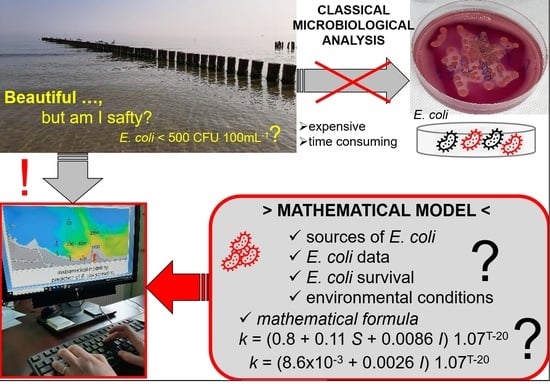
Difficulties in the Modeling of E. coli Spreading from Various Sources in a Coastal Marine Area
Abstract
:1. Introduction
- To present results of the application of the PM3Dhydrodynamic model with the use of the microbiological module to predict the level of E. coli concentrations around the point of discharge of treated wastewater into the waters of the Gulf of Gdańsk, Poland;
- To describe problems/barriers/obstacles encountered by researchers who undertake the development of a mathematical model of pathogen spread in the marine environment under conditions of discharging treated wastewater into brackish/saline water on the example of the Gulf of Gdańsk, Poland.
2. Results
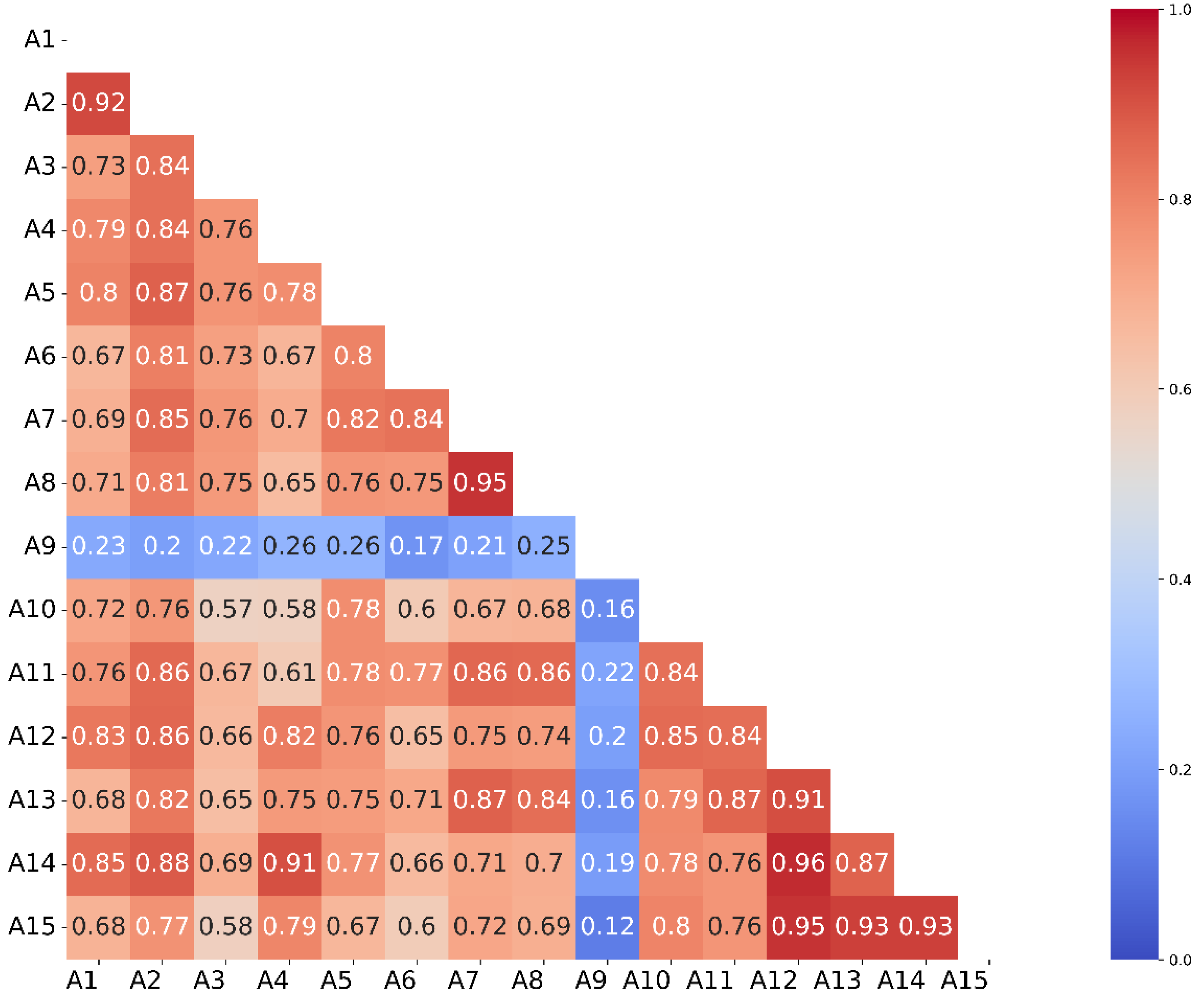
2.1. Validation of the Model
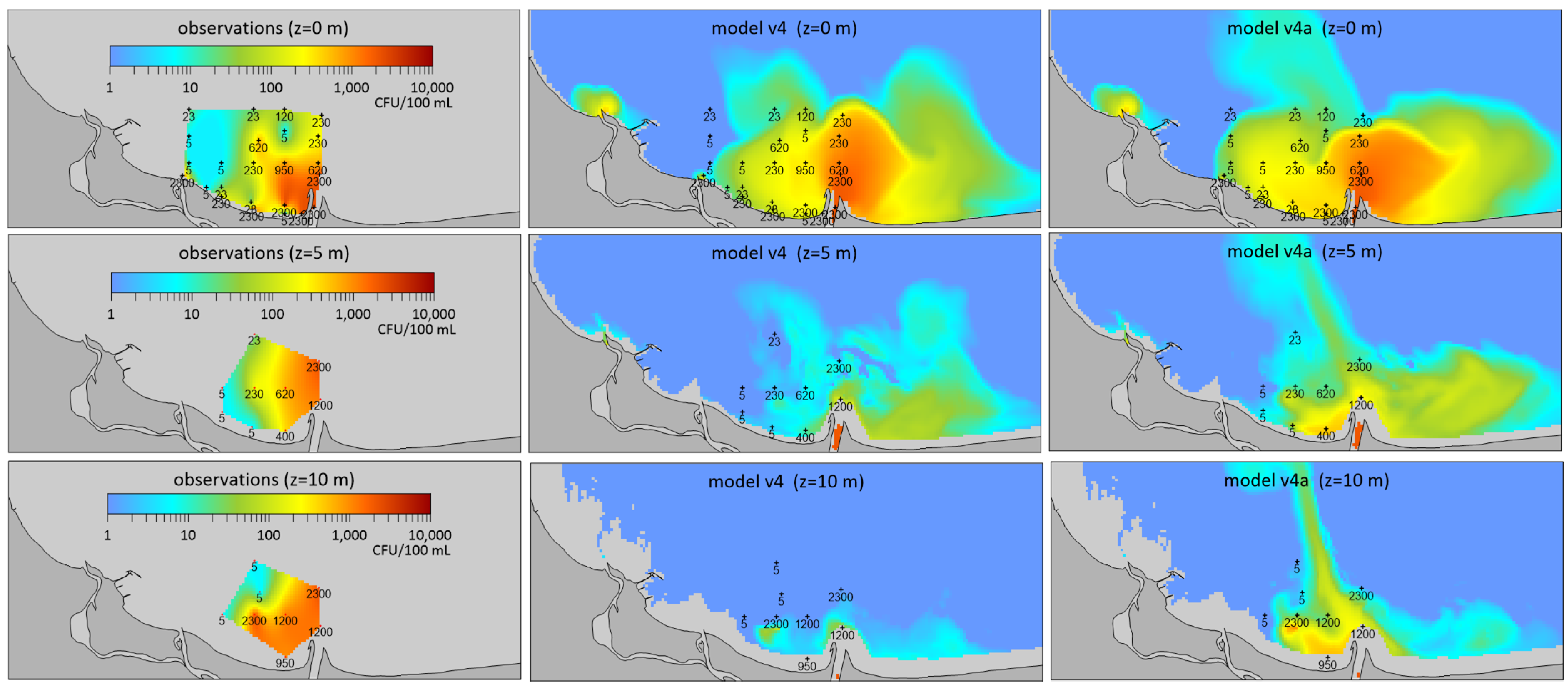
2.2. A Case Study Dated 12 August 2003—Spatial Distribution at Different Depths
3. Discussion
- E. coli concentration in river tributaries (including the largest one, the Vistula River);
- Results of systematic monitoring of E. coli concentration provided in treated wastewater discharged into the Gdańsk Bay.
4. Materials and Methods
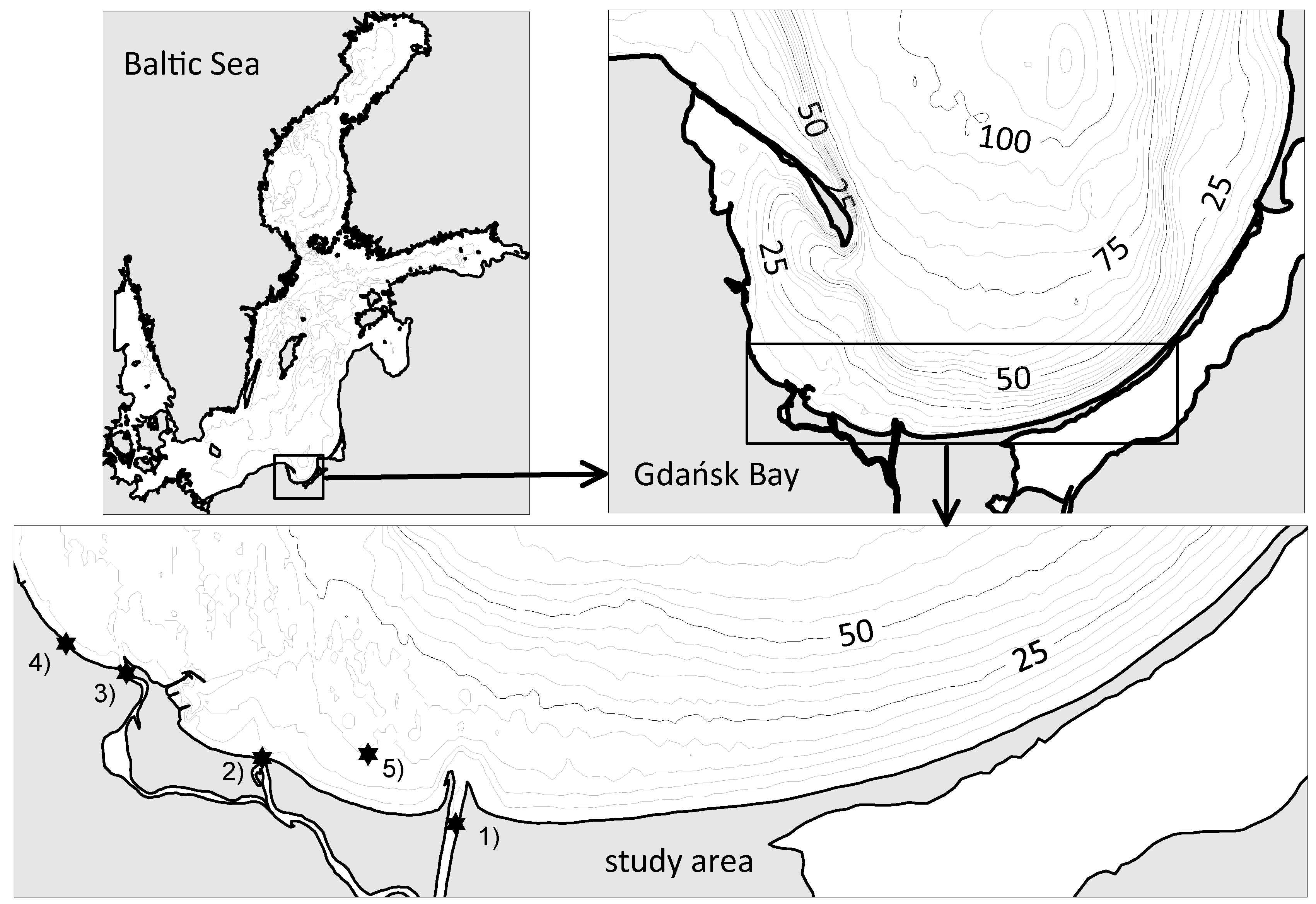
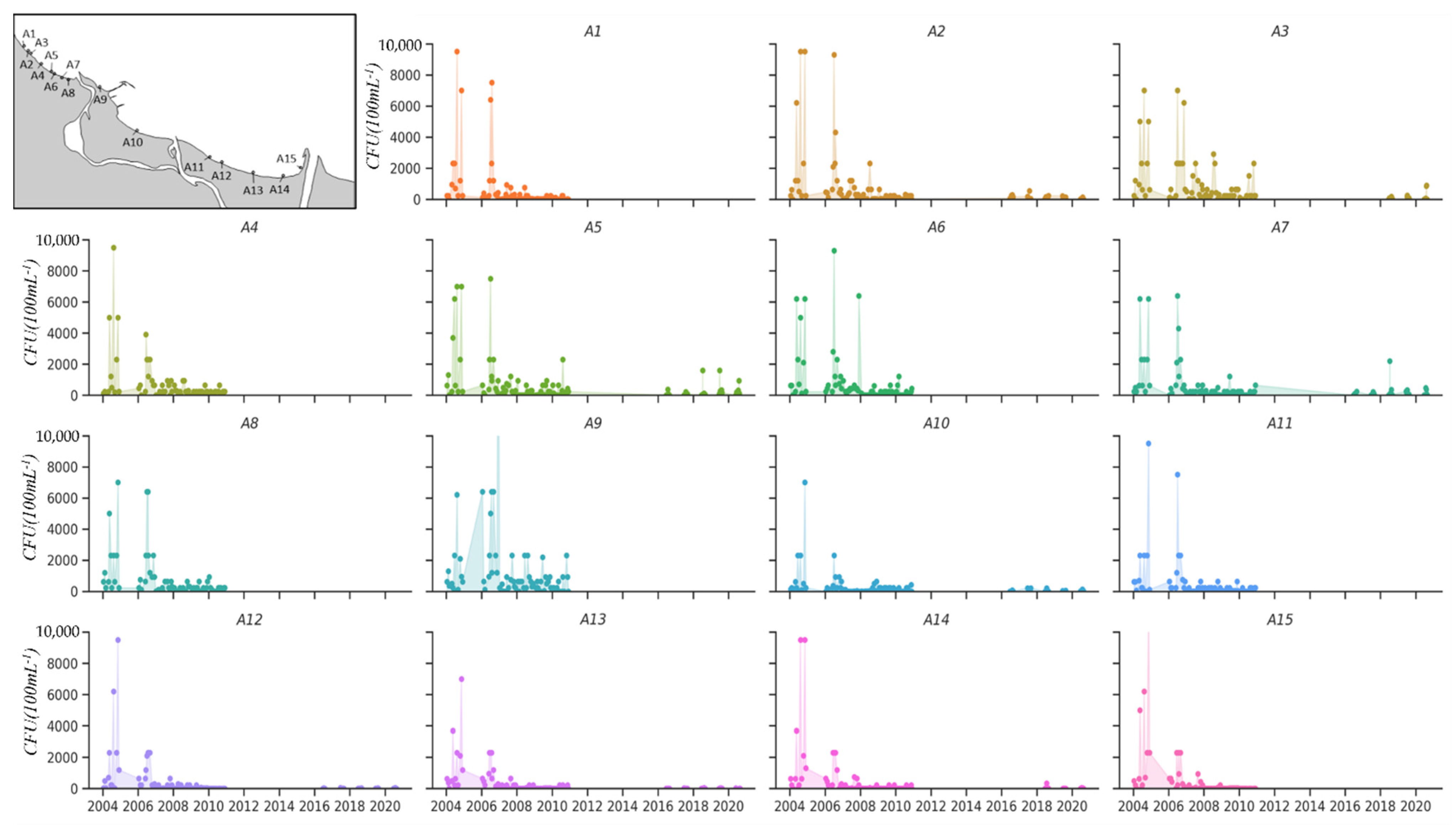
4.1. Description of the Study Area
4.2. Quality of the Bathing Water in the Gdańsk Bay Area
4.2.1. Point Sources
- Four WWTPs (of which only two have a biological treatment stage);
- the Vistula estuary and local water courses;
- storm sewer.
4.2.2. Area Sources
5. Description of the Model for E. coli Spread
5.1. General Assumptions
5.2. Faecal Coliforms (MPN of Faecal Coliforms)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chu, E.W.; Karr, J.R. Environmental Impact: Concept, Consequences, Measurement. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igere, B.E.; Okoh, A.I.; Nwodo, U.U. Wastewater treatment plants and release: The vase of Odin for emerging bacterial contaminants, resistance and determinant of environmental wellness. Emerg. Contam. 2020, 6, 212–224. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Dis. Mon. 2003, 49, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; van Kerrebroeck, P.E.; Nordenbo, A.; Van Poppel, H. Treatment of lower urinary tract dysfunction in patients with multiple sclerosis. Committee of the European Study Group of SUDIMS (Sexual and Urological Disorders in Multiple Sclerosis). J. Neurol. Neurosurg. Psychiatry 1992, 55, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Heyns, C.F.; Fisher, M. The urological management of the patient with acquired immunodeficiency syndrome. BJU Int. 2005, 95, 709–716. [Google Scholar] [CrossRef]
- Peeling, R.W.; Brunham, R.C. Chlamydiae as pathogens: New species and new issues. Emerg. Infect. Dis. 1996, 2, 307–319. [Google Scholar] [CrossRef]
- Chang, S.L.; Shortliffe, L.D. Pediatric urinary tract infections. Pediatric Clin. 2006, 53, 379–400. [Google Scholar] [CrossRef]
- Prakash, J. The kidney in pregnancy: A journey of three decades. Indian J. Nephrol. 2012, 22, 159–167. [Google Scholar] [CrossRef]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef]
- Ronald, A.; Ludwig, E. Urinary tract infections in adults with diabetes. Int. J. Antimicrob. Agents 2001, 17, 287–292. [Google Scholar] [CrossRef]
- Grüne, B.; Viehweger, V.; Waldbillig, F.; Nientiedt, M.; Kriegmair, M.C.; Rassweiler-Seyfried, M.-C.; Ritter, M. Preoperative urine culture—Is it necessary to prevent infectious complications following ureterorenoscopy? J. Microbiol. Methods 2020, 173, 105933. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.F.C.; Zhang, L.; Balfour, A.J.; Garside, R.; Hawkey, P.M.; Murray, A.K.; Ukoumunne, O.C.; Gaze, W.H. Exposure to and colonisation by antibiotic-resistant E. coli in UK coastal water users: Environmental surveillance, exposure assessment, and epidemiological study (Beach Bum Survey). Environ. Int. 2018, 114, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, M.V.; Borges, C.A.; Beraldo, L.G.; Maluta, R.P.; Pollo, A.S.; Borzi, M.M.; Dos Santos, L.F.; Kariyawasam, S.; de Ávila, F.A. Shigatoxigenic and atypical enteropathogenic Escherichia coli in fish for human consumption. Braz. J. Microbiol. 2018, 49, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Maluta, R.P.; Fairbrother, J.M.; Stella, A.E.; Rigobelo, E.C.; Martinez, R.; de Ávila, F.A. Potentially pathogenic Escherichia coli in healthy, pasture-raised sheep on farms and at the abattoir in Brazil. Vet. Microbiol. 2014, 169, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, 4, 37–51.
- European Environment Agency. State of Bathing Water. Details on All Member States Bathing Waters for the 2020 Season Can Be Found in the National Bathing Water Reports. 2020. Available online: https://www.eea.europa.eu/themes/water/europes-seas-and-coasts/assessments/state-of-bathing-water/country-reports-2020-bathing-season/polish-bathing-water-quality-in-2020 (accessed on 21 February 2020).
- Commission, T.E. Bathing Water Quality. Available online: https://ec.europa.eu/environment/water/water-bathing/ (accessed on 23 February 2022).
- Džal, D.; Kosović, I.N.; Mastelić, T.; Ivanković, D.; Puljak, T.; Jozić, S. Modelling Bathing Water Quality Using Official Monitoring Data. Water 2021, 13, 3005. [Google Scholar] [CrossRef]
- Locatelli, L.; Russo, B.; Acero Oliete, A.; Sánchez Catalán, J.C.; Martinez-Gomariz, E.; Martinez, M. Modeling of E. coli distribution for hazard assessment of bathing waters affected by combined sewer overflows. Nat. Hazards Earth Syst. Sci. 2020, 20, 1219–1232. [Google Scholar] [CrossRef]
- Daniel, D.; Iswarani, W.P.; Pande, S.; Rietveld, L. A Bayesian Belief Network model to link sanitary inspection data to drinking water quality in a medium resource setting in rural Indonesia. Sci. Rep. 2020, 10, 18867. [Google Scholar] [CrossRef]
- Srikandi, E.D.; Karnaningroem, N. Dynamic modelling of Escherichia coli growth in water distribution system (a case study: Water supply zone 3 in Surabaya Indonesia). Int. J. Adv. Res. 2017, 5, 1145–1149. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P. Modeling in- stream Escherichia coli concentrations. Diss. Abstr. Int. 2012, 74 (Suppl. B:1–336), 5. [Google Scholar]
- Stocker, M.D.; Jeon, D.J.; Sokolova, E.; Lee, H.; Kim, M.S.; Pachepsky, Y.A. Accounting for the Three-Dimensional Distribution of Escherichia coli Concentrations in Pond Water in Simulations of the Microbial Quality of Water Withdrawn for Irrigation. Water 2020, 12, 1708. [Google Scholar] [CrossRef]
- Herrig, I.; Seis, W.; Fischer, H.; Regnery, J.; Manz, W.; Reifferscheid, G.; Böer, S. Prediction of fecal indicator organism concentrations in rivers: The shifting role of environmental factors under varying flow conditions. Environ. Sci. Eur. 2019, 31, 59. [Google Scholar] [CrossRef]
- Hu, J.; Yang, M.; Gompper, G.; Winkler, R.G. Modelling the mechanics and hydrodynamics of swimming E. coli. Soft Matter 2015, 11, 7867–7876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Falconer, R.A.; Lin, B. Integrated hydro-bacterial modelling for predicting bathing water quality. Estuar. Coast. Shelf Sci. 2017, 188, 145–155. [Google Scholar] [CrossRef]
- Cho, K.H.; Pachepsky, Y.A.; Oliver, D.M.; Muirhead, R.W.; Park, Y.; Quilliam, R.S.; Shelton, D.R. Modeling fate and transport of fecally-derived microorganisms at the watershed scale: State of the science and future opportunities. Water Res. 2016, 100, 38–56. [Google Scholar] [CrossRef]
- Niu, J.; Phanikumar, M. Modeling watershed-scale solute transport using an integrated, process-based hydrologic model with applications to bacterial fate and transport. J. Hydrol. 2015, 529, 35–48. [Google Scholar] [CrossRef]
- De Brauwere, A.; Gourgue, O.; de Brye, B.; Servais, P.; Ouattara, N.K.; Deleersnijder, E. Integrated modelling of faecal contamination in a densely populated river-sea continuum (Scheldt River and Estuary). Sci. Total Environ. 2014, 468–469, 31–45. [Google Scholar] [CrossRef]
- Zhou, J.; Falconer, R.A.; Lin, B. Refinements To The Efdc Model For Predicting The Hydro-Environmental Impacts Of A Barrage Across The Severn Estuary. Renew. Energy 2014, 1, 458–476. [Google Scholar] [CrossRef]
- Bedri, Z.; Corkery, A.; O’Sullivan, J.J.; Alvarez, M.X.; Erichsen, A.C.; Deering, L.A.; Demeter, K.; O’Hare, G.M.P.; Meijer, W.G.; Masterson, B. An Integrated Catchment-Coastal Modelling System for Real-Time Water Quality Forecasts. Environ. Model. Softw. 2014, 61, 458–476. [Google Scholar] [CrossRef]
- Yakirevich, A.; Pachepsky, Y.A.; Guber, A.K.; Gish, T.J.; Shelton, D.R.; Cho, K.H. Modeling transport of Escherichia coli in a creek during and after artificial high-flow events: Three-year study and analysis. Water Res. 2013, 47, 2676–2688. [Google Scholar] [CrossRef]
- Chan, S.N.; Thoe, W.; Lee, J.H.W. Real-time forecasting of Hong Kong beach water quality by 3D deterministic model. Water Res. 2013, 47, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Soupir, M.L.; Ikenberry, C.D.; Rehmann, C.R. Predicting Streambed Sediment and Water Column Escherichia coli Levels at Watershed Scale. JAWRA J. Am. Water Resour. Assoc. 2016, 52, 184–197. [Google Scholar] [CrossRef]
- Weiskerger, C.J.; Phanikumar, M.S. Numerical Modeling of Microbial Fate and Transport in Natural Waters: Review and Implications for Normal and Extreme Storm Events. Water 2020, 12, 1876. [Google Scholar] [CrossRef]
- Petersen, F.; Hubbart, J.A. Physical Factors Impacting the Survival and Occurrence of Escherichia coli in Secondary Habitats. Water 2020, 12, 1796. [Google Scholar] [CrossRef]
- Nag, R.; Monahan, C.; Whyte, P.; Markey, B.K.; O’Flaherty, V.; Bolton, D.; Fenton, O.; Richards, K.G.; Cummins, E. Risk assessment of Escherichia coli in bioaerosols generated following land application of farmyard slurry. Sci. Total Environ. 2021, 791, 148189. [Google Scholar] [CrossRef] [PubMed]
- García-Alba, J.; Bárcena, J.F.; Ugarteburu, C.; García, A. Artificial neural networks as emulators of process-based models to analyse bathing water quality in estuaries. Water Res. 2019, 150, 283–295. [Google Scholar] [CrossRef]
- Michalska, M.; Zorena, K.; Bartoszewicz, M. Analysis of faecal bacteria isolated from air and seawater samples following an emergency sewage discharge into the Gulf of Gdansk in 2018-preliminary study. Int. Marit. Health 2019, 70, 239–243. [Google Scholar] [CrossRef]
- Matciak, M.; Nowicki, J. The hydrological and hydrochemical division of the surface observations. Oceanologia 1996, 37, 75–88. [Google Scholar]
- Jankowski, A. Matematyczne modelowanie pola przepływów w Morzu Bałtyckim. In Prace Habilitacyjne—Polska Akademia Nauk. Instytut Oceanologii; Ossolineum: Wroclaw, Poland, 1988; p. 281. [Google Scholar]
- Jędrasik, J.; Kowalewski, M. Mean annual and seasonal circulation patterns and long-term variability of currents in the Baltic Sea. J. Mar. Syst. 2019, 193, 1–26. [Google Scholar] [CrossRef]
- Taranowska, S. O kierunkach i prędkościach wiatrów dolnych na obszarze Środkowego i Południowego Bałtyku (1947–1960). Przegląd Geogr. 1968, 13, 75–88. [Google Scholar]
- Kullenberg, G. Physical Oceanograph7y. In The Baltic Sea; Voipio, A., Ed.; Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1981; Volume 30, pp. 135–181. [Google Scholar]
- Voipio, A. (Ed.) The Baltic Sea; Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1981; Volume 30. [Google Scholar]
- Kwiecień, K. Warunki klimatyczne. In Bałtyk Południowy; Ossolineum: Wrocław, Poland, 1987; pp. 219–288. [Google Scholar]
- Islam, M.M.M.; Hofstra, N.; Islam, M.A. The Impact of Environmental Variables on Faecal Indicator Bacteria in the Betna River Basin, Bangladesh. Environ. Processes 2017, 4, 319–332. [Google Scholar] [CrossRef]
- Ratajczak, W. Environmental Threats Resulting from the Discharge of Treated Sewage to Sea Waters. Ph.D. Thesis, Medical University of Gdańsk, Gdańsk, Poland, 2017. Unpublished. (In Polish). [Google Scholar]
- Majewski, W. General characteristics of the Vistula and its basin. Acta Energetica 2013, 2, 6–15. [Google Scholar] [CrossRef]
- Meissner, W.; Kośmicki, A.; Bzoma, S. Liczebność ptaków wodnych na Zatoce Gdańskiej w okresie wrzesień 2020–kwiecień 2021. Ornis Pol. 2021, 62, 357–363. [Google Scholar]
- Kowalewski, M. A three-dimensional hydrodynamic model of the Gulf of Gdansk. Oceanol. Stud. 1997, 26, 77–98. [Google Scholar]
- Ołdakowski, B.; Kowalewski, M.; Jędrasik, J.; Szymelfenig, M. Ecohydrodynamic model of the Baltic Sea. Part 1. Description of the ProDeMo model. Oceanologia 2005, 47, 477–516. [Google Scholar]
- Kowalewski, M. The flow of nitrogen into the euphotic zone of the Baltic Proper as a result of the vertical migration of phytoplankton: An analysis of the long-term observations and ecohydrodynamic model simulation. J. Mar. Syst. 2015, 145, 53–68. [Google Scholar] [CrossRef]
- Kowalewska-Kalkowska, H.; Kowalewski, M. Combining Satellite Imagery and Numerical Modelling to Study the Occurrence of Warm Upwellings in the Southern Baltic Sea in Winter. Remote Sens. 2019, 11, 2982. [Google Scholar] [CrossRef] [Green Version]
- Flint, K.P. The long-term survival of Escherichia coli in river water. J. Appl. Bacteriol. 1987, 63, 261–270. [Google Scholar] [CrossRef]
- Liang, C.; Yao, Z.; Du, S.; Hong, M.; Wang, K.; Zhang, D. Sediment pH, not the bacterial diversity, determines Escherichia coli O157:H7 survival in estuarine sediments. Environ. Pollut. 2019, 252, 1078–1086. [Google Scholar] [CrossRef]
- Wanjugi, P.; Fox, G.A.; Harwood, V.J. The Interplay Between Predation, Competition, and Nutrient Levels Influences the Survival of Escherichia coli in Aquatic Environments. Microb. Ecol. 2016, 72, 526–537. [Google Scholar] [CrossRef]
- Garcia-Armisen, T.; Servais, P. Partitioning and fate of particle-associated E. coli in river waters. Water Environ. Res. 2009, 81, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sagarduy, M.; Courtois, S.; Del Campo, A.; Garmendia, J.M.; Petrau, A. Differential decay and prediction of persistence of Enterococcus spp. and Escherichia coli culturable cells and molecular markers in freshwater and seawater environments. Int. J. Hyg. Environ. Health 2019, 222, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Rozen, Y.; Belkin, S. Survival of enteric bacteria in seawater. FEMS Microbiol. Rev. 2001, 25, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Korajkic, A.; Wanjugi, P.; Harwood, V.J. Indigenous microbiota and habitat influence Escherichia coli survival more than sunlight in simulated aquatic environments. Appl. Environ. Microbiol. 2013, 79, 5329–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lothigius, A.; Sjöling, A.; Svennerholm, A.-M.; Bölin, I. Survival and gene expression of enterotoxigenic Escherichia coli during long-term incubation in sea water and freshwater. J. Appl. Microbiol. 2010, 108, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Sáez, L.; Gasol, J.M.; Lefort, T.; Hofer, J.; Sommaruga, R. Effect of natural sunlight on bacterial activity and differential sensitivity of natural bacterioplankton groups in northwestern Mediterranean coastal waters. Appl. Environ. Microbiol. 2006, 72, 5806–5813. [Google Scholar] [CrossRef] [Green Version]
- Krolska, M.; Latala, A.; Michalska, M.; Nowacki, J.S.Z. Escherichia coli survival in the water from the Gulf of Gdansk under laboratory conditions. Oceanol. Stud. 1997, 26, 133–143. [Google Scholar]
- Jozić, S.; Morović, M.; Šolić, M.; Krstulović, N.; Ordulj, M. Effect of solar radiation, temperature and salinity on the survival of two different strains of Escherichia coli. Fresenius Environ. Bull. 2014, 23, 1852–1859. [Google Scholar]
- Thupaki, P.; Phanikumar, M.S.; Beletsky, D.; Schwab, D.J.; Nevers, M.B.; Whitman, R.L. Budget analysis of Escherichia coli at a Southern Lake Michigan Beach. Environ. Sci. Technol. 2010, 44, 1010–1016. [Google Scholar] [CrossRef]
- Szumilas, T.; Michalska, M.; Bartoszewicz, M. Changes of sanitary conditions of coastal waters near Gdańsk in years 1993–2002. Inżynieria Morska i Geotech 2004, 3, 131–138. [Google Scholar]
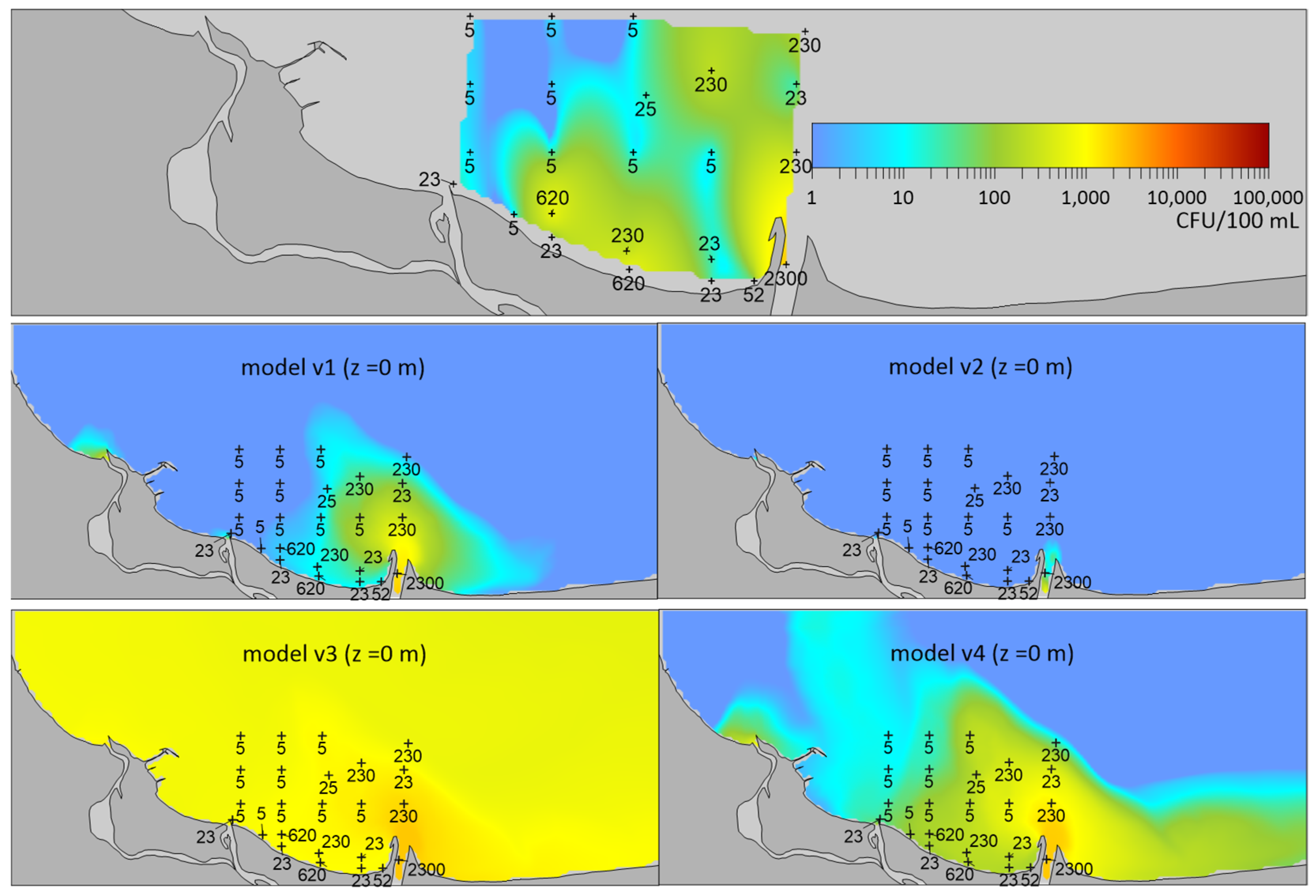

| DATE | BIAS | RMSE | R |
|---|---|---|---|
| 5 November 2002 | −1.94 | 0.28 | 0.96 |
| 10 December 2002 | −1.94 | 0.26 | −0.46 |
| 5 February 2003 | −1.25 | 1.01 | 0.58 |
| 25 March 2003 | −0.24 | 1.25 | 0.48 |
| 29 March 2003 | −0.29 | 1.47 | 0.35 |
| 8 May 2003 | 0.08 | 1.20 | 0.17 |
| 10 May 2003 | 0.32 | 1.08 | 0.13 |
| 29 May 2003 | 0.18 | 0.86 | 0.37 |
| 17 June 2003 | 0.08 | 0.88 | 0.54 |
| 4 July 2003 | −0.17 | 0.97 | 0.33 |
| 16 July 2003 | 0.33 | 1.00 | 0.19 |
| 12 August 2003 | −0.63 | 1.18 | 0.29 |
| 6 September 2003 | −0.14 | 1.17 | 0.26 |
| 26 September 2003 | −0.61 | 1.25 | 0.22 |
| 3 October 2003 | −0.41 | 0.98 | 0.23 |
| Date | Area of Accident | Description |
|---|---|---|
| 15 May 2018 | Gdańsk Ołowianka wastewater pumping station | Engine failure at the pumping station managing 60% of the wastewater from Gdańsk and transporting it to the Gdańsk Wschód (WWTP). At that time, about 2600 m3 of wastewater was discharged per hour directly into Gdańsk Bay. |
| 12 June 2019 | outlet from Swarzewo WWTP | Pipe bursting and wastewater spilling into Puck Bay and onto residents’ properties |
| 27 August 2019 | the Vistula River | First pipeline failure in Warsaw |
| 29 August 2020 | the Vistula River | Second pipeline failure in Warsaw |
| Version of Model | Formula | Reference |
|---|---|---|
| v1 | k = 6.466 − 0.195 T + 2.215·10−7 T2 I2 | [65] |
| v2 | [66] | |
| v3 | [67] | |
| v4 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, L.; Kowalewski, M.; Potrykus, M.; Redko, V.; Rybak, B. Difficulties in the Modeling of E. coli Spreading from Various Sources in a Coastal Marine Area. Molecules 2022, 27, 4353. https://doi.org/10.3390/molecules27144353
Wolska L, Kowalewski M, Potrykus M, Redko V, Rybak B. Difficulties in the Modeling of E. coli Spreading from Various Sources in a Coastal Marine Area. Molecules. 2022; 27(14):4353. https://doi.org/10.3390/molecules27144353
Chicago/Turabian StyleWolska, Lidia, Marek Kowalewski, Marta Potrykus, Vladyslav Redko, and Bartosz Rybak. 2022. "Difficulties in the Modeling of E. coli Spreading from Various Sources in a Coastal Marine Area" Molecules 27, no. 14: 4353. https://doi.org/10.3390/molecules27144353
APA StyleWolska, L., Kowalewski, M., Potrykus, M., Redko, V., & Rybak, B. (2022). Difficulties in the Modeling of E. coli Spreading from Various Sources in a Coastal Marine Area. Molecules, 27(14), 4353. https://doi.org/10.3390/molecules27144353