Abstract
Speciality malts and their extracts have physicochemical characteristics such as colour, flavour, and aroma sorted for in food production. Speciality malts used in food production are mostly produced from cereal grains. Hence, this study aimed to produce speciality malts from Bambara groundnut (BGN) seeds and analyse their physicochemical characteristics and metabolites. The base, toasted, caramel, and roasted malt were produced by drying at different temperatures and times. Syrups were produced isothermally from the speciality malts. The speciality malts and syrups were assessed for colour, pH, protein, α and β-amylases, total polyphenols, antioxidants, and metabolite profiling. The BGN speciality malts were assayed for fatty acid methyl esters (FAME), hydrocarbons, sugar alcohols, sugars, acids, amino acids, and volatile components using capillary gas chromatography-mass spectrometry (GC-MS) and gas chromatography with flame ionisation detection (GC-FID). The colours of the speciality malts and syrups were significantly (p = 0.000) different. The protein content of the BGN speciality malts was significantly different (p = 0.000), while the protein content of the syrups was not significantly different. The amylase activities of the BGN speciality malt decreased with the change in kilning temperatures and time. The α- and β-amylase activities for the specialty malts were 1.01, 0.21, 0.29, 0.15 CU/g and 0.11, 0.10, 0.10, 0.06 BU/g. The total polyphenols and antioxidant activities differed for all BGN speciality malts. There were twenty-nine volatiles detected in the BGN speciality malts. Fifteen amino acids consisted of seven essential amino acids, and eight non-essential amino acids were detected in the speciality malts. Fatty acid methyl esters (FAME) identified were palmitoleic, oleic, linolelaidic, linoleic, and arachidic acid. The sugars, organic acids, and sugar alcohols consisted of lactic acid, fructose, sucrose, and myo-inositol. The BGN speciality malts exhibited good physicochemical characteristics and metabolites that can make them useful as household and industrial ingredients for food production, which could be beneficial to consumers.
Keywords:
Bambara groundnut; α-amylase; β-amylase; total polyphenols; antioxidant; steeping; sprouting; metabolites 1. Introduction
Household processing such as dehulling, boiling/cooking, pressure cooking, milling, roasting, fermentation, soaking, and malting are applied to improve the physicochemical properties of cereals and legumes [1,2,3,4]. Malting is an inexpensive household food processing method that has recently gained attention from researchers to study legumes [4,5,6,7,8]. Malting consists of three simple steps: steeping, sprouting, and drying under controlled conditions [9,10].
Malting of legumes has been reported to encourage an increase in free amino acids and vitamins by the modification of the functional properties of the seed’s physical and chemical components [11,12,13]. In addition, malting promotes hydrolytic enzymes, which are not present in ungerminated grains [14,15]. Due to the activation of the hydrolytic enzyme, the malting process (soaking, sprouting, and kilning) gives malted grains their characteristic colour, taste, flavour, and nutritional components [16,17,18].
The final step of malting, kilning, is a biochemical process applied to cereals and legumes to enhance their physicochemical properties. The kilning temperature and time are increased to obtain desired malt properties such as enzymes, moisture removal for stabilisation, raw flavours removal, malty flavours, and colour development [19,20,21,22,23]. During the kilning process, the reaction of sugars and amino acids promotes melanoidins and reductones through the Maillard reaction [19,24,25,26]. The melanoidins formed are responsible for the antioxidant potential of the speciality malt types [21,24,26,27]. For example, the dark speciality malts’ Maillard reaction products (melanoidins) are significant antioxidants that increase with increasing malt colour due to changes in kilning temperature and time [23,25,28].
Producing speciality malts comes with different drying temperatures [24,29,30]. Base malts are produced at a low temperature between 50 and 80 °C for their high degree of diastatic power. The base malt mostly precedes other speciality malts such as roasted, toasted, and caramel malts used in food processing for various benefits [24,31,32]. Caramel, roasted, and toasted malts are termed speciality malts because they are produced primarily because of their characteristic high antioxidants, colour, and flavour [28,30,32,33]. Generally, speciality malts and their extracts (syrup) add sensory benefits to the final product by enhancing their colour, flavour, and taste [34,35].
Barley is the most used ingredient in malt production, and it is majorly used in the brewing and food industries [36]. Although barley malt is commonly used, other cereals and legumes are also malted to access their nutritional value [37]. Mung beans, soybeans, cowpea, black beans, lentils, chickpea, and Bambara groundnut are some of the legumes that have been sprouted and studied for their nutritional, physicochemical, and functional characteristics [38].
Bambara groundnut (BGN) is not a commonly known legume crop in many parts of the world. However, it is categorised as the third most crucial legume in Africa, after peanuts and cowpeas [39,40]. Sustainable food experts have gained interest in BGN because it is an underutilised and nutritious crop [41]. BGN is an indigenous plant cultivated in Africa on a small scale by subsistence farmers [42,43]. It is a good quality protein food containing substantially high proteins, carbohydrates, fats, and minerals [13,40].
Following the malting process, BGN seeds’ physicochemical, functional, thermal, health-promoting, and nutritional properties greatly improved while reducing their anti-nutritional factors [13,15]. Abba et al. [44] noted that malting BGN improved its protein content. Value-added snacks, weaning, ready-to-eat, and composite products have been made from malted BGN seeds and have shown improvement over the unmalted seeds [45,46]. The nutritional and functional characteristics of malted BGN in the production of Okpa, composite biscuits, flours, and infant formula, have been investigated [46,47], where the malted BGN samples were acceptable to consumers. Bambara groundnut was subjected to steeping (36 and 48 h) and sprouting (0 to 144 h) at different times to study its physicochemical characteristics [48]. The study reported that the BGN malt exhibited good physicochemical characteristics peculiar to malt products and can be used as a functional food ingredient in food and beverage formulations [48]. In this study, the amylase-rich BGN malt produced by steeping for 36 h and sprouting for 96 h was used to produce speciality malts. However, no documented study has reported the physicochemical characteristics of BGN speciality malts and syrup products. Thus, this work investigated the production of BGN speciality malts and syrups, their physicochemical characteristics, and metabolites.
2. Results and Discussion
2.1. Colour Characteristics of Bambara Groundnut Speciality Malts and Syrups
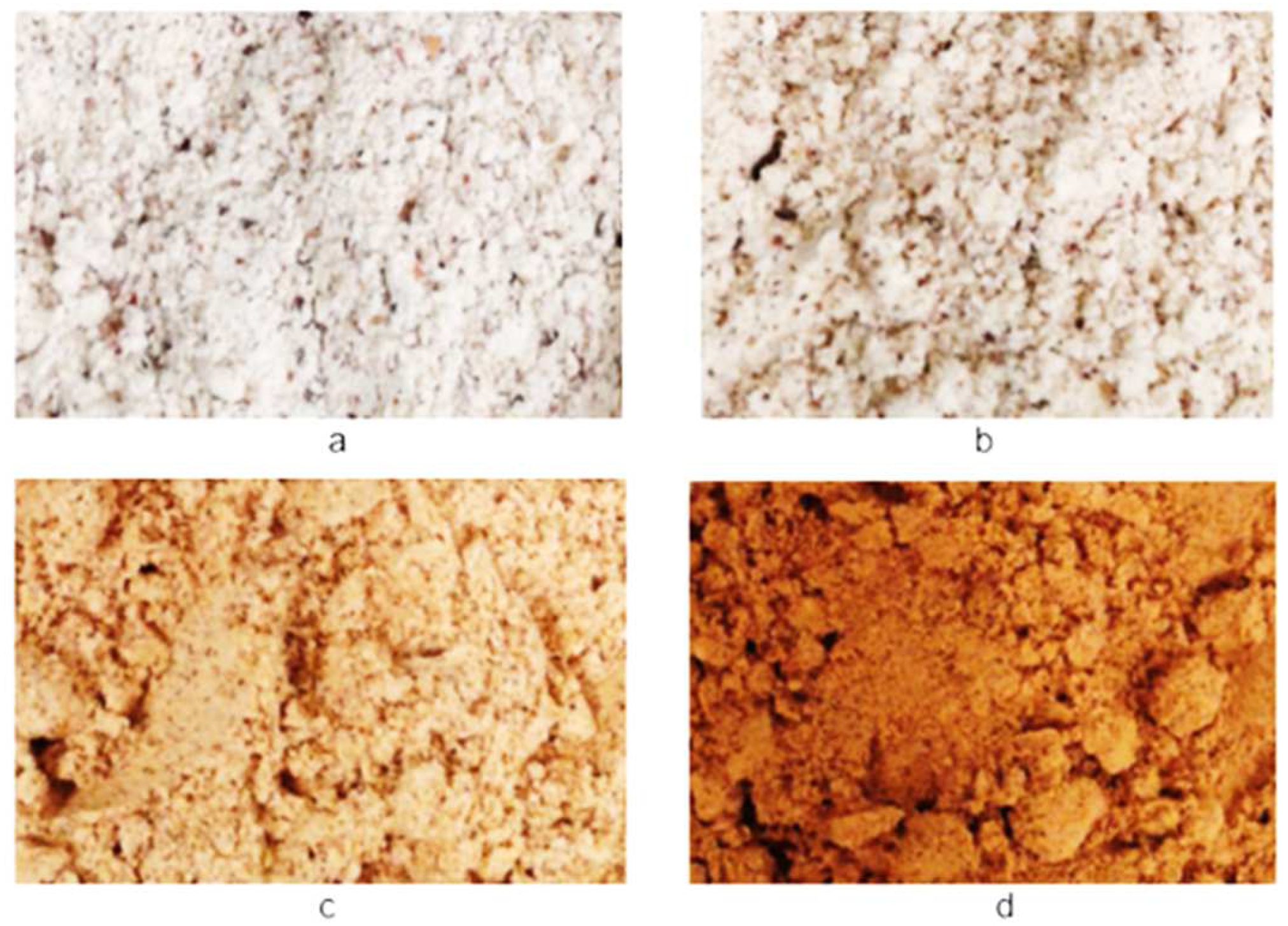
The CIE L*a*b* colour space coordinates, chroma, and hue of the BGN speciality malts consisting of base (BM), caramel (CM), roasted (RM), and toasted (TM) malts are shown in Table 1. The lightness (L*) and the hue angle (h°) decreased from 74.12 to 45.98 and 71.54 to 53.90 for the BGN speciality malt types. The redness (a*), yellowness (b*), and chroma increased for the BGN speciality malts, 3.96 to 16.44, 11.85 to 22.68, and 12.50 to 28.03, respectively. There was a significant difference across the lightness, redness, yellowness, chroma, and hue for all the speciality malts. As seen by the physical eye, the colour of the speciality malt was as shown in Figure 1.

Table 1.
Colour characteristics of Bambara groundnut speciality malt 1.

Figure 1.
Bambara groundnut speciality malts: (a) base malt, (b) caramel malt, (c) roasted malt, and (d) toasted malt.
The colour change has been attributed to the non-oxidative Maillard reaction due to heat [32]. The reaction between reducing sugars and amino acid contents of malted grains consists of the Maillard reaction’s complex reactions [49]. The reactions are important mechanisms of non-enzymatic browning during heat processing of malt [50]. The factors affecting the degree and magnitude of the Maillard reaction are temperature, time, water activity, and concentration [32]. These factors affect the end product and give the products their characteristic colour, flavour, and anti-oxidative activity, which are essential in industrial food products [51].
The colour of the malt is greatly affected by temperature and time [52]. Yahya et al. [53] reported a similar result in the production of barley and malt roasting operations where the product became darker as temperature increased above 150 °C, showing lightness (L*) reducing from 75 to 40. Furthermore, the evaluation of the colour coordinates in the study of barley speciality malt features showed a colour difference, whereby there was a decrease in lightness (L*) at high temperatures, while the redness (a*) and yellowness (b*) increased at higher temperatures [33]. Studies on the development of Maillard reaction during roasted (caramel) malt production demonstrated that the colour formation depends mainly on the time and temperature of kilning [24,28,54].
However, the colour changes were attributed to the measure of shorter chain melanoidins or caramelisation by conversion to darker-coloured malt with increased temperatures [22]. The base, caramel, roasted, and toasted malt syrup’s lightness values are indicated in Table 2. The chroma and hue angle (h°) for the base, caramel, roasted, and toasted malt syrups ranged from 8.68 to 21.75 and 59.20 to 73.53°, respectively. The hue angle (ho) of the BGN syrups represents the red to yellow colour range (0°–90°). There is, however, a significant (p = 0.000) difference in the speciality malt syrup’s lightness, redness, yellowness, and chroma, except for hue. The higher colour values signify the BGN speciality malt syrup colour’s intensity. The reduction in lightness of the syrups from base malt syrup to toasted malt syrup was attributed to the Maillard reaction developing Maillard reaction products during heating and caramelisation [55,56]. The parameter redness (a*) and yellowness (b*) were positive values indicating reddish and yellowish syrup colours. The redness was highest for the roasted malt syrup, and the yellowness was lowest for toasted malt syrup, attributed to the differences in the kilning temperatures and time [57]. The BGN speciality malts syrups’ chroma was lowest for the toasted BGN speciality malt syrup. The chroma values refer to the colour saturation, where low chroma values are weak, and high chroma values are highly saturated or strong [58,59]. The chroma values for the study on rice syrup were low, with a dark brown colour range compared to this study [25,60].

Table 2.
CIE L*a*b*, chroma, and hue for the Bambara groundnut speciality malt syrups 1.
The speciality malts’ hue angle was higher than that of the syrups due to heat application during mashing. Hue angle (h°) is the attribute of a colour distinguished by the red, yellow, blue, green, or purple object [58]. Hue angle (h°) can accurately determine how humans perceive colour, as shown in Figure 2 [60]. The BGN speciality malt syrup hue angle range of 59.20 to 73.53° indicated reddish-yellow. The hue angle (h°) of the speciality malt syrup is consistent with other studies that reported a decrease in h° value during heat application to syrup [57,58]. In addition, the colour of the BGN speciality malt syrup indicated that more colour developed during the wort boiling based on temperature and time [61].

Figure 2.
Bambara groundnut speciality malt syrups: (a) base malt syrup, (b) caramel malt syrup, (c) roasted malt syrup, (d) toasted malt syrup.
Malt extract boiling generally increases wort colour due to the formation of melanoidins, the caramelisation of sugars, and polyphenols’ oxidation [62]. The application of heat reduced the lightness (L*), redness (a*), yellowness (b*), and the hue of the syrup for all BGN speciality malt syrups, while hue angle (h°) increased. Moreover, the toasted malt syrup exhibited the darkest colour for the colour parameters (CIE L*a*b*, chroma). The decrease in lightness, redness, and yellowness of speciality malt syrup has been attributed to the formation of colour compounds (melanoidins) due to the Maillard reaction during kilning and further heating when producing syrups, thus providing desirable colours to food produced with them [60].
The speciality malt, extracts, and syrups are good sources of natural colour enhancement in food industries for beverages, baked items, and culinary recipes [63,64]. The colour enhancement can be attained using a base malt ratio with specialised malt flours, malt extracts, or syrup [63,65]. The speciality malts and syrups in this study exhibited colours desirable in the food industries, which could be used to impact the colours of baked goods and breakfast meals similar to the popular barley malt [22]. Furthermore, being significantly different could mean that the speciality malts and syrups could impact different shades of colours as ingredients in product formulation.
2.2. The pH Characteristics of the Bambara Groundnut Speciality Malts and Syrups
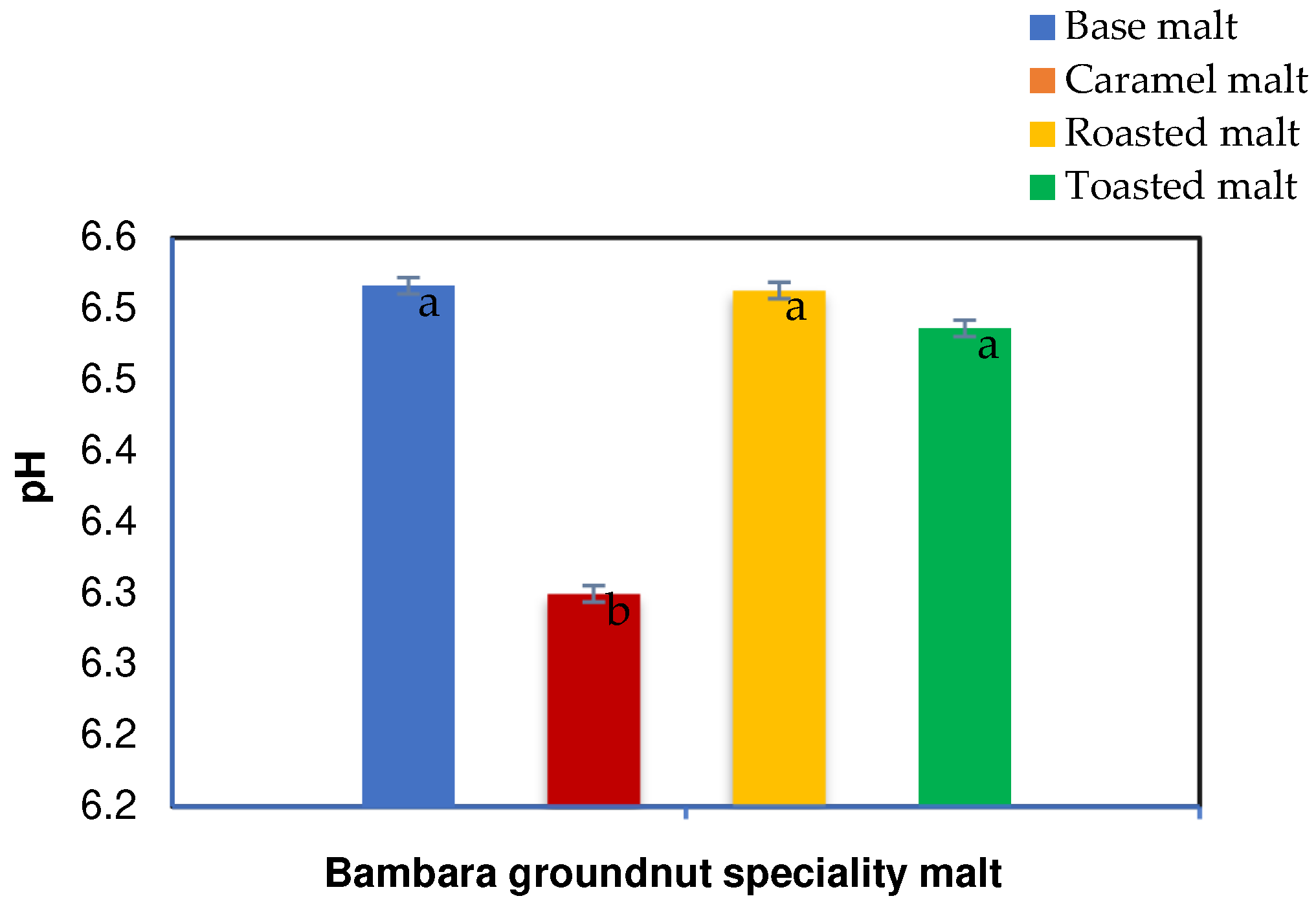
The pH for the BGN speciality base (BM), caramel (CM), roasted (RM), and toasted (TM) malts ranged from 6.30 to 6.52. The base, roasted, and toasted malts were not significantly different, as shown in Figure 3. However, the base, roasted, and toasted malts exhibited higher pH than the caramel malt. The caramel malt had the lowest pH value, while the higher pH in the three speciality malts has been attributed to variations in temperature and time [33]. However, the high pH of the base malt is a characteristic of base malt [66]. During their study, Vandecan et al. [33] showed that the time and temperature of roasting caramel malt resulted in a pH increase. Their results showed that malt pH decreased with increasing kilning temperature due to the acidic Maillard reaction products, reductones and melanoidins. However, there was a pH increase after the initial decrease with increased roasting temperature to 180 °C, similar to this study. The pH increase was ascribed to the decline in the concentration of acidic components due to evaporation, further conversion, and polymerisation reactions [24,33]. In their study, Geurts [67] noted that the malt pH depends on the production method used to create speciality malt. The effects of pH on speciality malts have been studied, where it was discovered that dark malts tend to exhibit higher pH than pale (base malt) and light caramel malt [67,68]. The high pH is due to the dark roasted and toasted malt products being roasted at high temperatures that are enough to use Maillard reaction, caramelisation, and pyrolysis, which can affect the pH of the speciality malt [67,69].

Figure 3.
pH characteristics of Bambara groundnut speciality malt. Values are mean ± standard deviation of triplicate values; mean values on the bars with different letters are significantly different (p < 0.05).
The characteristic pH values of the BGN speciality malt syrups for the base, caramel, roasted, and toasted malt syrups were 5.52, 5.13, 5.46, and 5.71, respectively, in Table 3. The pH of the toasted malt syrup was much higher than the base, caramel, and roasted malt syrups, with a significant (p = 0.000) difference. pH is crucial in wort production; it regulates the activity of the enzymes (external and internal) in the mash [70]. The mashing and wort boiling period is the application of heat treatment that can separate the calcium ion (Ca2+) bound with phosphates (K2PO4) and polypeptides to form insoluble compounds by the release of hydrogen ion (H+) and decrease the wort pH [70,71,72,73]. Due to boiling, the wort becomes acidic with a range of 0.1–0.3 pH units for a typical boiling process due to the melanoidins formation [61,62]. Moreover, the pH of the BGN caramel, roasted, and toasted speciality malt syrups was lower and was attributed to the formation of acids from sugars compared to the base malt syrup [62,67,72].

Table 3.
pH characteristics of Bambara groundnut speciality malt syrups 1.
Due to heat application during boiling, the pH is relatively low after malt syrup production [73]. The pH values of the wort produced from chickpea, yellow pea, common vetch, and green lentil were 5.44, 5.7, 5.53, and 5.51, respectively [74]. These are in the same range as this study’s BGN speciality malt syrup. The pH of the BGN speciality malt syrups is in the same range as that of the barley malt syrups under study in the specific European brewery convention range [74,75,76,77]. The BGN speciality malt syrups exhibiting a similar pH range might make them useful in brewing industries as a substitute for malted barley. Thus, producing BGN malt syrups isothermally, as described in this study, produced products that could be used in product formulation, promoting BGN as a functional ingredient.
2.3. The Protein Content of Bambara Groundnut Speciality Malts and Syrups
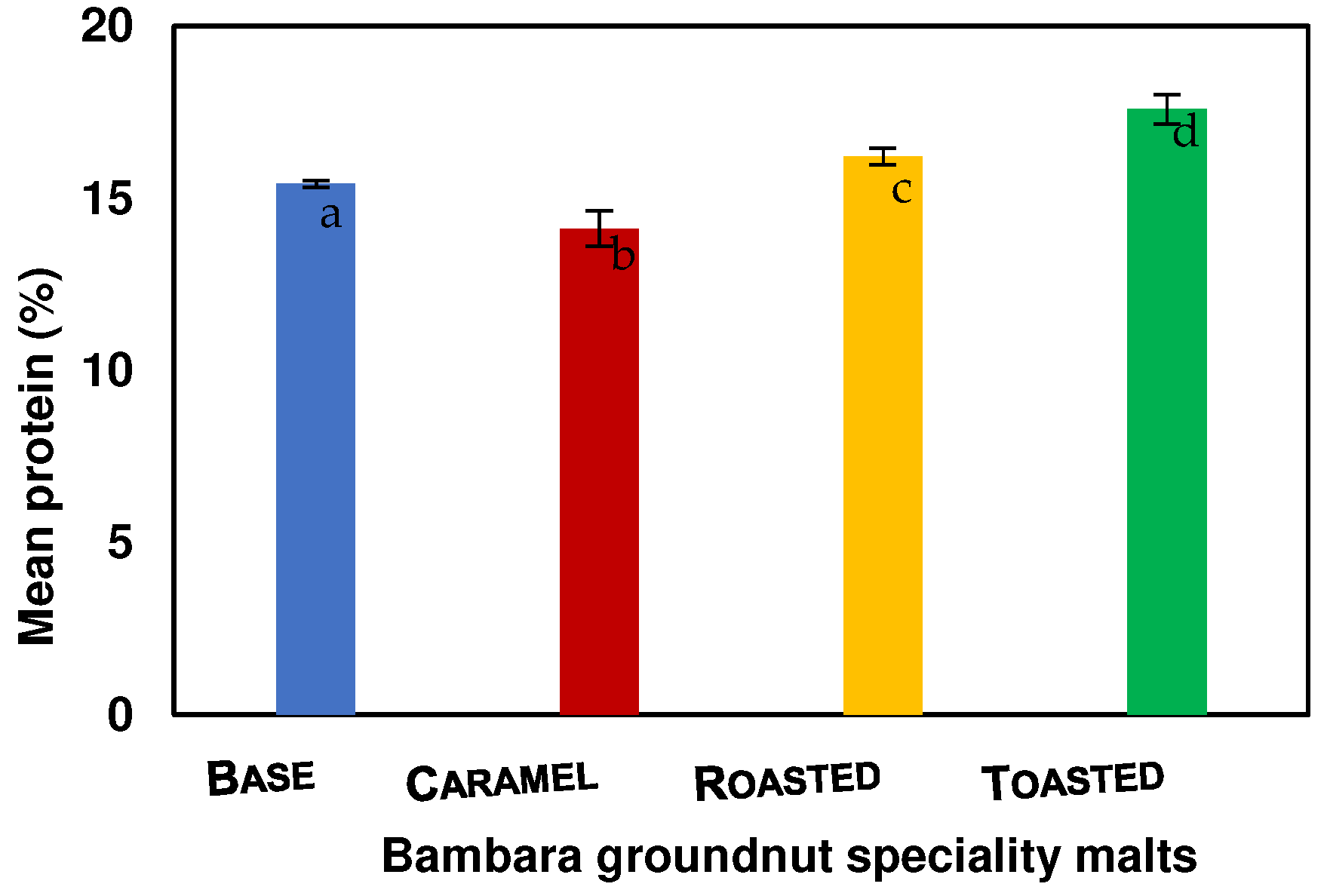
The protein content values of the base, caramel, roasted, and toasted BGN speciality malts are 15.41, 14.12, 16.22, and 17.58, respectively, as shown in Figure 4. The protein contents for the BGN speciality malts are significantly (p = 0.000) different, with caramel malt having the lowest protein content. The difference in the protein content could be due to different kilning temperatures and times. It was noted that high kilning temperatures of malted HomChaiya rice influenced the protease enzymes similar to this study, which invariably increased the soluble protein and amino acids [51]. Therefore, the increase is attributed to the soluble protein as kiln temperature increased due to an acceleration of proteolytic activities.

Figure 4.
Protein content of Bambara groundnut speciality malts. Values are the mean of triplicates ± standard deviation; mean values on the bars with different letters are significantly different (p < 0.05).
In contrast to the results of this study, Diedericks et al. [40] reported a reduction in the protein of BGN seeds subjected to roasting from 70 to 179 °C for soaked and unsoaked BGN seeds. They attributed the reduction to the exposure to high temperature due to denaturation of proteins depending on their thermal stability. However, [78] recorded no difference in protein content at different temperatures up to 100 °C in Greek barley.
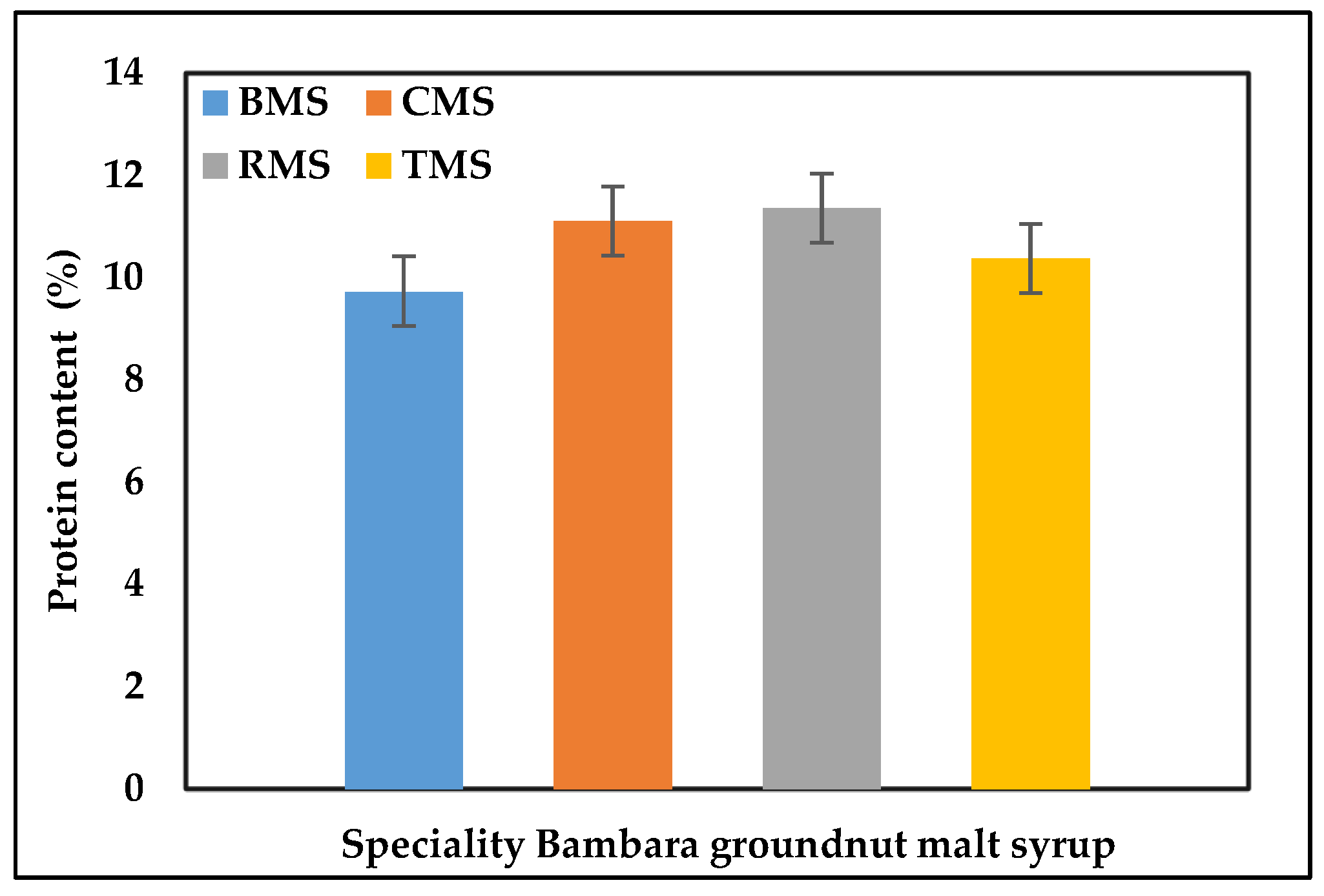
The Bambara groundnut speciality malt syrups protein content was lower after mashing and boiling, ranging from 9.73, 10.37, 11.10 to 11.35 for the base, caramel, roasted, and toasted malt speciality syrups, respectively, as shown in Figure 5. Based on the Kruskal–Wallis test, protein distribution was the same across the syrups, showing no significant difference. However, [74,79,80] produced gluten-free worts and malt extracts from legumes, resulting in high protein content with reduced anti-nutritional constituents and increased antioxidants. Wort boiling is a thermal process whereby various chemical, physicochemical, physical, and biochemical reactions occur. The boiling of the wort is important for sterilising the wort, stopping enzymatic reactions, water evaporation from the wort, unwanted aroma compounds removal, and hot break or hot trub, which is the precipitation of the wort protein contents’ insoluble coagulum [62,81].

Figure 5.
The protein content of the BGN speciality malt syrup, BMS—base malt syrup, CMS—caramel malt syrup, RMS—roasted malt syrup, TMS—toasted malt syrup.
The protein content of malt is dependent on the enzyme-to-substrate ratio, that is, the ratios of α- and β-amylases/starch and endo-peptidases/proteins [62]. The protein contents of the malt extract decrease after boiling, which matches the results reported by [82] and [83]. The reduction in protein can be attributed to protein degradation during mashing and wort boiling [84,85]. In contrast to the reduction in protein observed in this study and literature, [83] established that the barley wort protein content increased, and this was ascribed to the elevated stability of the soluble proteins. However, the BGN speciality malt syrup exhibited a good proportion of protein content that could benefit consumers.
2.4. Amylase Activities of Bambara Groundnut Speciality Malts and Syrups
The α and β-amylase activities for the base, caramel, roasted, and toasted BGN speciality malts were 1.01, 0.21, 0.29, 0.15 CU/g and 0.11, 0.10, 0.10, 0.06 BU/g, respectively, as shown in Table 4. The amylase activities of the BGN speciality malts differed significantly (p = 0.000) across the malt types. Studies have shown that amylase activities change with changes in kilning temperature and time, which is similar to this study’s results [36,51,86,87]. Kilning temperature and time of the germinated sorghum grains reduced the α- and β-amylase activities [88]. Uriyo [89] observed that kilning black-eyed peas at higher temperatures reduced the α-amylase activities, and β-amylase activity could not be detected in the germinated cowpea. The α and β-amylase of cowpea, buckwheat, sorghum, teff, and barley malt were found to decrease linearly with an increase in drying temperature [87,90,91,92]. As with other studies on sorghum, buckwheat, teff, barley, and cowpea, β-amylase was low or absent after kilning, which correlates with this study on BGN speciality malt [89,93]. In addition, α- and β-amylase decreased with a change in kilning temperature and time, where the β-amylase showed the lowest value. The resultant reduction in α and β-amylase regarding kilning temperature and time was because diastatic enzymes can only survive in mild kilning due to the formation of heat-stable complexes in the starch granules [94]. The decrease in the enzymatic activity could thus be due to the heat denaturation of grains, known as the enzyme-inactivating phase [86,90,95]. The barley α-amylase is more thermostable than β-amylase; the α and β-amylase of the BGN speciality malts have shown similar thermostability [87,91]. There was an increased inactivation by kilning due to denaturation by heat application [87,95,96]. Despite the heat application during kilning, mashing, and boiling of malt wort to produce syrup, some amylase survives [94,97,98,99].

Table 4.
Bambara groundnut specialty malts α and β-amylase activities 1.
The base, caramel, roasted, and toasted BGN speciality malt syrups (BMS, CMS, RMS, and TMS) α-amylase values were 0.39, 0.31, 0.30, 0.31 CU/g, and β-amylase values were 0.14, 0.13, 0.15, 0.21 BU/g, respectively, as shown in Table 5. There is a significant (p = 0.000) difference across the amylase activities of the speciality malt syrups. The increase in the α and β-amylase activities observed in the BGN speciality malt syrups after wort boiling is due to the activities of the enzymes [61,100]. The production of malt-based syrups involves producing the malt, the mashing process to produce wort from the malt, and the concentration of the wort to malt syrup by boiling [74,101]. Characteristics of malt syrup are brown, sweet, gluey liquids with diastatic enzymes (base malt) or without diastatic enzymes (speciality malt) [102]. Speciality malts are very important for enhancing and improving malt wort (syrup) by improving its colour and flavour [102].

Table 5.
Amylase activities of Bambara groundnut speciality malt syrups 1.
The α-amylase activity of the base malt syrup was the highest, while the roasted malt syrup activity was shown to be the lowest. The β-amylase is thermally unstable; it is denatured at high temperatures, thus the low content in this study [97]. The mashing and wort boiling temperature could have affected the β-amylase content due to the mashing temperature of 60 °C in this study [100]. In their study, De Schepper et al. [103] noted that α-amylase and β-amylase are temperature-dependent. α-amylase is inactivated at 63–71 °C and β-amylase at 54–66 °C [103]. These two enzymes are very important as α-amylase breaks complex, insoluble starch molecules into smaller, soluble molecules that are more stable thermally. α-amylase produces low molecular weight sugars, glucose, maltose, and maltotriose. β-amylase, being an unstable enzyme at high temperatures, produces only maltose. Once its activity reaches a peak, it declines and then drops at an increase in temperature [104,105,106]. The activities of these enzymes (α- and β-amylase) are relatively dependent on the temperature and time of mashing and wort boiling [103], as shown in this study. The inactivation is thus attributed to the starch hydrolyses by the two enzymes. α-amylase is an endo-acting enzyme that degrades starch during mashing and cleaving α-1,4-D-glucosidic linkages to produce oligosaccharides and limit dextrins [97,107,108]. On the other hand, β-amylase is an exo-acting enzyme, hydrolysing starch and oligosaccharide α-1,4-D-glucosidic linkages from the non-reducing end to produce maltose [109,110,111]. Thus, having enzyme-rich malt and syrup would greatly depend on the extraction temperature due to the heat-sensitive nature of the α- and β-amylases. However, boiling the BGN syrup at temperatures lower than 60 °C could increase amylase concentrations.
2.5. Total Polyphenols Content and Antioxidant Activities of Bambara Groundnut Speciality Malts and Syrups
Total polyphenols content and antioxidant activities of BGN speciality malts are illustrated in Table 6. There was a significant (p = 0.000) difference with an increase in total polyphenols and antioxidants content from 1.50 to 3.11 mg GAE/g. Moreover, the antioxidants increased, where FRAP ranged from 4.89 to 15.89 µmol AAE/g and DPPH ranged from 6.36 to 14.13 µmol TE/g. The increase in total polyphenols and antioxidants during kilning may be attributed to the extraction and release of bound phenolic compounds ((+)-catechin and ferulic acid) due to friable tissue created by kilning [10,30,112]. This friable tissue made it easy to extract the phenolic compounds better by synthesising some hydrolytic enzymes in studied grains such as barley, quinoa, millet, and sorghum [21,113,114,115,116,117,118].

Table 6.
Total polyphenols and antioxidant activities of Bambara groundnut speciality malts 1.
The total polyphenols by Folin–Ciocâlteu reagent (FCR), antioxidant activities by ferric-reducing antioxidant power (FRAP), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay showed an increase with the increase in kilning time and temperature. Increased antioxidant properties are contributed by the Maillard reaction products (MRPs) produced during kilning of the malting process [23]. Mainly, the roasting processes exhibit heat-induced antioxidants MRP called melanoidins [31,119]. Continuous research on antioxidants during the malting process, especially the kilning time and temperature, has clearly shown that dark speciality malt had the most significant antioxidant activities [31,120].
The BGN speciality malt syrups exhibited total polyphenols of 0.72, 0.65, 1.20, and 1.60 mg GAE/g, FRAP 2.00, 1.20, 2.42, and 4.43 µmol AAE/g, and 1.56 1.51, 2.11, and 2.96 µmol TE/g, for the base, caramel, roasted, and toasted BGN speciality malt syrups, respectively, in Table 7. There was a significant difference across the BGN speciality syrups. The total polyphenols activity in the toasted malt syrup was the highest, while the caramel malt had the lowest value.

Table 7.
Total polyphenols and antioxidant activities of Bambara speciality malt syrups 1.
Since Maillard reaction activities enhance the colour of the speciality malt during kilning of malt and boiling of wort, there is an increase in total polyphenols after boiling the malt extracts to produce syrup [28]. Coghe et al. [31] showed in their investigation that dark speciality malts and their extracts had the highest antioxidant activities due to higher heat application, as heat treatment is linked with an increase in antioxidant activity. The antioxidant activity of speciality malt wort increase was attributed to redox-reducing antioxidants developed during curing and roasting, giving rise to malt colour change and antiradical antioxidant activity formed during the Maillard reaction [22,121]. Samaras et al. [19] noted that the antioxidant activity of phenolic compounds and antioxidants was higher for the darkly kilned malts as Maillard reaction products increased. Maillard reaction products have antioxidant properties that influence the oxidative stability of wort [22,24,28]. However, studies have shown that malt kilned at high temperatures has the most increased antioxidant activity, contributing to higher intensities of Maillard reaction products [36,117]. Congress worts produced from vetch, green lentil, chickpea, and yellow pea malts had high phenolic and antioxidant components [74]. In the Folin–Ciocalteu, DPPH, and FRAP assays, vetch had the highest total polyphenols and antioxidants [74]. The high content of total polyphenols and antioxidants is attributed to the dark colour and hardcover characteristics of this type of legume seed, having higher flavonoids and condensed tannins, which may increase antioxidant activity [74,122]. Research works and reports have noted that legumes with dark-coloured and tough seed coats have strong antioxidant characteristics [122,123,124,125,126]. BGN is characterised by tough and coloured (black, dark brown, red, white, and speckled) varieties that could be attributed to the increased antioxidant in BGN speciality toasted malt activities in this study [124,127]. Thus, a desirable high total polyphenolic and antioxidant food product could be produced from the BGN toasted malt and syrup.
2.6. Total Soluble Solid of Bambara Groundnut Malt Syrups
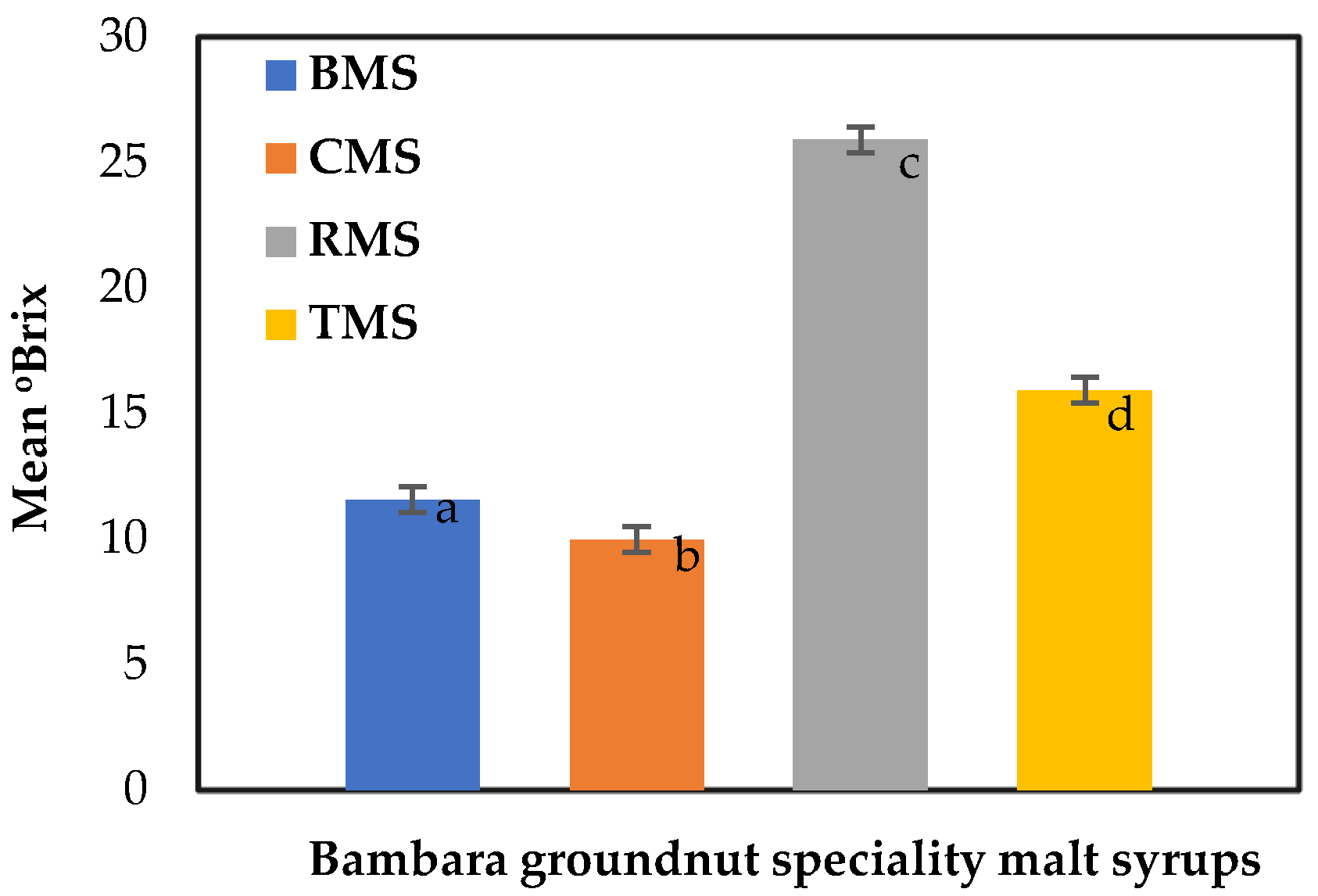
The degree Brix (°Brix) of the BGN speciality malt syrups was 11.57, 9.97, 25.90, and 15.93 °Brix, as illustrated in Figure 6. The °Brix for roasted malt syrup was the highest, indicating the highest total soluble solids content. A degree Brix (°Brix) is a gram of sucrose in 100 g of solution. The soluble solids recorded in the legume malt worts by Gasiński et al. [74], without the addition of enzyme consisting of vetch, green lentil, chickpea, and yellow pea (2.40, 1.59, 2.39, and 2.80 Plato° (≈°Brix)), were lower than the values for BGN malt syrups. Meanwhile, in the malted and unmalted rice syrups production by Ofoedu et al. [69], the °Brix was higher for the malted rice syrups, peaking at 72.10 °Brix. The high °Brix value was attributed to increased hydrolytic activity during germination and mashing by releasing more hydrolysates.

Figure 6.
Degree Brix of Bambara speciality malt syrups. Values are the mean of triplicates ± standard deviation; mean values on the bars with different letters are significantly different (p < 0.05). BMS—base malt syrup, CMS—caramel malt syrup, RMS—roasted malt syrup, TMS—toasted malt syrup.
Furthermore, it was recognised that the physicochemical characteristics and quality of malts depend on the kilning duration and intensity, which will affect the mashing and wort quality [78]. The quality of the extract and malt extract syrup will add value to the production of foods by serving as a source of sweetener, flavour, colour, and enzymes [128,129]. The high total soluble content of the roasted malt syrup could be desirable in producing a non-alcoholic beverage that will add natural sweetness to the product and benefit consumers’ well-being.
2.7. Metabolites of the Bambara Groundnut Speciality Malts
The BGN speciality malts (base malt, caramel malt, roasted malt, and toasted malt) were profiled for metabolites, including the amino acids, sugars, sugar alcohol, organic acids, fatty acids methyl esters (FAME), and volatiles, as illustrated in the following sections.
2.7.1. Amino Acid Compositions of Bambara Groundnut Speciality Malts
The amino acid of the BGN speciality malts was significantly (p = 0.000) different from the base, caramel, roasted to toasted except for leucine which was not significantly different across the BGN speciality malts, as shown in Table 8. The non-essential amino acids consist of aspartic acid, glutamic acid, cysteine, serine, proline, alanine, glycine, and tyrosine. Lysine was the highest amino acid for the base, caramel, and roasted malts at 61.97, 52.67, and 38.89 mg/g, respectively, while aspartic acid was the highest for toasted malt at 14.46 mg/g. On the other hand, methionine was the lowest amino acid for all BGN speciality malt types. This is because methionine, a sulphur-containing essential amino acid, is more deficient in legumes than other essential amino acids while rich in lysine [130,131,132]. However, raw BGN has a considerably high amount of methionine, ranging from 1.30 to 2.90 g/100 g compared to other legumes [133,134,135,136].

Table 8.
Amino acids concentrations of Bambara groundnut speciality malts 1.
The amino acid profile for the BGN speciality malt (BM, CM, RM, and TM) showed higher amino acid contents than the raw BGN seeds. Nzelu [137] and Chinma et al. [15] noted that germination increases the amino acid content of BGN due to protease activity. However, there was a consistent decline in the amino acids of the BGN speciality malts. The decline has been attributed to different kilning temperatures and the initiation of Maillard reactions between reducing sugars and amino compounds in barley malts [138]. Samaras et al. [19] noted that the concentrations of amino acids decreased with increased heat treatment applied to barley grains in the production of speciality malts. The decrease in amino acids was also attributed to the Maillard reaction level and sugar caramelisation by Strecker degradation at higher temperatures [32,139]. This study showed that BGN speciality malts varied in amino acid concentration due to the drying conditions; hence, the base malt with the highest amino acid concentration could be optimised for production to use as functional ingredients in food and beverage production.
2.7.2. Acids, Sugars, and Sugar Alcohol of Bambara Groundnut Speciality Malts
Lactic acid, a non-volatile organic acid, was present in all the BGN speciality malts, where toasted malt (0.06 mg/g) had the highest content. There was a significant (p = 0.000) difference in the lactic acid concentration for the base, caramel, roasted to toasted BGN speciality malts. The higher lactic acid contents have been attributed to the kilning time and temperature by [140]. Comparing two malting regimes of barley, South [141] noted that kilning time is important for final lactic acid levels in malts, where long kilning times lead to high levels of lactic acids. It was suggested that lactic acid must have been produced by dividing the grain microbes of the malt during kilning. The concentrations of the acid, sugar, and sugar alcohol in the BGN speciality malts are illustrated in Table 9.

Table 9.
Acid, sugars, and sugar alcohol concentration of speciality Bambara groundnut malts 1.
Sugars and sugar alcohols consisting of fructose, sucrose, and myo-inositol were also present in the BGN speciality malts in appreciable concentrations, as is illustrated in Table 9. The toasted malt (0.76 mg/g) had the highest concentration of myo-inositol, while the roasted malt had a higher concentration of fructose and sucrose at 0.34 and 9.08 mg/g, respectively. However, the fructose and sucrose concentrations for the BGN speciality malts were not significantly different. The varying concentration of sugars in the BGN speciality malt was attributed to the intensity and duration of the heat applied during kilning, resulting in Maillard reaction formation and sugar caramelisation common in extremely roasted malts [19]. However, Almeida et al. [142] noted that sucrose is more abundant in the pilsner malt (base malt variety) profiled by high-performance–liquid chromatography (HPLC). However, it was suggested that the heat application during kilning increased the sugar composition of the final malt product as sugar was used as precursor for the thermally generated compounds [51]. Therefore, based on the sugar, sugar alcohol, and acid concentration of the BGN speciality malt in this study, toasted malt could be produced for its use in the production of various food products, particularly in beverage industries.
2.7.3. Fatty Acids Methyl Esters (FAME) of Bambara Groundnut Speciality Malts
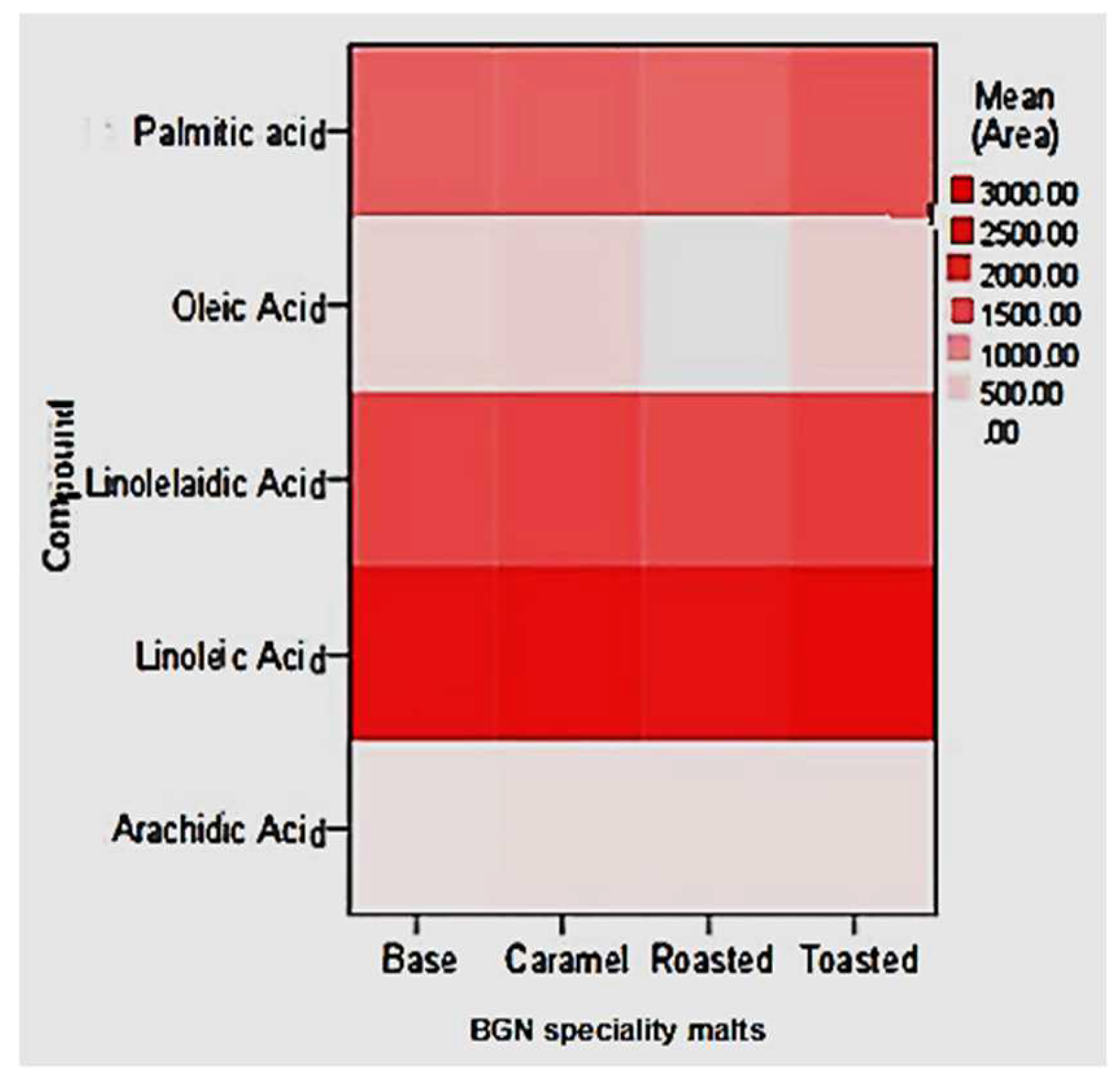
The FAME identified in the base, caramel, roasted, and toasted Bambara groundnut speciality malts were palmitic, oleic, linolelaidic, linoleic, and arachidic acid, as illustrated in Figure 7. The metabolite levels on the heatmap correspond to the colour temperature, and higher temperatures indicate higher levels of FAME compounds. The BGN speciality malts exhibited FAME in different concentrations. Linoleic acid was abundant in all the BGN speciality malt types, while oleic acid was the lowest and was absent in the roasted malt.

Figure 7.
Heat plots of saturated, monosaturated, and polyunsaturated FAME of speciality Bambara groundnut malts.
The major fatty acid components in raw BGN are caprylic, capric, lauric, palmitic, palmitoleic, oleic, and linoleic acids [143,144]. Whereby linoleic acid was found to be the highest fatty acid in raw BGN seeds [145], which could contribute to its concentration in the speciality malts. Similar to this study, Özcan et al. [146] reported that linoleic acid content in barley malt increased during the malting process (steeping, sprouting, and drying), whereas oleic and palmitic acid content decreased. Bravi et al. [147] also noted that the linoleic acid increased in barley malt after kilning, which could be why the BGN speciality malt in this study exhibited high concentration. Furthermore, an increase in heat application to linoleic acid has been found to increase its concentration, which was attributed to varying lipids biosynthesis during the malting process [148,149,150]. Linoleic acid, oleic acid, and palmitoleic acids are essential unsaturated fatty acids necessary in human food to prevent certain heart diseases [143,151,152,153,154]. Therefore, being abundant in the BGN speciality malt across all products could benefit human health and encourage its production in large quantities.
2.7.4. Volatile metabolites in Bambara groundnut speciality malts
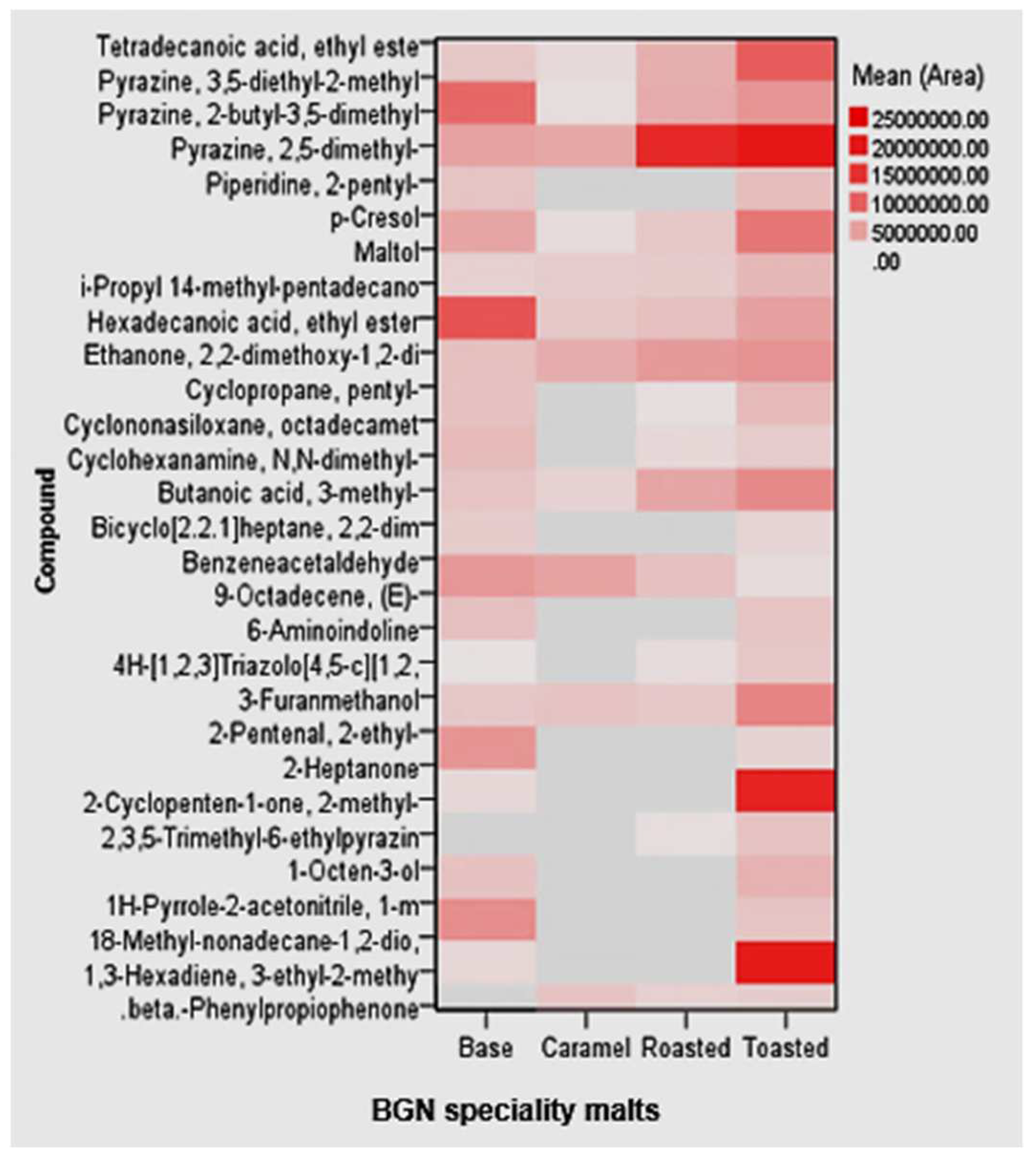
A total of 29 volatile metabolites were identified in the BGN speciality malts based on retention times and mass spectrometric data from MS libraries by HS-GC-FID. The volatile compounds consisted of pyrazine, furans, aldehydes, ketones, esters, and alcohols. The most abundant volatile compound in the BGN speciality malts was the pyrazine, 2,5-dimethyl, higher in the toasted malt. Conversely, the lowest volatile compound was the 2,3,5-Trimethyl-6-ethylpyrazine in all the BGN speciality malts. The volatile compounds in the speciality malts are on the heatmap illustrated in Figure 8.

Figure 8.
Heat plots of the volatile metabolites of Bambara groundnut speciality malts.
The most abundant volatiles in the BGN speciality malts were the pyrazines. Pyrazines are volatile compounds with monocyclic aromatic rings with two nitrogen atoms. Foods can contain different groups of pyrazines, which consist of alkyl, methoxy, and sulphur-containing chains [155]. However, pyrazine, 2,5-dimethyl, is the most abundant in the BGN speciality malts. It is characterised by chocolate and roasted nut flavours [156]. Thus, it is an essential flavour compound in roasted food products, especially roasted coffee [157]. In addition, it is used as a flavour additive and odorant in foods such as cereals; it also occurs naturally in asparagus, green tea, crispbread, malt, raw shrimp, soya, and wheat bread [158,159,160,161]. Its high concentration in the BGN toasted malt could be attributed to the subjection to higher temperatures after initial drying of 50 °C. Methylpyrazine volatile compounds, heterocyclic volatiles, are formed by the Maillard reaction and are also common in the pyrolysis process at higher temperatures and with very low moisture contents [139,162]. The pyrolysis process thus suggested that the maltol present in the BGN speciality malts was formed in addition to Maillard reactions, which accounts for its higher concentration in toasted malt due to its low moisture and high-temperature drying.
Maltol (3-Hydroxy-2-methyl-4-pyrone), a naturally occurring organic compound used as a flavour enhancer, is found only in highly roasted speciality malts such as roasted and toasted malts [163]. Maltol is formed due to the Maillard reaction and is characterised by a sweet baked aroma typical in highly heated malts [139]. The impact of different times and temperatures applied during the caramelisation process of roasted and toasted malt developed the caramel-like flavour maltol [33]. Maltol is a safe, reliable, natural antioxidant, food preservative, and flavour [164]. It is found in baked products, red ginseng root, coffee, chicory, soybeans, bread crusts, and caramelised foods [35,165]. It has also been used in catalysis, cosmetics, pharmaceutical formulation, and food chemistry [166,167]. In addition, it can be used to treat anaemia, tumour, nerve cell oxidative stress, and kidney damage [164,168]. Studies have also shown that maltol reduced acute alcohol-induced liver injury, prevented oxidative injury through activating some signalling pathways, and prevented cisplatin-induced acute kidney injury [169,170].
The lowest volatile compound was the 2,3,5-Trimethyl-6-ethylpyrazine, mainly in roasted malt. It is a nitrogen-containing compound in the pyrazines group of volatile heterocyclic [160]. It is characterised by an earthy, nutty, roasted flavour formed during roasting at high temperatures between 135 and 250 °C [155]. It is also a chocolate enhancer used in foods containing coffee, cocoa, meat, and potatoes as a roasted flavour [171].
The volatiles in the BGN speciality malts have flavour characteristics used in the food industries to enhance and improve acceptability of food products for consumers [155,171]. These days, organic and natural labels have been gaining popularity as consumers become more aware of the ingredients in their food. Due to the high demand of consumers to eat organically grown food, the need for volatile flavours has increased, and there is a need to extract these volatiles from natural products for use in food production [171,172,173]. Thus, toasted malt with more abundant volatiles, such as maltol and pyrazine, 2,5-dimethyl, could be used for food and beverage production. Moreover, the physicochemical and biochemical characteristics of the speciality BGN malts and their syrups produced from optimum amylase malt showed good characteristics that can be incorporated into food production as ingredients or condiments.
3. Materials and Methods
3.1. Materials, Reagents, and Equipment Sources
The amylase-rich BGN malt, speciality malt, and syrups were obtained from the Department of Food Science and Technology, Cape Peninsula University, South Africa. Chemicals and reagents were of analytical standards. Alpha and beta-amylase kits were from Megazyme Ltd., Ireland. The equipment was from the Department of Food Science and Technology and Oxidative Stress Research Centre, Cape Peninsula University of Technology, Cape Town, South Africa. Equipment included the Dumas nitrogen analyser LECO CN 628 (Leco Corp., St Joseph, MI, USA). The Avanti® J-E centrifuge JSE111330 (Beckman Coulter Inc., Indianapolis, IN, USA), and Thermo Scientific MultiSkan plate reader spectrophotometer (Thermo Scientific, Waltham, MA, USA). The other equipment included the pH meter (Hanna Checker pH meter, Model HI1270), water bath, Colour Flex EZ (Model TC-P III-A, Tokyo Denshoku Co., Ltd., Tokyo, Japan), and an Excalibur Food Dehydrator (Excalibur, Sacramento, CA, USA).
3.2. Bambara Groundnut Speciality Malts and Syrups Physicochemical Analysis
3.2.1. Colour Determination of Speciality Bambara Groundnut Malts and Syrups
Following the method of [174], the colour measurement of the Bambara groundnut speciality malts and their respective extract syrups were analysed using Colour Flex EZ (Hunter Lab, Reston, VA, USA) with daylight illumination set at D65, 10° standard observer angle, and 25 mm aperture. The standard black (L* = 8.47, a* = −0.96, b* = 2.79) and white (L* = 8.47, a* = −0.96 b* = 2.75) tiles were used for the instrument’s calibration. Five grams (5 g) of the samples in triplicate was measured into a glass sample cup (Hunter Lab 04720900, 6.4 cm) with an internal diameter of 6.4 cm following the method by [175]. The CIEL*a*b* (Commission Internationale de l’Eclairage’s) was used to measure the colour parameters, where L* is 0 = black and 100 = white, a* is −a* = greenness, and +a* = redness and b* is −b* = blueness and +b* = yellowness, respectively. As shown in Equations 1 and 2, the chroma and hue angle (h°) were calculated following the method of [176].
where C = chroma; a*2 = redness; b*2 = greenness.
where h° = hue angle; a*2 = redness; b*2 = greenness.
3.2.2. Determination of Speciality Bambara Groundnut Malts and Syrups pH
Ten milligrams (10 mg) of milled BGN speciality malts (BM, CM, RM, and TM) was separately mixed with 40 mL of distilled water in a 50 mL centrifuge tube for 5 min using a vortex mixer by following the [177] method with some differences. After mixing, the centrifuge tubes containing the mixtures were kept for 1 h at ambient temperature and centrifuged for 10 min at 1500× g. The supernatant’s (at room temperature) pH was measured in triplicate using a laboratory pH meter calibrated with buffers 4 and 7 (Hanna Checker pH meter, Model HI1270).
3.2.3. Protein Content Determination of Bambara Groundnut Speciality Malts and Syrups
Bambara groundnut speciality malts and syrups’ crude protein was determined using the LECO CN 628 Dumas nitrogen analyser (Leco Corp, St Joseph, MI, USA). The samples in triplicate were analysed after five blanks, EDTA standard, and ProNutro control sample. The samples to the value of 0.09 mg were wrapped and tightly folded in tin foil cups, P/N: 502-186-200, and combustion was carried out in pure oxygen at a temperature of 950 °C in the reactor consisting of the combustion catalyst. A mixture of gases containing CO2, H2O, NO, and NO2 (carbon dioxide, water, and nitrogen) was created during the fast combustion reaction. Designated columns absorbed the gases, where oxygen was removed, and nitrogen oxides were converted into nitrogen. The remaining carbon dioxide (CO2) and water (H2O) were removed via a thermal conductivity column carried by helium gas. The nitrogen content was then measured by the Dumas Nitrogen analyser. Following the [178] method, the crude protein was calculated by multiplying the protein factor of 6.25 expressed in percentage with the measured nitrogen.
3.2.4. Determination of Apparent Degree BRIX (°Brix) of Bambara Groundnut Syrups
The method of Ofoedu et al. [69] was used to measure the total soluble sugar of the syrups at a temperature of 20 °C with a handheld KERN-SOHN refractometer (KERN ORA 10 BA/BB Kern & Sohn, GmbH, Germany). First, the standardisation of the handheld refractometer was carried out with distilled water at 20 °C until the Brix value read zero. Then, one drop of each BGN syrup was dropped on the lens (sensitive surface) using plastic filling pipettes to take measurements. Finally, the total sugar contents (°Brix) were read from the refractometer scale in triplicate.
3.2.5. α- and β-Amylases Activities of Bambara Groundnut Speciality Malt Determination
Following the method of [179], the alpha and beta-amylase (α- and β-amylase) activities of the BGN speciality malts and syrups were determined in triplicates. Section 1 and Section 2 detailed the determination of the α- and β-amylase enzymes through the enzymatic Ceralpha Method (K-CERA, Megazyme) and the enzymatic kit Beta-amylase (Megazyme, K-BETA3).
Alpha-Amylase Assay Procedure (Ceralpha Method)
The milled BGN speciality malts and syrups of 3.0 g were measured separately into 50 mL conical flasks. Twenty millilitres (20 mL) of extraction buffer solution of pH 5.4 was added to each flask and was stirred vigorously using the vortex mixer. The samples were then extracted for 20 min at 40 °C in the incubator and occasionally stirred with a vortex mixer. After extraction, 25 mL of each sample was measured into 50 mL centrifuge tubes and centrifuged with Centrifuge 5810R at 1000× g for 10 min. Finally, the sample extracts were separated into 25 mL centrifuge tubes for the assay procedure
The assay was carried out by measuring 0.2 mL aliquots of Megazyme unbuffered amylase HR reagent containing blocked p-nitrophenyl maltoheptaoside (BPNPG7, 54.5 mg) and thermostable α-glucosidase (125 U at pH 6.0) into 25 mL centrifuge test tubes. Then, the 0.2 mL amylase HR reagent and the sample extracts were preincubated at 40 °C for 5 min. Next, the preincubated 0.2 mL of the samples was added directly to the tubes’ bottom containing the 0.2 mL of the amylase HR reagent solution and incubated at 40 °C for 20 min. Exactly 3 mL of stopping reagent (10 g of tri-sodium phosphate in 1 L of distilled water pH adjusted to 11.0) was added immediately after incubation; the tubes were vigorously stirred using a vortex mixer. The Thermo Electron Corporation MultiSkan Spectrum was set at 400 nm against distilled water to read the absorbance of the solution in triplicate.
Beta-Amylase Assay Procedure (Betamyl-3 Method)
The BGN speciality malt and syrups (0.5 g) were weighed into 25 mL centrifuge tubes, and 5 mL extraction buffer containing Tris/HCl 25 mL, 1 M, pH 8.0 plus disodium EDTA of 20 mM and sodium azide of 0.02% w/v diluted in distilled water was added. The enzymes were allowed to extract for one hour at room temperature, with repeated stirring on the vortex mixer. Then, the mixtures were centrifuged using the Eppendorf Centrifuge 5810 R at 2000× g for 10 min. Immediately after centrifugation, 0.2 mL of the filtrate was added to 4 mL of the dilution buffer containing MES dilution buffer 48 mL, 1 M, pH 6.2 plus disodium EDTA 20 mM, BSA 10 mg/mL, and sodium azide of 0.09% w/v. The mixed solution was then used for the assay of β-amylase activities.
The assay of the β-amylase was performed by placing an aliquot of 0.2 mL of the diluted samples into the bottom of 25 mL centrifuge tubes. The tubes were preincubated at 40 °C for 5 min, and after incubation, 0.2 mL of preincubated Megazyme Betamyl-3 substrate solution (p-nitrophenyl-β-D-maltotrioside (PNPβ-G3) plus β-glucosidase (50 U)) was added. Then, stabilisers were added to each diluted sample, and the vortex mixer was used to stir the mixture. These mixtures were incubated at 40 °C for 10 min, after which 3.0 mL of the stopping reagent (10 g of Tris buffer (Megazyme cat. No. B-TRIS500)) in 900 mL of distilled water, pH adjusted to 8.5 was added, and the contents stirred with the use of the vortex mixer. The absorbance of the solution and reagent blank reading was read at 400 nm against distilled water with a Thermo Scientific MultiSkan microplate spectrophotometer.
3.2.6. Total Polyphenols and Antioxidants Activities of Bambara Groundnut Speciality Malt Determination
Followed the methods of [116,180,181], the total polyphenols and antioxidants activities of the BGN speciality malts determinations were carried out with the Folin–Ciocâlteu reagent (FCR), ferric-reducing antioxidant power (FRAP), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays, as explicated in the following three sections.
Total Polyphenols Content by Folin–Ciocâlteu Reagent (FCR) Assay
Five hundred milligrams (500 mg) of BGN speciality malt and syrups was measured into screw-cap tubes to determine the total polyphenols with gallic acid as the standard. The BGN speciality malt and syrups were extracted with 10 mL of 70% methanol mixed with 0.1% HCL. The samples were then centrifuged after mixing with a vortex mixer using the Eppendorf Centrifuge 5810 R at 4000× g, 21 °C for 5 min. The Folin–Ciocalteu assay was carried out by measuring 25 mL of the decanted liquids and mixing it with 125 µL of 0.2 M Folin–Ciocalteu reagent and 100 µL of 7.5% Na2CO3 solution in 96-well transparent plate. The absorbance was read in triplicate with a Thermo Scientific MultiSkan microplate spectrophotometer reader (734 nm at 25 °C) after a 2 h incubation period. The standard calibration curve was constructed with 40 mg gallic acid (Sigma Cat Nr: G7384). The results were expressed as mg Gallic acid equivalents (GAE)/g).
Antioxidant Activities by Ferric-Reducing Antioxidant Power (FRAP) Assay
Bambara groundnut speciality malt and syrups of 500 mg were weighed into 50 mL screw-cap tubes. Ten millilitres of 70% methanol (containing 0.1% HCl) was added to the samples in the screw-cap tubes. The samples were mixed with a vortex, then centrifuged at 4000 rpm for 5 min and the supernatants (10 μL each) were pipetted into microplate wells in triplicates. Three hundred microlitres (300 µL) of the FRAP reagent was added to each sample in the microplate wells. Ascorbic acid was the standard, and distilled water was the blank. The samples were incubated for 30 min at 37 °C, and absorbance was read at 593 nm. The Thermo Scientific MultiSkan microplate spectrophotometer was used for reading absorbance. The results were expressed as mg ascorbic acid equivalents (AAE)/g.
Antioxidant Activities by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
The Bambara groundnut speciality malts and their syrup free radical scavenging ability were determined using the DPPH radical (25 mg/L) in 70% methanol. Each of the samples was mixed with 0.275 mL DPPH solutions. The samples and standards were incubated at 37 °C for 30 min in the dark, and absorbance reactions were read at 517 nm. The Thermo Scientific MultiSkan microplate spectrophotometer was used for reading absorbance. The standard was Trolox, and results were expressed as µmole Trolox/g.
3.2.7. Metabolite Profiling of Bambara Groundnut Speciality Malt
The metabolite profiling was carried out on the Bambara groundnut speciality malts. The sugars, sugar alcohols, organic acids, and amino acids were profiled by capillary gas chromatography–mass spectrometry (GCMS) [182,183,184,185,186]. A gas chromatography–flame ionisation detector (GC-FID) was used to analyse the fatty acid methyl esters (FAME) [187]. A headspace gas chromatography–flame ionisation detector (GC-FID) was used to analyse the fatty acid methyl esters (FAME) and volatile compounds [188].
Determination of Fatty Acids Methyl Esters (FAME) and Hydrocarbons by Gas Chromatography–Flame Ionisation Detection (GC-FID)
The analysis of fatty acid and hydrocarbons was carried out by extracting and converting the BGN speciality malt lipids into fatty acid methyl esters (FAME). The extraction was carried out using diethyl ether and petroleum ether in methanol. A model Agilent 7890A gas chromatography (GC) coupled with Flame Ionisation Detection (GC-FID) was used for detection according to the [188] method, 996.06 with some modifications.
The BGN speciality malts of 1.5 mg were weighed into the separate 70 mL test tubes to digest. The tube’s contents were thoroughly mixed with 100 mg of pyrogallic acid, 2 mL internal standard solution of 5 mg/mL undecanoic acid dissolved in hexane, and 2 mL ethanol. Immediately after mixing, 10 mL of 32% HCL was mixed into each tube. The tubes were then placed in a 70–80 °C water bath for 40 min, and the contents were mixed every 10 min. After digestion, the tubes were removed and allowed to cool to room temperature. The 25 mL diethyl ether was added to each tube and shaken for 5 min for extraction. Petroleum ether of 25 mL was further added and shaken for 5 min. After separating the two layers, the clear upper layer was decanted into 150 mL beakers, and ether was evaporated in the fume hood to dryness.
Derivatisation of the samples was carried out by reconstituting the residues in 3 mL chloroform and diethyl ether. The solutions were transferred into 10 mL tubes and evaporated under the nitrogen stream to dry. Immediately after drying, 2 mL of 2% H2SO4 in methanol reagent and 1 mL toluene were added. The tubes were tightly closed and placed in the incubator at 100 °C for 45 min, then cooled to room temperature. After cooling, 5 mL distilled water and 1 mL hexane were added and thoroughly shaken, using the vortex mixer for 1 min. The layers were left to separate, and the top layers were carefully transferred to 20 mL test tubes. Approximately 1 g anhydrous Na2SO4 was added to each tube to have a clear solution. The clear solutions were then transferred into 2 mL clear vials, and GC analysis was carried out. Fatty acids were identified by comparing their retention times to the retention times of the standard.
Sugars, Acids, and Sugar Alcohols Determination by Gas Chromatography–Mass Spectrometry (GC-MS)
Sugars, sugar alcohols, and organic acids were analysed using GCMS by measuring 1 mL of 70% methanol (MeOH), then adding approximately 100 mg of the BGN speciality malts and extracting at 45 °C in the oven for 3 hours. The extracted samples of 130 µL were dried completely with a gentle stream of nitrogen and derivatised with 100 µL of methoxamine at 40 °C for 2 h. Then, 30 µL of N, O-Bis (trimethylsilyl) trifluoroacetamide (BSTFA) was added and derivatised at 60 °C for 30 min. Finally, the samples were transferred into 2 mL GC vials, and 1 µL was injected onto the GC-MS in spit-less mode.
Separation was performed on a gas chromatograph (Trace 1300, Thermo Fisher Scientific S.p.A., Strada Rivoltana 20090 Rodano-Milan, Italy) coupled with a mass spectrometer (TSQ 8000, Thermo Scientific). The carbohydrates were separated on a non-polar capillary column Rxi-5Sil MS (30 m, 0.25 mm ID, 0.25 µm film thickness). Helium was used as the carrier gas at a 1 mL/min flow rate. The injector temperature was maintained at 250 °C. The oven temperature was 80 °C for 1 min and was ramped up to 300 °C at a rate of 7 °C/min and held for 2 min.
Amino Acids Determination by Gas Chromatography–Mass Spectrometry (GC-MS)
Following the method of [189] with a little difference, 3 mL of 6M HCl was added to ca. 500 mg of the BGN speciality malts (BM, CM, RM, and TM). They were hydrolysed for 24 h at 110 °C, cooled down to room temperature, and diluted at a ratio of 1:9 with 70% methanol (v/v). Next, 100 µL was transferred into a 2 mL tube and dried completely under a gentle stream of nitrogen. Then, the samples were reconstituted and derivatised with 30 µL silylation reagent N-tert-butyldimethylsilyl- N-methyl trifluoroacetamide (MTBSTFA) and 100 µL acetonitrile at 100 °C for 1 h. After which, they were cooled down to room temperature and injected into the GC-MS instrument for analysis.
Component separation was performed on a gas chromatograph (Trace1300, Thermo Fisher Scientific S.p.A., Strada Rivoltana 20090 Rodano-Milan, Italy) coupled to a TSQ8000 mass spectrometer (Thermo Scientific). The GC-MS system was connected to a TriPLUS autosampler. Amino acids were separated on a capillary column Rxi-5Sil MS (30 m, 0.25 mm ID, 0.25 µm film thickness). Helium was used as the carrier gas at a 1 mL/min flow rate, and the injector temperature was maintained at 250 °C. In addition, 1 µL of the sample was injected in spit-less mode. The oven temperature was programmed to 100 °C for 1 min and ramped up to 300 °C at a rate of 15 °C/min and held for 6 min. The Agilent mass spectrometer detector (MSD) was operated in scan mode, and the source and quad temperatures were maintained at 250 °C and 150 °C, respectively. The transfer line temperature was maintained at 250 °C. The mass spectrometer was operated under electron impact (EI) mode at ionisation energy of 70 eV by scanning from 35 to 650 m/z.
Volatile Compounds Determination by Headspace Gas Chromatography–Mass Spectrometry (HS-GC-MS)
The headspace gas chromatography–mass spectrometry (HS-GC-MS) analyses were performed using a model Agilent 7890B Gas Chromatography–5977A coupled with a Mass Spectrometer detector system (Agilent Technologies, Santa Clara, CA, USA) with a split-less injector that is suitable for GC analysis by following the method of [187] with some differences. The Agilent J&W GC HP-5ms capillary column of 30 m × 0.25 mm × 0.25 µm was used to separate the volatiles. The carrier gas was helium, with a 0.6 mL/min flow rate. Two hundred and fifty microlitres of the speciality malts volume was injected with a split ratio of 50:1 and weighed into 10 mL glass headspace vials covered with silicon septum with a purge flow of 3 mL/min and screw-capped. The oven temperature was 50 °C, held for 5 min, increased at 10 °C/min to 200 °C and held for 5 min with a running time of 25 min. The injector temperature, pressures, and volume were set at 240 °C, 2.6149 psi, and 250 μL, respectively. The incubation temperature and time were set at 120 °C and 300 s, respectively. The samples were then run concurrently.
The compounds were identified through Wiley mass spectral (MS) library and Golm metabolome database search. The volatile compounds identification was performed by comparing the mass spectra with the spectra of the reference compounds in both the Wiley MS library and was verified based on mass spectra obtained from the literature. The volatile results were provided based on the compounds’ quality and peak area counts.
3.3. Identification of Metabolite Compounds
Identification of BGN speciality malt constituents was performed by comparing the retention times and mass spectra with reference compounds. Moreover, it was conducted by comparing mass spectra with the entries of the National Institute of Standards and Technology mass spectra library NIST02 and the GOLM metabolome database [183,184,186,190].
3.4. Statistical Data Analysis
All results were reported as mean ± standard deviation of three independent trials. Multivariate analysis of variance (MANOVA) was used to establish differences between treatments. Duncan’s multiple range test was used to separate means where significant differences existed ((SPSS version 26.0, IBM Corporation, Armonk, NY, USA)). Kruskal–Wallis test was used to test the distribution of protein when normality is violated to determine the mean differences between treatments.
4. Conclusions
This study successfully produced speciality Bambara groundnut malts and their corresponding syrups from the amylase-rich green BGN malts steeped at 36 h and sprouted at 96 h. The speciality malts and syrups exhibited colours desirable in the food industries, which could be used to impact different shades of colours as ingredients in product formulation in baked goods. The BGN speciality malt syrups exhibited a similar pH range to malted barley syrup, making it a functional ingredient in the beverage industries. Bambara groundnut speciality roasted malt and toasted malt syrup exhibited favourable protein concentration compared to base and caramel malts, which could benefit human health when consumed. The enzyme activities were affected by heat application during malt kilning and extract boiling due to the heat-sensitive nature of the α- and β-amylases. However, boiling at temperatures lower than 60 °C could be recommended for the production of BGN syrups with higher amylase concentrations. The toasted malt and its syrup exhibited the highest total polyphenolic and antioxidant activities, which could make it a desirable functional food product ingredient. The °Brix of the roasted malt syrup was the highest, which could be a desirable attribute in producing non-alcoholic beverages by adding natural sweetness to the product, and which can be of benefit to consumers who are health-conscious. The profile of metabolite components in the speciality BGN malt included amino acids, fatty acid methyl esters, sugars, sugar alcohol, acid, and volatiles. The metabolites identified in the BGN speciality malt could add value to the sensory properties and nutritional and functional characteristics of BGN seeds. Thus, the speciality Bambara groundnut malt possesses components that can be incorporated into human diets for their health benefits. Hence, its use in the food and beverage industries should be encouraged.
Author Contributions
Conceptualisation, V.A.J.; Data collection, A.H.A.; Conducted the experiment, A.H.A.; Data Analyses and Interpretation, V.A.J.; Resources, V.A.J. and A.O.O.; Writing—Original Draft Preparation, A.H.A.; Writing—Review and Editing, A.H.A., V.A.J. and A.O.O.; Visualisation, A.H.A.; Supervision, V.A.J. and A.O.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial assistance from the Cape Peninsula University of Technology University and Food and Beverages Manufacturing Sector Education and Training Authority (FoodBev Seta 2019/2020 bursary).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No additional data were generated other than the data reported in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Alain, M.; Israël, M.; René, M.S. Improving the nutritional quality of cowpea and Bambara bean flours for use in infant feeding. Pak. J. Nutr. 2007, 6, 660–664. [Google Scholar]
- Subuola, F.; Widodo, Y.; Kehinde, T. Processing and Utilization of Legumes in the Tropics. In Trends in Vital Food and Control Engineering; InTech: West Palm Beach, FL, USA, 2012; ISBN 9789535104490. [Google Scholar]
- Kavitha, S.; Parimalavalli, R. Effect of processing methods on proximate composition of cereals and legumes flour. J. Hum. Nutr. Food Sci. 2014, 2, 1051. [Google Scholar]
- Serventi, L. Upcycling Legume Water: From Wastewater to Food Ingredients; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-42467-1. [Google Scholar]
- Xue, Z.; Wang, C.; Zhai, L.; Yu, W.; Chang, H.; Kou, X.; Zhou, F. Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.), soybean (Glycine max L.) and black bean (Phaseolus vulgaris L.) during the germination process. Czech J. Food Sci. 2016, 34, 68–78. [Google Scholar] [CrossRef]
- Tang, D.; Dong, Y.; Ren, H.; Li, L.; He, C. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem. Cent. J. 2014, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Sarmento, T.; Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of cooking and germination on phenolic composition and dietary fibre fractions in dark beans (Phaseolus vulgaris L.) and lentils (Lens culinaris L.). LWT Food Sci. Technol. 2016, 66, 72–78. [Google Scholar] [CrossRef]
- Viktorinová, K.; Petřeková, K.; Šimek, J.; Hartman, I.; Hertel, V. Nutrition and Sensory Evaluation of Germinated Legumes. Kvas. Prum. 2020, 66, 270–276. [Google Scholar] [CrossRef]
- Erba, D.; Angelino, D.; Marti, A.; Manini, F.; Faoro, F.; Morreale, F.; Pellegrini, N.; Casiraghi, M.C. Effect of sprouting on nutritional quality of pulses. Int. J. Food Sci. Nutr. 2019, 70, 30–40. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; den Broeck, H.C.; Brouns, F.J.P.H.; Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2018, 18, 1541–4337. [Google Scholar] [CrossRef]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Owuamanam, C.; Ogueke, C.; Iwouno, J.; Edom, T. Use of Seed Sprouting in Modification of Food Nutrients and Pasting Profile of Tropical Legume Flours. Niger. Food J. 2014, 32, 117–125. [Google Scholar] [CrossRef][Green Version]
- Manickavasagan, A.; Thirunathan, P. (Eds.) Pulses; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-41375-0. [Google Scholar]
- Aguilera, Y.; Díaz, M.F.; Jiménez, T.; Benítez, V.; Herrera, T.; Cuadrado, C.; Martín-Pedrosa, M.; Martín-Cabrejas, M.A.; Jiménez, T.; Benítez, V.; et al. Changes in nonnutritional factors and antioxidant activity during germination of nonconventional legumes. J. Agric. Food Chem. 2013, 61, 8120–8125. [Google Scholar] [CrossRef] [PubMed]
- Chinma, C.E.; Abu, J.O.; Asikwe, B.N.; Sunday, T.; Adebo, O.A. Effect of germination on the physicochemical, nutritional, functional, thermal properties and in vitro digestibility of Bambara groundnut flours. LWT 2021, 140, 110749. [Google Scholar] [CrossRef]
- Boukid, F.; Zannini, E.; Carini, E.; Vittadini, E. Pulses for bread fortification: A necessity or a choice? Trends Food Sci. Technol. 2019, 88, 416–428. [Google Scholar] [CrossRef]
- Asuk, A.A.; Ugwu, M.N.; Idole, B. The Effect of Different Malting Periods on the Nutritional Composition of Malted Sorghum-Soy Composite Flour. J. Food Sci. Nutr. Res. 2020, 3, 217–230. [Google Scholar] [CrossRef]
- Murugkar, D.A. Effect of sprouting of soybean on the chemical composition and quality of soymilk and tofu. J. Food Sci. Technol. 2014, 51, 915–921. [Google Scholar] [CrossRef]
- Samaras, T.S.; Camburn, P.A.; Chandra, S.X.; Gordon, M.H.; Ames, J.M. Antioxidant Properties of Kilned and Roasted Malts. J. Agric. Food Chem. 2005, 53, 8068–8074. [Google Scholar] [CrossRef]
- Lloyd, W.J.W. Environmental Effects on the Biochemical Phases of Malt Kilning. J. Am. Soc. Brew. Chem. 1988, 46, 8–13. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Correia, E.; Lopes, L.; Guido, L.F. Further insights into the role of melanoidins on the antioxidant potential of barley malt. Food Chem. 2014, 160, 127–133. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Gonçalves, L.M.; Guido, L.F. Overall Antioxidant Properties of Malt and How They Are Influenced by the Individual Constituents of Barley and the Malting Process. Compr. Rev. Food Sci. Food Saf. 2016, 15, 927–943. [Google Scholar] [CrossRef]
- Sharma, P.; Goudar, G.; Longvah, T.; Gour, V.S.; Kothari, S.L.; Wani, I.A. Fate of Polyphenols and Antioxidant Activity of Barley during Processing. Food Rev. Int. 2020, 8, 163–198. [Google Scholar] [CrossRef]
- Coghe, S.; Gheeraert, B.; Michiels, A.; Delvaux, F.R. Development of Maillard Reaction Related Characteristics During Malt Roasting. J. Inst. Brew. 2006, 112, 148–156. [Google Scholar] [CrossRef]
- Woffenden, H.M.; Ames, J.M.; Chandra, S. Relationships between Antioxidant Activity, Color, and Flavor Compounds of Crystal Malt Extracts. J. Agric. Food Chem. 2001, 49, 5524–5530. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Kun, S.; Hegyesné Vecseri, B.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2019, 56, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.W. Brewing New Technologies; Woodhead Publishing Limited: Cambridge, UK, 2006; ISBN 9781855734906. [Google Scholar]
- Coghe, S.; Vanderhaegen, B.; Pelgrims, B.; Basteyns, A.V.; Delvaux, F.R. Characterization of Dark Specialty Malts: New Insights in Color Evaluation and Pro- and Antioxidative Activity. J. Am. Soc. Brew. Chem. 2004, 61, 125–132. [Google Scholar] [CrossRef]
- Cortés, N.; Kunz, T.; Suárez, A.F.; Hughes, P.; Methner, F.-J. Development and Correlation between the Organic Radical Concentration in Different Malt Types and Oxidative Beer Stability. J. Am. Soc. Brew. Chem. 2010, 68, 107–113. [Google Scholar] [CrossRef]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef]
- Coghe, S.; Adriaenssens, B.; Leonard, S.; Delvaux, F.R. Fractionation of colored maillard reaction products from dark specialty malts. J. Am. Soc. Brew. Chem. 2004, 62, 79–86. [Google Scholar] [CrossRef]
- Coghe, S.; Derdelinckx, G.; Delvaux, F.R. Effect of non-enzymatic browning on flavour, colour and antioxidative activity of dark specialty malts—A review. Monatsschr. Brauwiss 2004, 57, 25–38. [Google Scholar]
- Vandecan, S.M.G.; Daems, N.; Schouppe, N.; Saison, D.; Delvaux, F.R. Formation of Flavor, Color, and Reducing Power during the Production Process of Dark Specialty Malts. J. Am. Soc. Brew. Chem. 2011, 69, 150–157. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Arendt, E.K. Nonbrewing Applications of Malted Cereals, Pseudocereals, and Legumes: A Review. J. Am. Soc. Brew. Chem. 2015, 73, 223–227. [Google Scholar] [CrossRef]
- Rögner, N.S.; Mall, V.; Steinhaus, M. Odour-active compounds in liquid malt extracts for the baking industry. Eur. Food Res. Technol. 2021, 247, 1263–1275. [Google Scholar] [CrossRef]
- Guido, L.; Moreira, M. Malting. In Engineering Aspects of Cereal and Cereal-Based Products; Guine, R.D., dos Reis Correia, P.M., Eds.; CRC Press: Boca Raton, FI, USA, 2013; pp. 51–70. [Google Scholar]
- Dziki, D.; Gawlik-Dziki, U. Processing of germinated grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–90. ISBN 9780128115251. [Google Scholar]
- Mayes, S.; Ho, W.K.; Chai, H.H.; Gao, X.; Kundy, A.C.; Mateva, K.I.; Zahrulakmal, M.; Hahiree, M.K.I.M.; Kendabie, P.; Licea, L.C.S.; et al. Bambara groundnut: An exemplar underutilised legume for resilience under climate change. Planta 2019, 250, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.S.; Chai, H.H.; Basu, S.; Sri Redjeki, E.; Moreton, J.; Mayes, K.; Ho, W.K.; Massawe, F.; Mayes, S. Exploring the domestication of Bambara groundnut. Acta Hortic. 2015, 1101, 183–190. [Google Scholar] [CrossRef]
- Diedericks, C.F.; Venema, P.; Mubaiwa, J.; Jideani, V.A.; van der Linden, E. Effect of Processing on the Microstructure and Composition of Bambara Groundnut (Vigna subterranea (L.) Verdc.) Seeds, Flour and Protein Isolates. Food Hydrocoll. 2020, 108, 106031. [Google Scholar] [CrossRef]
- Drewnowski, A. 50 Foods for Healthier People and a Healthier Planet. World Wildl. Found. Knorr Foods 2020, 59. [Google Scholar]
- Nwokolo, E. Bambara groundnut (Vigna subterranea (L.) Verdc.). In Food and Feed from Legumes and Oilseeds; Springer: Boston, MA, USA, 1996; Volume 52, pp. 216–221. ISBN 9781461380504. [Google Scholar]
- Oyeyinka, A.T.; Pillay, K.; Tesfay, S.; Siwela, M. Physical, nutritional and antioxidant properties of Zimbabwean Bambara groundnut and effects of processing methods on their chemical properties. Int. J. Food Sci. Technol. 2017, 52, 2238–2247. [Google Scholar] [CrossRef]
- Abba, R.Z.; Imam, A.A.; Atiku, M.K.; Muhammad, Y.Y. Effect of Sprouting on the Functional Properties and Amino Acid Profile of Two Bambara Groundnut (Vigna subterranea) Protein Isolates. Asian Food Sci. J. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Jideani, V.A. Utilizing bambara groundnut in Value-Added products. Food Technol. 2016, 70, 48–52. [Google Scholar]
- Agu, H.O.; Onuoha, G.O.; Elijah, O.E.; Jideani, V.A. Consumer Acceptability of Acha and Malted Bambara Groundnut (BGN) Biscuits Sweetened with Date Palm. Heliyon 2020, 6, e05522. [Google Scholar] [CrossRef]
- Ding, J.; Feng, H. Controlled germination for enhancing the nutritional value of sprouted grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–112. ISBN 9780128115251. [Google Scholar]
- Adetokunboh, A.H.; Obilana, A.O.; Jideani, V.A. Enzyme and Antioxidant Activities of Malted Bambara Groundnut as Affected by Steeping and Sprouting Times. Foods 2022, 11, 783. [Google Scholar] [CrossRef]
- Kramer, P. Management of Malting for Flavor Development and its Impact on Malt Analyses. In Proceedings of the Barley Improvement Conference, Davis, CA, USA, 12 January 2015. [Google Scholar]
- Woffenden, H.M.; Ames, J.M.; Chandra, S.; Anese, M.; Nicoli, M.C. Effect of Kilning on the Antioxidant and Pro-oxidant Activities of Pale Malts. J. Agric. Food Chem. 2002, 50, 4925–4933. [Google Scholar] [CrossRef] [PubMed]
- Lekjing, S.; Venkatachalam, K. Effects of germination time and kilning temperature on the malting characteristics, biochemical and structural properties of HomChaiya rice. RSC Adv. 2020, 10, 16254–16265. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, T. Malt specifications & brewing performance. Brew. Int. 2002, 2, 27–30. [Google Scholar]
- Yahya, H.; Linforth, R.S.T.; Cook, D.J. Flavour generation during commercial barley and malt roasting operations: A time course study. Food Chem. 2014, 145, 378–387. [Google Scholar] [CrossRef]
- Mayer, H.; Ceccaroni, D.; Marconi, O.; Sileoni, V.; Perretti, G.; Fantozzi, P. Development of an all rice malt beer: A gluten free alternative. LWT Food Sci. Technol. 2016, 67, 67–73. [Google Scholar] [CrossRef]
- Murevanhema, Y.Y.; Jideani, V.A. Production and Characterization of Milk Produced from Bambara Groundnut (Vigna subterranea) Varieties. J. Food Process. Preserv. 2015, 39, 1485–1498. [Google Scholar] [CrossRef]
- Simons, C. Color Determination in Food. Available online: https://cwsimons.com/color-determination-in-food/ (accessed on 16 March 2021).
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Agustini, S. Color development in complex model system on various time and temperature. J. Din. Penelit. Ind. 2017, 28, 1–9. [Google Scholar]
- Jha, S.N. (Ed.) Colour Measurements and Modeling. In Nondestructive Evaluation of Food Quality; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–40. ISBN 978-3-642-15795-0. [Google Scholar]
- Osuji, C.M.; Ofoedu, C.E.; Omeire, G.C.; Ojukwu, M. Colour analysis of syrup from malted and unmalted rice of different varieties. Croat. J. Food Sci. Technol. 2020, 12, 130–138. [Google Scholar] [CrossRef]
- Willaert, R.G.; Baron, G. V Wort Boiling Today-Boiling Systems with Low Thermal Stress in Combination with Volatile Stripping. Cerevisia 2001, 26, 217–230. [Google Scholar]
- Willaert, R. The Beer Brewing Process: Wort Production and Beer Fermentation. In Handbook of Food Products Manufacturing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 443–506. [Google Scholar]
- Giebel, J. Food product development: Malt ingredient solutions. Available online: https://www.preparedfoods.com/articles/116693-food-product-development-malt-ingredient-solutions (accessed on 29 January 2019).
- Felix, O.E. Production of Malt-based Sugar Syrup from Enzymatic Hydrolysis of Malted Sorghum and Millet Grains. Asian Food Sci. J. 2020, 14, 1–17. [Google Scholar] [CrossRef]
- Hansen, B.; Wasdovitch, B. Malt Ingredients in Baked Goods. Cereal Foods World 2005, 50, 18–22. [Google Scholar]
- DeLange, A.J. Estimating Mash pH. Available online: http://themodernbrewhouse.com/wp-content/uploads/2016/11/DeLange-Estimating-Mash-pH.pdf (accessed on 15 December 2021).
- Geurts, J. Specialty Malt Acidity. In Proceedings of the World Brewing Congress 2016, Chilton, WI, USA, 13–17 August 2016. [Google Scholar]
- Liscomb, C.; Bies, D.; Hansen, R. Specialty Malt Contributions to Wort and Beer. J. Am. Soc. Brew. Chem. 2015, 52, 181–190. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; Osuji, C.M.; Omeire, G.C.; Ojukwu, M.; Okpala, C.O.R.; Korzeniowska, M. Functional properties of syrup from malted and unmalted rice of different varieties: A comparative study. J. Food Sci. 2020, 85, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Cela, N.; Condelli, N.; Caruso, M.C.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten-free brewing: Issues and perspectives. Fermentation 2020, 6, 53. [Google Scholar] [CrossRef]
- Palmer, G. Barley and Malt. In Handbook of Brewing, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 139–160. ISBN 9781498751926. [Google Scholar]
- DeLange, A.J. Understanding pH and Its Application in Small- Scale Brewing—Part I: Fundamentals and Relevance to Brewhouse Procedures. Available online: https://www.morebeer.com/articles/understanding_ph_in_brewing (accessed on 16 October 2020).
- Ofoedu, C.E.; Akosim, C.Q.; Iwouno, J.O.; Obi, C.D.; Shorstkii, I.; Okpala, C.O.R. Characteristic changes in malt, wort, and beer produced from different Nigerian rice varieties as influenced by varying malting conditions. PeerJ 2021, 9, e10968. [Google Scholar] [CrossRef]
- Gasiński, A.; Błażewicz, J.; Kawa-Rygielska, J.; Śniegowska, J.; Zarzecki, M. Analysis of Physicochemical Parameters of Congress Worts Prepared from Special Legume Seed Malts, Acquired with and without Use of Enzyme Preparations. Foods 2021, 10, 304. [Google Scholar] [CrossRef]
- Fox, G.P. Chemical Composition in Barley Grains and Malt Quality. In Genetics and Improvement of Barley Malt Quality; Springer Science & Business Media: Berlin/Heidelberg, Germany; Springer Science & Business Media: New York, NY, USA, 2009; pp. 63–98. [Google Scholar]
- Stewart, G.G. Adjuncts. In Brewing Materials and Processes; CRC Press: Boca Raton, FL, USA, 2017; pp. 129–144. [Google Scholar]
- Gebeyaw, M. Impact of Malt Barley Varieties on Malt Quality: A Review. Agric. Rev. 2021, 42, 116–119. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M. Influence of kilning temperature on chemical composition of a Greek barley malt and its wort properties. Millenium J. Educ. Technol. Health 2018, 2, 49–58. [Google Scholar] [CrossRef]
- Black, K.; Distillery, A.; Scotland, A. Legumes in Distilleries and Breweries—Novel Beverages and High Protein Co-products—TRUE Project. Available online: https://www.true-project.eu/legume-brewing/ (accessed on 13 June 2020).
- Iannetta, P.P.M.; Black, K.; Tziboula-Clarke, A.; White, P.; Walker, G.M. Why Bean Beer? 2018. Available online: https://rke.abertay.ac.uk/ws/portalfiles/portal/15422461/Walker_BeenBeer_Published_2018.pdf (accessed on 13 June 2020).
- Kü, F.; Back, W.; Krottenthaler, M.; Kurz, T. Particle Size Distribution in Wort During Large Scale Brewhouse Operations. Am. Inst. Chem. Eng. AIChE J. 2007, 53, 1373–1388. [Google Scholar] [CrossRef]
- Jin, B.; Li, L.; Liu, G.-Q.; Li, B.; Zhu, Y.-K.; Liao, L.-N. Structural Changes of Malt Proteins During Boiling. Molecules 2009, 14, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Coverdale, S.M.; Onley-Watson, K.; Bell, D.; Healy, P. The gel filteration chromatographic-profiles of protein and peptides of wort and beer: Effects of processing—Malting, Mashing, kettle boiling, fermebntation and filtering. Inst. Guild Brew. 2003, 109, 41–50. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F.; Ferreira, I.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Parkes, S. Wort Boiling Science. Available online: https://byo.com/article/wort-boiling-homebrew-science/ (accessed on 16 August 2020).
- Karababa, E.; Schwarz, P.B.; Horsley, R.D. Effect of Kiln Schedule on Micromalt Quality Parameters. J. Am. Soc. Brew. Chem. 1993, 51, 163–167. [Google Scholar] [CrossRef]
- Gebremariam, M.M.; Zarnkow, M.; Becker, T. Effect of Drying Temperature and Time on Alpha-Amylase, Beta-Amylase, Limit Dextrinase Activities and Dimethyl Sulphide Level of Teff (Eragrostis tef) Malt. Food Bioprocess Technol. 2013, 6, 3462–3472. [Google Scholar] [CrossRef]
- Uvere, P.O.; Adenuga, O.D.; Mordi, C. The effect of germination and kilning on the cyanogenic potential, amylase and alcohol levels of sorghum malts used for burukutu production. Sci. Food Agric. 2000, 80, 352–358. [Google Scholar] [CrossRef]
- Uriyo, M.G. Changes in enzyme activities during germination of cowpeas (Vigna unguiculata, cv. California blackeye). Food Chem. 2001, 73, 7–10. [Google Scholar] [CrossRef]
- Evans, D.E.; Wallace, W.; Lance, R.C.M.; MacLeod, L.C. Measurement of beta-amylase in malting barley (Hordeum vulgare L.). II. The effect of germination and kilning. J. Cereal Sci. 1997, 26, 241–250. [Google Scholar] [CrossRef]
- Blaise, P.; Phiarais, N.; Wijngaard, H.H.; Arendt, E.K.; Brew, J.I. The Impact of Kilning on Enzymatic Activity of Buckwheat Malt. J. Inst. Brew. 2005, 111, 290–298. [Google Scholar]
- Yousif, A.M.; Evans, D.E. Changes in malt quality during production in two commercial malt houses. J. Inst. Brew. 2020, 126, 233–252. [Google Scholar] [CrossRef]
- Abuajah, C.I. Malting Properties of Sorghum: Kilning Temperatures, Heat and Resultant Effects; LAP LAMBERT Academic Publishing: Saarbrucken, Germany, 2013. [Google Scholar]
- Bathgate, G.N. A review of malting and malt processing for whisky distillation. J. Inst. Brew. 2016, 122, 197–211. [Google Scholar] [CrossRef]
- Ferrari-John, R.S.; Katrib, J.; Zerva, E.; Davies, N.; Cook, D.J.; Dodds, C.; Kingman, S. Electromagnetic Heating for Industrial Kilning of Malt: A Feasibility Study. Food Bioprocess Technol. 2017, 10, 687–698. [Google Scholar] [CrossRef]
- Rani, K. Extraction and study of kinetic parameters of variety of sprouted pulses β-amylases. Int. J. Pharm. Life Sci. 2012, 3, 1893–1896. [Google Scholar]
- Lewis, M.J.; Young, T.W. Malting biochemistry. In Brewing; Springer: Boston, MA, USA, 2001; pp. 191–204. [Google Scholar]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of its By-Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Poisson, G.; Kortz, H.; Field, J.; Fleury, N.; Kmiotek, S.J. Brewing Process Optimization: Mash Efficiency. Ph.D. Dissertation, Worcester Polytechnic Institute, Worcester, MA, USA, 2021. [Google Scholar]
- Parés Viader, R.; Yde, M.S.H.; Hartvig, J.W.; Pagenstecher, M.; Carlsen, J.B.; Christensen, T.B.; Andersen, M.L. Optimization of Beer Brewing by Monitoring α-Amylase and β-Amylase Activities during Mashing. Beverages 2021, 7, 13. [Google Scholar] [CrossRef]
- Gholami Aghel, M.; Hashemiravan, M.; Khorshidpoor, B. Production of Functional Beverage based on Carrot juice, Malt Extract and Ginger Extract. Adv. Biores. 2016, 7, 130–134. [Google Scholar] [CrossRef]
- Lewis, M.J.; Young, T.W. Malting technology: Malt, specialized malts and non-malt adjuncts. In Brewing; Springer: Boston, MA, USA, 2001; pp. 163–190. [Google Scholar]
- De Schepper, C.F.; Michiels, P.; Buvé, C.; Van Loey, A.M.; Courtin, C.M. Starch hydrolysis during mashing: A study of the activity and thermal inactivation kinetics of barley malt α-amylase and β-amylase. Carbohydr. Polym. 2021, 255, 117494. [Google Scholar] [CrossRef]
- O’Rourke, T. The function of enzymes in brewing. Brew. Int. 2002, 2, 14–18. [Google Scholar]
- German Brewing Infusion Mashing. Available online: http://braukaiser.com/wiki/index.php/Infusion_Mashing (accessed on 17 March 2021).
- Mosher, M.; Trantham, K. Brewing Science: A Multidisciplinary Approach; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-46393-3. [Google Scholar]
- Henson, C.A.; Duke, S.H.; Bockelman, H.E. Comparisons of modern U.S. and Canadian malting barley cultivars with those from pre-prohibition: II. amylolytic enzyme activities and thermostabilities. J. Am. Soc. Brew. Chem. 2018, 76, 38–49. [Google Scholar] [CrossRef]
- Rani, H.; Bhardwaj, R.D. Quality attributes for barley malt: “The backbone of beer”. J. Food Sci. 2021, 86, 3322–3340. [Google Scholar] [CrossRef]
- Taylor & Francis Group, LLC. Enzymes in Food and Beverage Processing; Chandrasekaran, M., Ed.; Taylor & Francis Group, LLC: Abingdon, UK, 2015; ISBN 9781482221282. [Google Scholar]
- Montanari, L.; Floridi, S.; Marconi, O.; Tironzelli, M.; Fantozzi, P. Effect of mashing procedures on brewing. Eur. Food Res. Technol. 2005, 221, 175–179. [Google Scholar] [CrossRef]
- Herrera-Gamboa, J.G.; López-Alvarado, C.B.; Pérez-Ortega, E.; Damas-Buenrostro, L.C.; Cabada-Amaya, J.C.; Pereyra-Alférez, B. Proteomic analysis of two malting barleys (Hordeum vulgare L.) and their impact on wort quality. J. Cereal Sci. 2018, 80, 150–157. [Google Scholar] [CrossRef]
- Ambra, R.; Pastore, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, H.; Chen, J.; Fan, W.; Dong, J.; Kong, W.; Sun, J.; Cao, Y.; Cai, G. Evolution of phenolic compounds and antioxidant activity during malting. J. Agric. Food Chem. 2007, 55, 10994–11001. [Google Scholar] [CrossRef] [PubMed]
- Jannat, B. Antioxidant activity of Iranian barley grain cultivars and their malts. Artic. Afr. J. Food Sci. 2015, 9, 534–539. [Google Scholar] [CrossRef]
- Dvořáková, M.; Guido, L.F.; Dostálek, P.; Skulilová, Z.; Moreira, M.M.; Barros, A.A. Antioxidant Properties of Free, Soluble Ester and Insoluble-Bound Phenolic Compounds in Different Barley Varieties and Corresponding Malts. J. Inst. Brew. 2008, 114, 27–33. [Google Scholar] [CrossRef]
- Nemzer, B.; Lin, Y.; Huang, D. Antioxidants in sprouts of grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–68. [Google Scholar]
- Inns, E.L.; Buggey, L.A.; Booer, C.; Nursten, H.E.; Ames, J.M. Effect of modification of the kilning regimen on levels of free ferulic acid and antioxidant activity in malt. J. Agric. Food Chem. 2011, 59, 9335–9343. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Dimitrov, K.; Galván D´Alessandro, L. Effect of malting conditions on phenolic content, Maillard reaction products formation, and antioxidant activity of quinoa seeds. J. Food Sci. Technol. 2016, 53, 3978–3985. [Google Scholar] [CrossRef]
- Sovrano, S.; Buiatti, S.; Anese, M. Influence of malt browning degree on lipoxygenase activity. Food Chem. 2006, 99, 711–717. [Google Scholar] [CrossRef]
- Pejin, J.; Grujić, O.; Canadanovic-Brunet, J.; Vujic, D.; Tumbas, V. Investigation of phenolic acids content and antioxidant activity in malt production. J. Am. Soc. Brew. Chem. 2009, 67, 81–88. [Google Scholar] [CrossRef]
- Woffenden, H.M.; Ames, J.M.; Chandra, S. Antioxidant activity, colour and flavour of crystal malt extracts. Int. Congr. Ser. 2002, 1245, 483–484. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Guleria, P. Sustainable Agriculture Reviews 45; Sustainable Agriculture Reviews; Guleria, P., Kumar, V., Lichtfouse, E., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 45, ISBN 978-3-030-53016-7. [Google Scholar]
- Segev, A.; Badani, H.; Kapulnik, Y.; Shomer, I.; Oren-Shamir, M.; Galili, S. Determination of Polyphenols, Flavonoids, and Antioxidant Capacity in Colored Chickpea (Cicer arietinum L.). J. Food Sci. 2010, 75, S115–S119. [Google Scholar] [CrossRef]
- Jideani, V.A.; Diedericks, C.F. Nutritional, Therapeutic, and Prophylactic Properties of Vigna subterranea and Moringa oleifera. In Antioxidant-Antidiabetic Agents and Human Health; InTech: West Palm Beach, FL, USA, 2014; pp. 188–207. [Google Scholar]
- López-Cortez, M.D.S.; Rosales-Martínez, P.; Arellano-Cárdenas, S.; Cornejo-Mazón, M. Antioxidants Properties and Effect of Processing Methods on Bioactive Compounds of Legumes. In Grain Legumes; InTech: London, UK, 2016. [Google Scholar]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Mandizvo, T.; Odindo, A.O. Seed coat structural and imbibitional characteristics of dark and light coloured Bambara groundnut (Vigna subterranea L.) landraces. Heliyon 2019, 5, 1249. [Google Scholar] [CrossRef] [PubMed]
- Krstanović, V.; Habschied, K.; Dvojković, K.; Mastanjević, K. Research on the Malting Properties of Domestic Wheat Varities. Fermentation 2020, 7, 1. [Google Scholar] [CrossRef]
- Baranwal, D. Malting: An indigenous technology used for improving the nutritional quality of grains- A review. Asian J. Dairy Food Res. 2017, 36, 179–183. [Google Scholar] [CrossRef]
- Limwiwattana, D.; Tongkhao, K.; Na Jom, K. Effect of Sprouting Temperature and Air R elative Humidity on Metabolic Profiles of Sprouting Black Gram (Vigna mungo L.). J. Food Process. Preserv. 2016, 40, 306–315. [Google Scholar] [CrossRef]
- Zhou, K.; Slavin, M.; Lutterodt, H.; Whent, M.; Eskin, N.A.M.; Yu, L. Cereals and Legumes. In Biochemistry of Foods; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–48. [Google Scholar]
- Tiwari, B.K.; Gowen, A.; McKenna, B. (Eds.) Pulse Foods: Processing, Quality and Nutraceutical Applications; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-12-382018-1. [Google Scholar]
- Mazahib, A.M.; Nuha, M.O.; Salawa, I.S.; Babiker, E.E. Some nutritional attributes of Bambara groundnut as influenced by domestic processing. Int. Food Res. J. 2013, 20, 1165–1171. [Google Scholar]
- Hardy, Z.; Jideani, A. Functional and Nutritional Characteristics of Bambara Groundnut Milk Powder as an Ingredient in Yoghurt. Masters Dissertation, Cape Peninsula University of Technology, Cape Town, South Africa, 2016. [Google Scholar]
- Duodu, K.G.; Apea-Bah, F.B. African Legumes: Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains; Elsevier: Amsterdam, The Netherlands, 2017; pp. 223–269. ISBN 9780081008911. [Google Scholar]
- Sarkar, A.; Sensarma, S.R. Sustainable Solutions for Food Security: Combating Climate Change by Adaptation, 2019th ed.; Sarkar, A., Sensarma, S.R., VanLoon, G.W., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-319-77877-8. [Google Scholar]
- Nzelu, I.C. Effect of malting on the amino acid profile of red and white/cream colored Bambara Groundnut Seeds’ Flour. Bioglobia 2016, 3, 68–72. [Google Scholar]
- Nie, C.; Wang, C.; Zhou, G.; Dou, F.; Huang, M. Effects of malting conditions on the amino acid compositions of final malt. Afr. J. Biotechnol. 2010, 9, 9018–9025. [Google Scholar] [CrossRef]
- Parr, H.; Bolat, I.; Cook, D. Modelling flavour formation in roasted malt substrates under controlled conditions of time and temperature. Food Chem. 2021, 337, 127641. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, Y.; Li, Q.; Gu, G. Factors influencing the organic acids content in final malt. J. Am. Soc. Brew. Chem. 2006, 64, 222–227. [Google Scholar] [CrossRef]
- South, J.B. Changes in organic acid levels during malting. J. Inst. Brew. 1996, 102, 161–166. [Google Scholar] [CrossRef]
- Almeida, C.; Viegas, O.; Meireles, S.; Brandão, T.; Ferreira, I.M.P.L.V.O. Quantification of sugars in Specialty malts by HPLC-IR. In IJUP—Book of Abstracts of the 7th Meeting of Young Researchers of University of Porto/Livro de Resumos do 7. Encontro de Investigação Jovem da U. Porto; Instituto de Ciências Biomédicas Abel Salazar: Rua de Jorge Viterbo Ferreira, Portugal, 2014; Volume 5, p. 83. [Google Scholar]
- Okonkwo, S.I.; Opara, M.F. The analysis of Bambara nut (Voandzeia subterranea (L.) thouars) for sustainability in Africa. Res. J. Appl. Sci. 2010, 5, 394–396. [Google Scholar] [CrossRef]
- Ibrahin, H.; Ogunwusi, I. Industrial Potentials of Bambara Nut. J. Poverty Invest. Dev. 2016, 22, 12–18. [Google Scholar]
- Adeleke, O.R.; Adiamo, O.Q.; Fawale, O.S. Nutritional, physicochemical, and functional properties of protein concentrate and isolate of newly-developed Bambara groundnut (Vigna subterrenea L.) cultivars. Food Sci. Nutr. 2018, 6, 229–242. [Google Scholar] [CrossRef]
- Özcan, M.M.; Aljuhaimi, F.; Uslu, N. Effect of malt process steps on bioactive properties and fatty acid composition of barley, green malt and malt grains. J. Food Sci. Technol. 2018, 55, 226–232. [Google Scholar] [CrossRef]
- Bravi, E.; Marconi, O.; Perretti, G.; Fantozzi, P. Influence of barley variety and malting process on lipid content of malt. Food Chem. 2012, 135, 1112–1117. [Google Scholar] [CrossRef]
- Gerčar, N.; Šmidovnik, A. Kinetics of geometrical isomerization of unsaturated FA in soybean oil. J. Am. Oil Chem. Soc. 2002, 79, 495–500. [Google Scholar] [CrossRef]
- Mateos, L.E.; Tonetto, G.M.; Crapiste, G.H. Isomerization of fatty acids in sunflower oil during heat treatment. Lat. Am. Appl. Res. 2010, 40, 213–217. [Google Scholar]
- Li, Y.; Yu, Y.; Luo, Q.; He, Y.; Tian, Z.; Zhao, Y. Thermally induced isomerization of linoleic acid and α-linolenic acid in Rosa roxburghii Tratt seed oil. Food Sci. Nutr. 2021, 9, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Tomislav, M. What is Linoleic Acid? Available online: https://www.news-medical.net/health/What-is-Linoleic-Acid.aspx P (accessed on 28 March 2021).
- Halimi, R.A.; Barkla, B.J.; Mayes, S.; King, G.J. Characteristics of the Underutilised Pulse Bambara Groundnut (Vigna subterranea (L.) Verdc.) Relevant to Food & Nutritional Security. Proceedings 2020, 36, 199. [Google Scholar] [CrossRef]
- Boye, J.I. Nutraceutical and Functional Food Processing Technology; John Wiley & Sons, Ltd: West Sussex, UK, 2015; ISBN 9781118504949. [Google Scholar]
- Bagchi, D.; Preuss, H.G.; Swaroop, A.; Bagchi, D. (Eds.) Nutraceuticals and Functional Foods in Human Health and Disease Prevention; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429156939. [Google Scholar]
- García-Lomillo, J.; González-SanJosé, M.L. Pyrazines in thermally treated foods. Encycl. Food Chem. 2018, 2, 353–362. [Google Scholar] [CrossRef]
- Hui, Y.H.; Chandan, R.C.; Clark, S.; Cross, N.; Dobbs, J.; Hurst, W.J.; Nollet, L.M.L.; Shimoni, E.; Sinha, N.; Smith, E.B.; et al. Handbook of Food Products Manufacturing; Hui, Y.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 9780470113554. [Google Scholar]
- Farah, A. Coffee as a Speciality and Functional Beverage; Woodhead Publishing Limited: Sawston, UK, 2009; ISBN 9781845693428. [Google Scholar]
- Maga, J.A.; Sizer, C.E. Pyrazines in foods. CRC Crit. Rev. Food Technol. 1973, 4, 39–115. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, S.; Nie, Y.; Xu, Y. Quantitative analysis of pyrazines and their perceptual interactions in Soy Sauce aroma type Baijiu. Foods 2021, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of pyrazines in maillard model systems: Effects of structures of lysine-containing dipeptides/tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef]
- Channell, G.A.; Yahya, H.; Cook, D.J. Thermal volatile generation in barley malt: On-line MS studies. J. Am. Soc. Brew. Chem. 2010, 68, 175–182. [Google Scholar] [CrossRef]
- Oxford University Press, Inc. The Oxford Companion to Beer; Oliver, G., Ed.; Oxford University Press, Inc.: New York, NY, USA, 2012; ISBN 9780195367133. [Google Scholar]
- Mi, X.-J.; Hou, J.-G.; Wang, Z.; Han, Y.; Ren, S.; Hu, J.-N.; Chen, C.; Li, W. The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt and p53 signaling pathways. Sci. Rep. 2018, 8, 15922. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, Y.; Wang, L.; Zhang, Y.; Zhou, J. Multispectroscopic studies on the interaction of maltol, a food additive, with bovine serum albumin. Food Chem. 2012, 133, 264–270. [Google Scholar] [CrossRef]
- Anwar-Mohamed, A.; El-Kadi, A.O.S. Induction of cytochrome P450 1a1 by the food flavoring agent, maltol. Toxicol. Vitr. 2007, 21, 685–690. [Google Scholar] [CrossRef] [PubMed]
- KrishnaKumar, V.; Barathi, D.; Mathammal, R.; Balamani, J.; Jayamani, N. Spectroscopic properties, NLO, HOMO–LUMO and NBO of maltol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hong, S.; Iizuka, Y.; Kim, C.Y.; Seong, G.J. The Neuroprotective Effect of Maltol against Oxidative Stress on Rat Retinal Neuronal Cells. Korean J. Ophthalmol. 2015, 29, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Q.; Hu, J.; Han, X.; Li, W.; Zhao, L. Maltol, a Food Flavoring Agent, Attenuates Acute Alcohol-Induced Oxidative Damage in Mice. Nutrients. 2015, 7, 682–696. [Google Scholar] [CrossRef]
- Li, W.; Su, X.-M.; Han, Y.; Xu, Q.; Zhang, J.; Wang, Z.; Wang, Y.-P. Maltol, a Maillard reaction product, exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. RSC Adv. 2015, 5, 101850–101859. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Hashem, C.; Vranková, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of these Valuable Flavor and Fragrance Compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Fan, W.; Qian, M.C. Characterization of Aroma Compounds of Chinese “Wuliangye” and “Jiannanchun” Liquors by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef]
- Ismarti, I.; Triyana, K.; Fadzillah, N.A.; Nordin, N.F.H. The significance of Maillard reaction for species-specific detection gelatine in food industry. J. Phys. Conf. Ser. 2021, 1731, 012018. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Chhikara, N.; Panghal, A.; Gat, Y. Quality characterization of gluten free noodles enriched with chickpea protein isolate. Food Biosci. 2020, 36, 100626. [Google Scholar] [CrossRef]
- Panghal, A.; Kaur, R.; Janghu, S.; Sharma, P.; Sharma, P.; Chhikara, N. Nutritional, phytochemical, functional and sensorial attributes of Syzygium cumini L. pulp incorporated pasta. Food Chem. 2019, 289, 723–728. [Google Scholar] [CrossRef]
- Medhe, S.; Jain, S.; Anal, A.K. Effects of sprouting and cooking processes on physicochemical and functional properties of moth bean (Vigna aconitifolia) seed and flour. J. Food Sci. Technol. 2019, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Atudorei, D.; Stroe, S.-G.; Codină, G.G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Plants 2021, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Awobusuyi, T.D.; Siwela, M. Nutritional properties and consumer’s acceptance of provitamin a-biofortified amahewu combined with Bambara (Vigna subterranea) flour. Nutrients 2019, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Montanuci, F.D.; Ribani, M.; de Matos Jorge, L.M.; Matos Jorge, R.M. Effect of steeping time and temperature on malting process. J. Food Process Eng. 2017, 40, e12519. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Total Phenolics, Phenolic Acids, Isoflavones, and Anthocyanins and Antioxidant Properties of Yellow and Black Soybeans As Affected by Thermal Processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef]
- Rico, D.; Peñas, E.; del Carmen García, M.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted barley flour as a nutritious and functional ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef]
- Frank, T.; Meuleye, B.S.; Miller, A.; Shu, Q.-Y.; Engel, K.-H. Metabolite Profiling of Two Low Phytic Acid ( lpa ) Rice Mutants. J. Agric. Food Chem. 2007, 55, 11011–11019. [Google Scholar] [CrossRef]
- Jom, K.N.; Frank, T.; Engel, K.-H. A metabolite profiling approach to follow the sprouting process of mung beans (Vigna radiata). Metabolomics 2011, 7, 102–117. [Google Scholar] [CrossRef]
- Frank, T.; Nörenberg, S.; Engel, K. Metabolite Profiling of Two Novel Low Phytic Acid ( lpa ) Soybean Mutants. J. Agric. Food Chem. 2009, 57, 6408–6416. [Google Scholar] [CrossRef]
- Frank, T.; Scholz, B.; Peter, S.; Engel, K.-H. Metabolite profiling of barley: Influence of the malting process. Food Chem. 2011, 124, 948–957. [Google Scholar] [CrossRef]
- Shu, X.-L.; Frank, T.; Shu, Q.; Engel, K. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I.; Loeza-Serrano, S.; Pérez-Vega, S.; Pandiella, S.S. Headspace gas chromatography (HS-GC) analysis of imperative flavor compounds in Lactobacilli-fermented barley and malt substrates. Food Sci. Biotechnol. 2015, 24, 1363–1371. [Google Scholar] [CrossRef]
- Lopez, A.R. AOAC Official Method 996.06 Fat (Total, Saturated, and Unsaturated) in Foods, hydrolytic extraction gas chromatographic method. In Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Stenerson, K.K. The Derivatization and Analysis of Amino Acids by GC-MS; Merck KGaA: Darmstadt, Germany, 2021. [Google Scholar]
- McGough, M.M.; Sato, T.; Rankin, S.A.; Sindelar, J.J. Reducing sodium levels in frankfurters using naturally brewed soy sauce. Meat Sci. 2012, 91, 69–78. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).