in dalfampridine using liquid chromatography. J. Pharm. Biomed. Anal. 2017, 133, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kalauz, A.; Kapui, I. Determination of potentially genotoxic impurities in crotamiton active pharmaceutical ingredient by gas chromatography. J. Pharm. Biomed. Anal. 2022, 210, 114544. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.M. Guideline on the limits of genotoxic impurities. In Evaluation of Medicines for Human Use; European Medicines Agency: London, UK, 2006; pp. 1–8. [Google Scholar]
- Guidance, F.D. Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches; US Food and Drug Administration, Department of Health and Human Services: Washington, DC, USA, 2008.
- Guideline, I.H. Assessment and control of dna reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7. In Proceedings of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human use (ICH), Geneva, Switzerland, 31 March 2017. [Google Scholar]
- Reddy, A.V.; Jaafar, J.; Umar, K.; Majid, Z.A.; Aris, A.B.; Talib, J.; Madhavi, G. Identification, control strategies, and analytical approaches for the determination of potential genotoxic impurities in pharmaceuticals: A comprehensive review. J. Sep. Sci. 2015, 38, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Christofi, M.; Markopoulou, C.K.; Tzanavaras, P.D.; Zacharis, C.K. UHPLC-fluorescence method for the determination of trace levels of hydrazine in allopurinol and its formulations: Validation using total-error concept. J. Pharm. Biomed. Anal. 2020, 187, 113354. [Google Scholar] [CrossRef] [PubMed]
- Grigori, K.; Loukas, Y.L.; Malenović, A.; Samara, V.; Kalaskani, A.; Dimovasili, E.; Kalovidouri, M.; Dotsikas, Y. Chemometrically assisted development and validation of LC-MS/MS method for the analysis of potential genotoxic impurities in meropenem active pharmaceutical ingredient. J. Pharm. Biomed. Anal. 2017, 145, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Chen, Y.; Xia, H.; Chen, Z.; Cheng, Q. Development and Validation of a Sensitive GC-MS/MS Method for the Determination of Five Potential Genotoxic Impurities in Abiraterone Acetate. J. Chromatogr. Sci. 2022, 60, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.; Hanser, T.; Judson, P.; Williams, R. Distinguishing between expert and statistical systems for application under ICH M7. Regul. Toxicol. Pharmacol. RTP 2017, 84, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Kashif, M. Use of Box-Behnken design for the nuclear magnetic resonance study of molecular complex of anticonvulsant drug with N,N-dimethylformamide and its application in quantitative analysis. J. Mol. Liq. 2021, 326, 115308. [Google Scholar] [CrossRef]
- Taylor, G.E.; Gosling, M.; Pearce, A. Low level determination of p-toluenesulfonate and benzenesulfonate esters in drug substance by high performance liquid chromatography/mass spectrometry. J. Chromatogr. A 2006, 1119, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Shi, Y.; Zheng, L.; Feng, F.; Zheng, F.; Liu, W. Rapid and simultaneous determination of sulfonate ester genotoxic impurities in drug substance by liquid chromatography coupled to tandem mass spectrometry: Comparison of different ionization modes. J. Chromatogr. A 2014, 1355, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, H.; Zhou, Y.; Qian, X.; Tu, J.; Chen, B.; Duan, G. Development and validation of a sensitive method for alkyl sulfonate genotoxic impurities determination in drug substances using gas chromatography coupled to triple quadrupole mass spectrometry. J. Pharm Biomed. Anal. 2019, 168, 23–29. [Google Scholar] [CrossRef] [PubMed]
- ICH, I. Q2 (R1): Validation of analytical procedures: Text and methodology. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, November 2005. [Google Scholar]
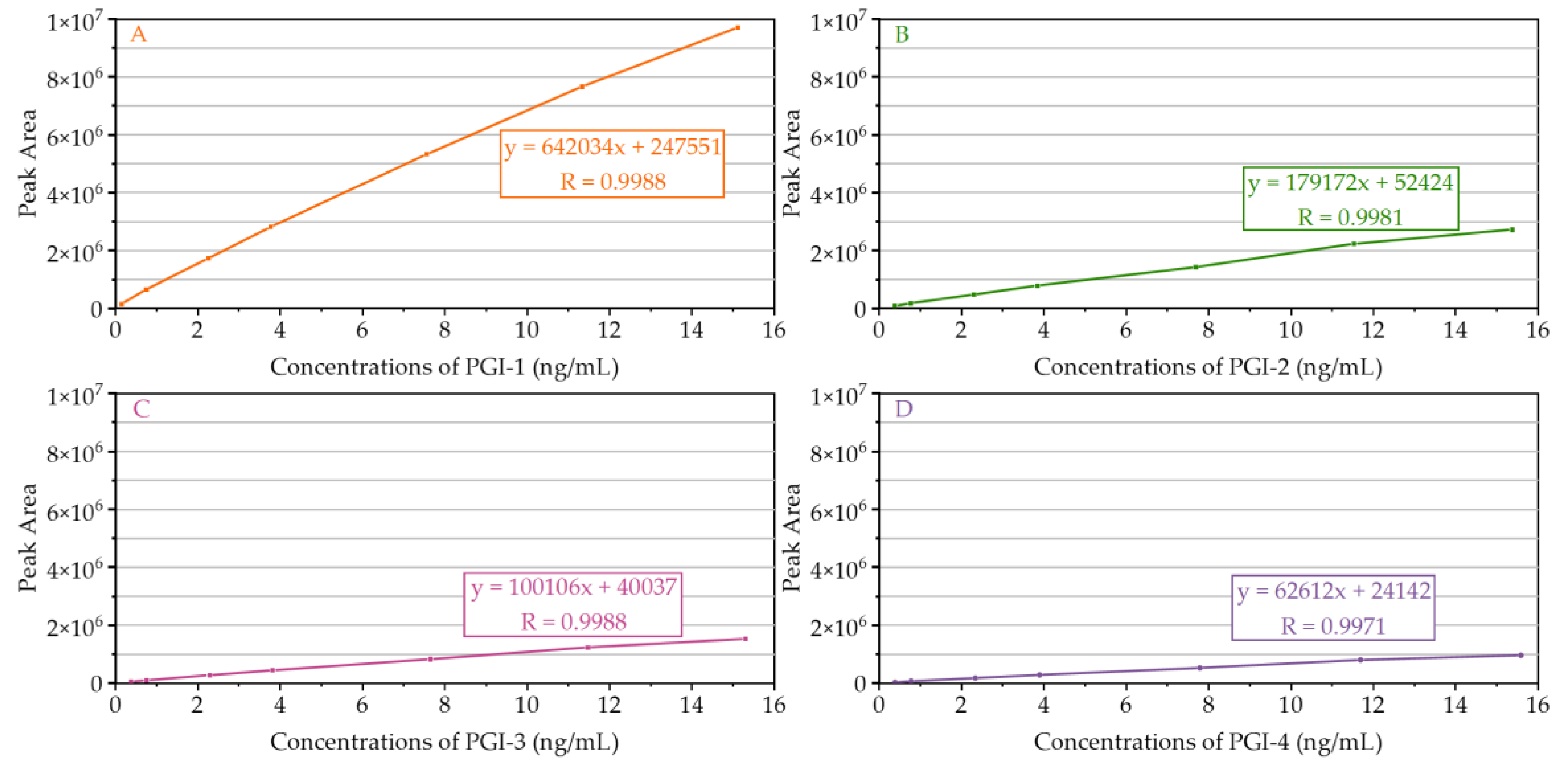
| Compound | Linear Range (ng/mL) | Calibration Curve Equation | Correlation Coefficient (R) | LOD (ng/mL) ± RSD (%) | LOQ (ng/mL) ± RSD (%) |
|---|---|---|---|---|---|
| PGI-1 | 0.1512–15.1158 | y = 642034x + 247551 | 0.9988 | 0.0453 ± 1.9 | 0.1512 ± 1.1 |
| PGI-2 | 0.3844–15.3778 | y = 179172x + 52424 | 0.9981 | 0.1153 ± 2.5 | 0.3844 ± 1.4 |
| PGI-3 | 0.3826–15.3046 | y = 100106x + 40037 | 0.9988 | 0.1148 ± 0.9 | 0.3826 ± 1.5 |
| PGI-4 | 0.3897–15.5864 | y = 62612x + 24142 | 0.9971 | 0.1169 ± 1.4 | 0.3897 ± 2.8 |
| Spike Level | PGI-1 | PGI-2 | PGI-3 | PGI-4 | |
|---|---|---|---|---|---|
| LOQ | Mean ± SD (%) | 94.9 ± 3.4 | 106.2 ± 4.7 | 111.4 ± 4.1 | 115.5 ± 2.8 |
| RSD (%) | 3.6 | 4.4 | 3.7 | 2.4 | |
| 100% | Mean ± SD (%) | 101.3 ± 0.5 | 102.0 ± 1.4 | 100.0 ± 1.0 | 102.1 ± 0.6 |
| RSD (%) | 0.5 | 1.4 | 1.0 | 0.6 | |
| 150% | Mean ± SD (%) | 98.6 ± 0.7 | 100.3 ± 1.9 | 100.3 ± 3.2 | 100.9 ± 0.5 |
| RSD (%) | 0.7 | 1.9 | 3.2 | 0.5 | |
| Compound | PGI-1 | PGI-2 | PGI-3 | PGI-4 | |
|---|---|---|---|---|---|
| Analyst 1 | Mean ± SD (%) | 97.7 ± 1.5 | 111.4 ± 1.0 | 92.1 ± 1.1 | 97.5 ± 2.0 |
| RSD (%) | 1.5 | 0.9 | 1.2 | 2.1 | |
| Analyst 2 | Mean ± SD (%) | 98.6 ± 2.1 | 101.2 ± 3.4 | 97.8 ± 2.0 | 102.9 ± 3.3 |
| RSD (%) | 2.1 | 3.3 | 2.1 | 3.2 | |
| Compound | Original Condition | Flow Rate (mL/min) | Column Temperature (°C) | Percentage of Acetonitrile (%) | Mean ± SD (%) | RSD (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| 0.28 | 0.32 | 28 | 32 | 18 | 22 | ||||
| PGI-1 | 99.7 | 99.9 | 99.5 | 99.3 | 99.4 | 98.5 | 99.6 | 99.4 ± 0.5 | 0.5 |
| PGI-2 | 112.5 | 107.1 | 123.0 | 127.6 | 112.5 | 97.8 | 96.2 | 111.0 ± 11.8 | 10.6 |
| PGI-3 | 90.4 | 94.7 | 102.7 | 101.7 | 94.1 | 96.4 | 99.1 | 97.0 ± 4.4 | 4.6 |
| PGI-4 | 94.3 | 106.2 | 99.8 | 99.5 | 106.4 | 107.6 | 102.0 | 102.3 ± 4.8 | 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Yang, H.; Yang, J.; Guo, N.; Wu, G.; Xu, X.; An, M. Quantitative Determination of Four Potential Genotoxic Impurities in the Active Pharmaceutical Ingredients in TSD-1 Using UPLC-MS/MS. Molecules 2022, 27, 4129. https://doi.org/10.3390/molecules27134129
Wang T, Yang H, Yang J, Guo N, Wu G, Xu X, An M. Quantitative Determination of Four Potential Genotoxic Impurities in the Active Pharmaceutical Ingredients in TSD-1 Using UPLC-MS/MS. Molecules. 2022; 27(13):4129. https://doi.org/10.3390/molecules27134129
Chicago/Turabian StyleWang, Taiyu, Hailong Yang, Jie Yang, Ningjie Guo, Guodong Wu, Xueyu Xu, and Ming An. 2022. "Quantitative Determination of Four Potential Genotoxic Impurities in the Active Pharmaceutical Ingredients in TSD-1 Using UPLC-MS/MS" Molecules 27, no. 13: 4129. https://doi.org/10.3390/molecules27134129





