Experimental Study on the Mechanical Properties and Microstructure of Metakaolin-Based Geopolymer Modified Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Soil
2.1.2. Metakaolin
Kaolinite Metakaolin
2.1.3. Alkali-Activator
2.2. Sample Preparation
3. Result and Discussion
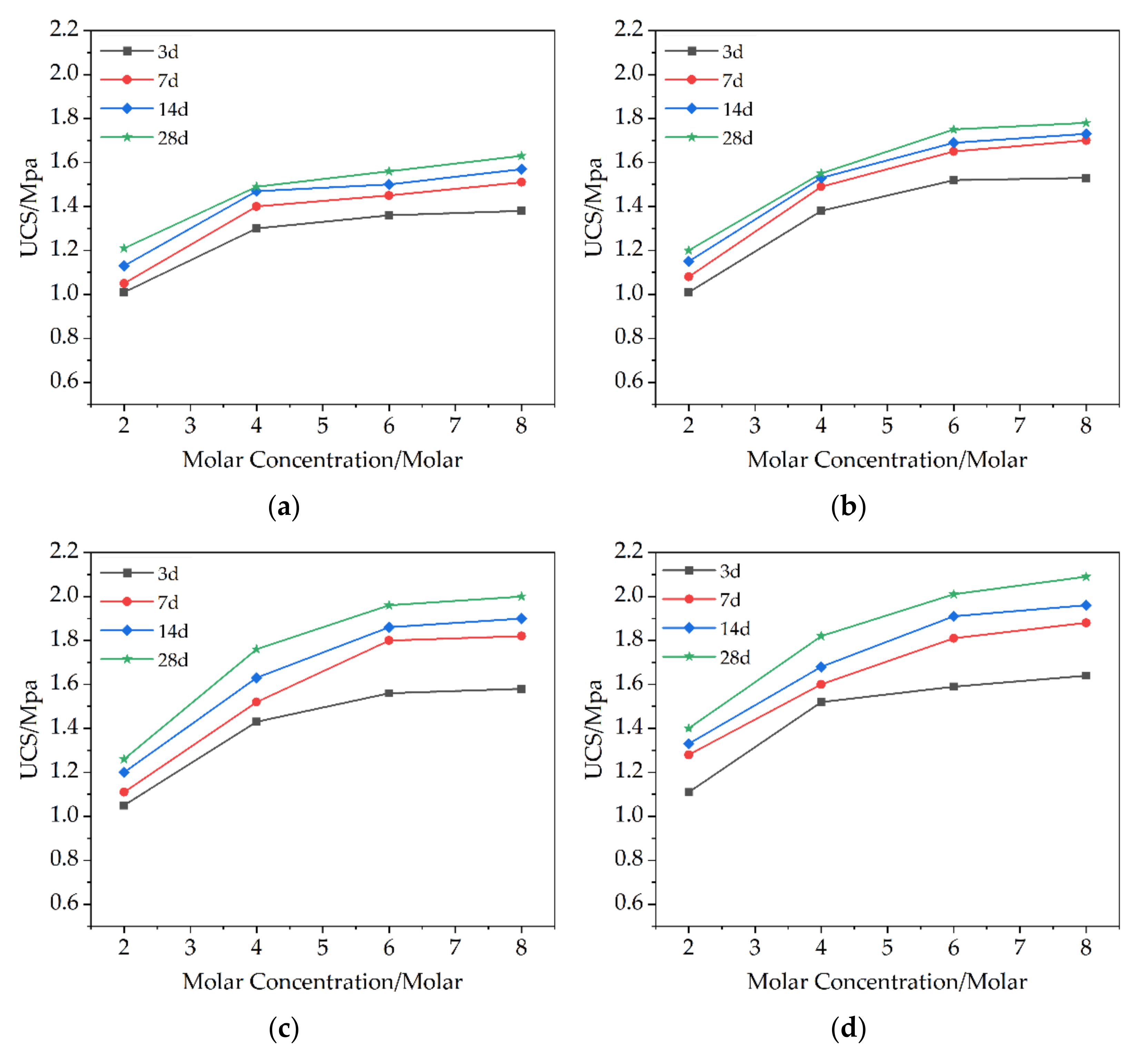
3.1. Unconfined Compression Strength
3.1.1. UCS of Soil-Geopolymer
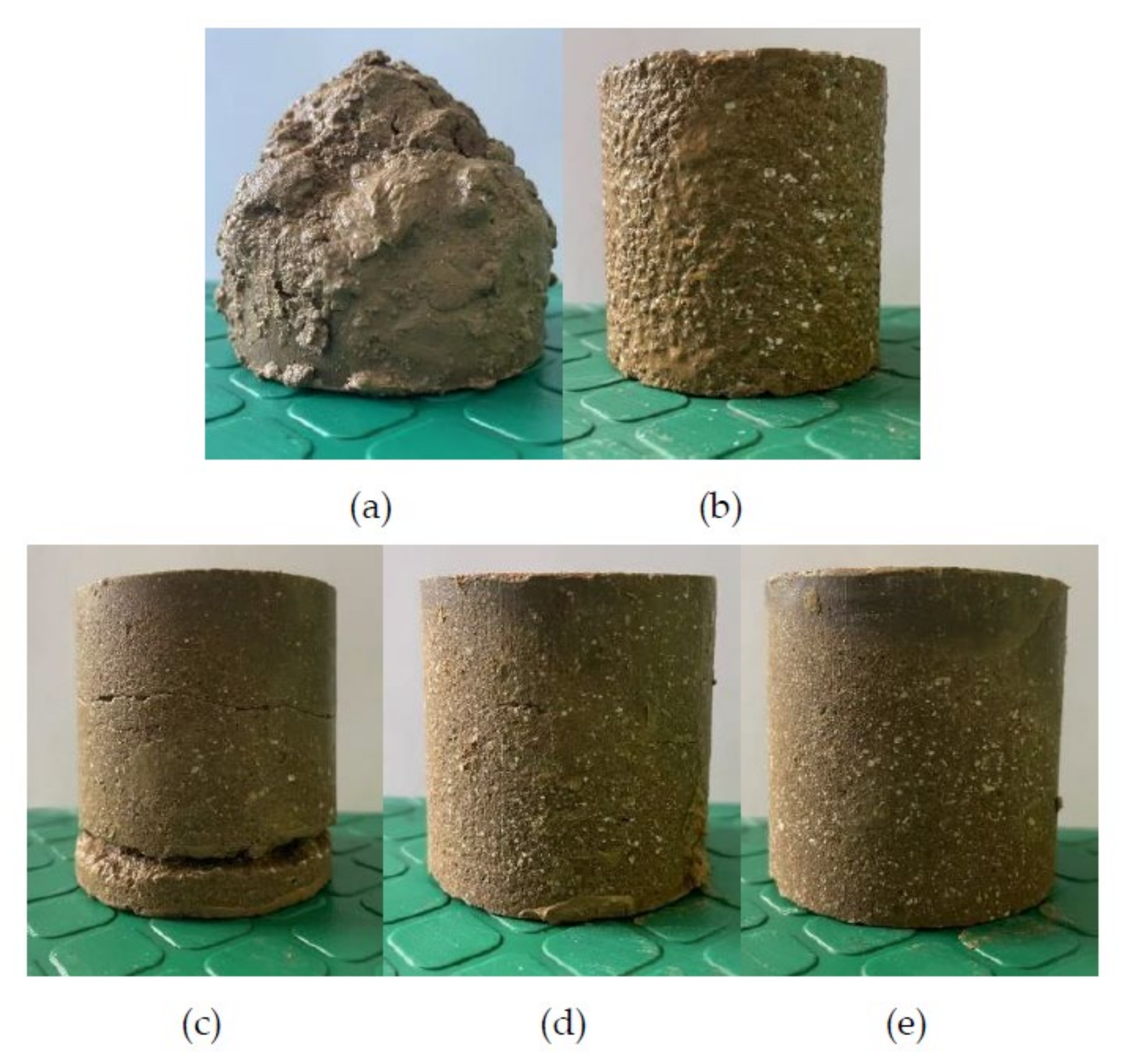
3.1.2. Failure Mechanism of Geopolymer-Improved Soil
3.2. Test of Water Resistance
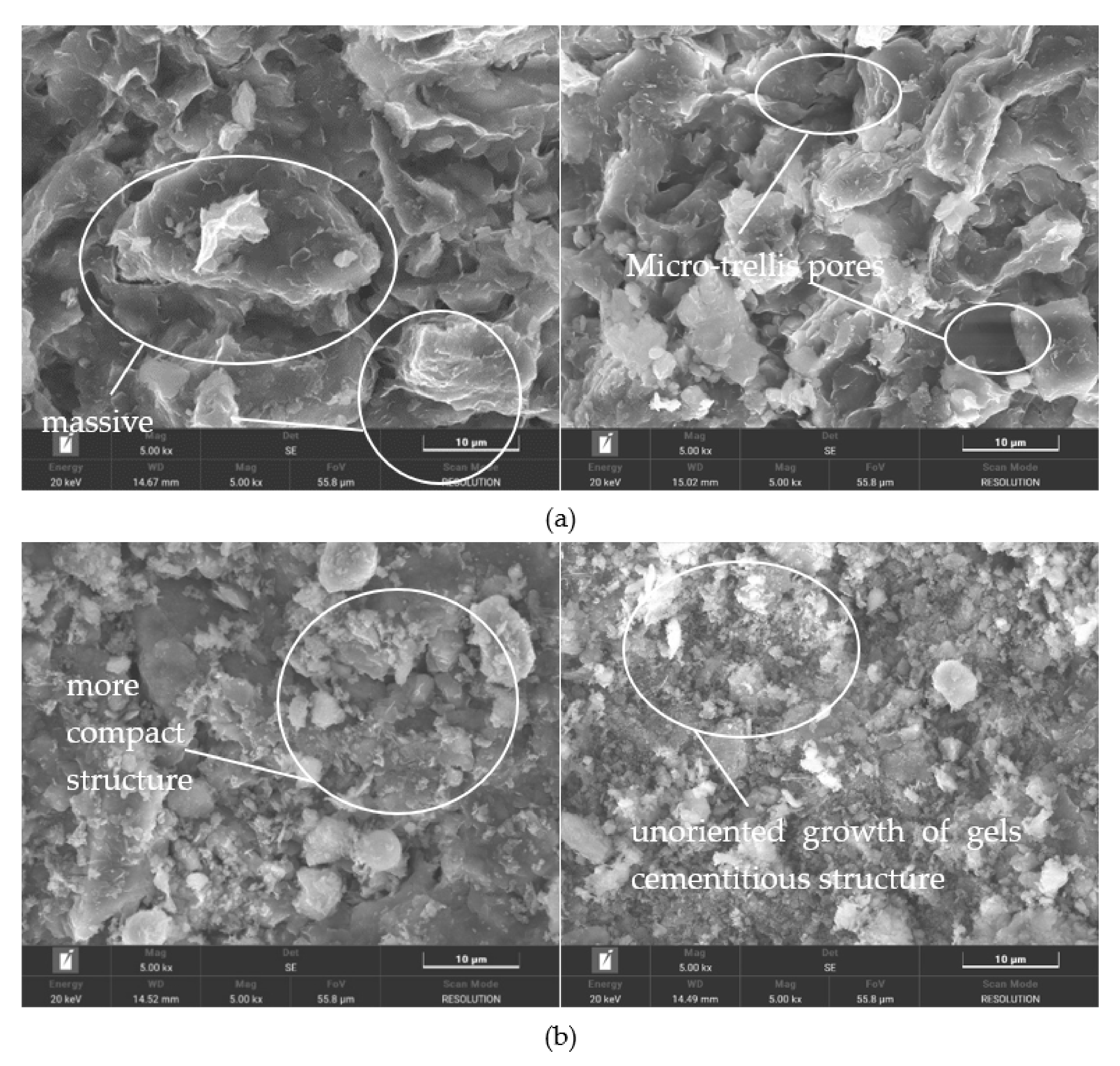
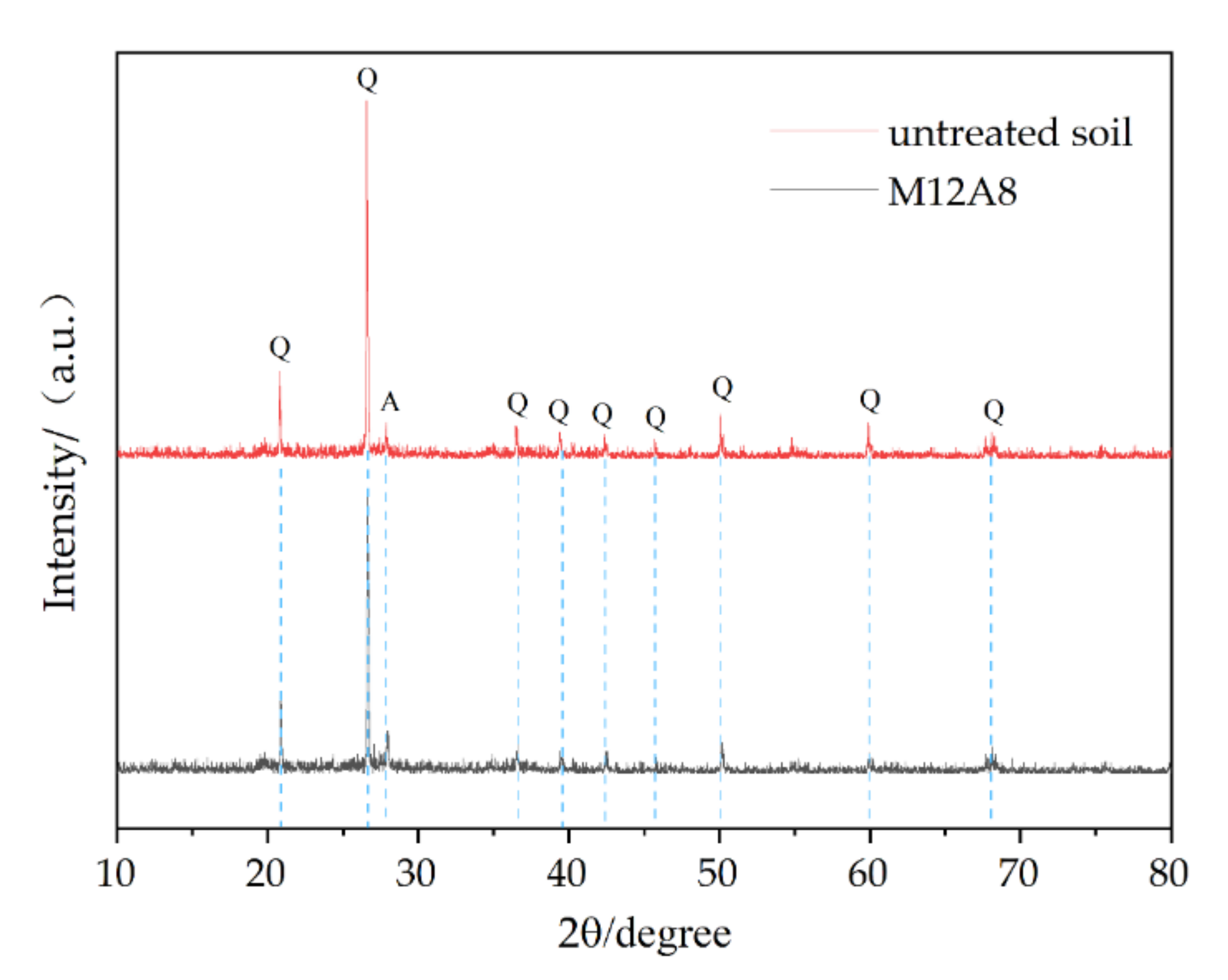
3.3. Microstructure Characteristics of Geopolymer-Improved Soil Using SEM and XRD
4. Conclusions
- (1)
- The unconfined compressive strength of clay increases with the increase in the metakaolin-based geopolymer and the value is 4109 kN when MKG content is 12%.
- (2)
- As the curing time increases from 3 days to 28 days, the unconfined compressive strength continues to increase, but tends to moderate. The increase was faster in the first 14 days. This may be due to the rapid reaction of MKG precursor.
- (3)
- The molar concentration of sodium hydroxide is too low to fully dissolve the active substance. When the molar concentration reaches 4M, the strength is significantly improved, while the higher concentration of sodium hydroxide does not greatly improve the strength.
- (4)
- With the increase in MKG and the molar concentration of the alkali activator, the failure of treated soil changed from plastic failure to brittle failure.
- (5)
- Scanning electron microscopy demonstrated that the treated soil has a denser structure, and the clay particles are covered with gelling compounds produced by the geopolymer, which contributes to its strength.
- (6)
- X-ray diffraction showed that clay provides a source of silica for the formation of geopolymers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Disu, A.A.; Kolay, P.K. A critical appraisal of soil Stabilization using geopolymers: The past, present and future. Int. J. Geosynth. Ground Eng. 2021, 7, 1–16. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental feasibility study of geopolymer as the next-generation soil stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- Khadka, S.D.; Jayawickrama, P.W.; Senadheera, S. Strength and shrink/swell behavior of highly plastic clay treated with geopolymer. Transp. Res. Rec. 2018, 2672, 174–184. [Google Scholar] [CrossRef]
- Abdila, S.R.; Abdullah, M.M.A.B.; Ahmad, R.; Rahim, S.Z.A.; Rychta, M.; Wnuk, I.; Nabiałek, M.; Muskalski, K.; Tahir, M.F.M.; Isradi, M.; et al. Evaluation on the mechanical properties of ground granulated blast slag (GGBS) and fly ash stabilized soil via geopolymer process. Materials 2021, 14, 2833. [Google Scholar] [CrossRef] [PubMed]
- Tigue, A.A.S.; Dungca, J.R.; Hinode, H.; Kurniawan, W.; Promentilla, M.A.B. Synthesis of a one-part geopolymer system for soil stabilizer using fly ash and volcanic ash. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 05017. [Google Scholar]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. Strength development of soil–fly ash geopolymer: Assessment of soil, fly ash, alkali activators, and water. J. Mater. Civ. Eng. 2018, 30, 04018171. [Google Scholar] [CrossRef] [Green Version]
- Syed, M.; GuhaRay, A.; Kar, A. Stabilization of expansive clayey soil with alkali activated binders. Geotech. Geol. Eng. 2020, 38, 6657–6677. [Google Scholar] [CrossRef]
- Gupta, S.; GuhaRay, A.; Kar, A.; Komaravolu, V.P. Performance of alkali-activated binder-treated jute geotextile as reinforcement for subgrade stabilization. Int. J. Geotech. Eng. 2018, 15, 1–15. [Google Scholar] [CrossRef]
- Odeh, N.A.; Al-Rkaby, A.H.J. Strength, Durability, and Microstructures characterization of sustainable geopolymer improved clayey soil. Case Stud. Constr. Mater. 2022, 16, e00988. [Google Scholar] [CrossRef]
- Sukprasert, S.; Hoy, M.; Horpibulsuk, S.; Arulrajah, A.; Rashid, A.S.A.; Nazir, R. Fly ash based geopolymer stabilisation of silty clay/blast furnace slag for subgrade applications. Road Mater. Pavement Des. 2021, 22, 357–371. [Google Scholar] [CrossRef]
- Mahrous, M.A.; Šegvić, B.; Zanoni, G.; Khadka, S.D.; Senadheera, S.; Jayawickrama, P.W. The role of clay swelling and mineral neoformation in the stabilization of high plasticity soils treated with the fly ash-and metakaolin-based geopolymers. Minerals 2018, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Samuel, R.; Puppala, A.J.; Radovic, M. Sustainability benefits assessment of metakaolin-based geopolymer treatment of high plasticity clay. Sustainability 2020, 12, 10495. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Q.; Zhu, Y.; Li, G.; Wu, Z.; Zhao, K. Experimental study on material ratio and strength performance of geopolymer-improved soil. Constr. Build. Mater. 2021, 267, 120469. [Google Scholar] [CrossRef]
- Samuel, R.; Puppala, A.J.; Banerjee, A.; Huang, O.; Radovic, M.; Chakraborty, S. Improvement of Strength and Volume-Change Properties of Expansive Clays with Geopolymer Treatment. Transp. Res. Rec. 2021, 2675, 308–320. [Google Scholar] [CrossRef]
- Adhikari, B.; Khattak, M.J.; Adhikari, S. Mechanical and durability characteristics of flyash-based soil-geopolymer mixtures for pavement base and subbase layers. Int. J. Pavement Eng. 2021, 22, 1193–1212. [Google Scholar] [CrossRef]
- Gokul, V.; Steffi, D.A.; Kaviya, R.; Harni, C.V.; Dharani, S.M.A. Alkali activation of clayey soil using GGBS and NaOH. Mater. Today Proc. 2020, 43, 1707–1713. [Google Scholar] [CrossRef]
- GB/T50123-2019; Standard for Geotechnical Testing Method. China Planning Press: Beijing, China, 2019.
- Abdulkareem, S.O.; Abbas, J.M. Effect of Adding Metakaolin Based Geopolymer to Improve Soft Clay under Different Conditions. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 856, p. 012011. [Google Scholar]
- ASTM D2166-06; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. American Society for Testing Materials: West Conshohocken, PA, USA, 2006.
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Yu, J.; Hua, S.; Qian, L.; Ren, X.; Zuo, J.; Zhang, Y. Development of Steel Slag-Based Solidification/Stabilization Materials for High Moisture Content Soil. J. Renew. Mater. 2022, 10, 735. [Google Scholar]



| Property | Specific Gravity | Liquid Limit (%) | Plastic Limit (%) | Plasticity Index (%) | Maximum Dry Density (g/cm3) | Optimum Moisture Content (%) |
|---|---|---|---|---|---|---|
| Index value | 2.65 | 41.52 | 13.85 | 27.67 | 1.91 | 12.81 |
| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| Ratio (%) | 54.31 | 43.73 | 0.53 | 0.67 | 0.26 | 0.19 | 0.08 | 0.22 |
| Metakaolin Content | Alkali-Activator (Sodium Hydroxide with Different Molar Concentrations) | |||
|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |
| 6 | M6A2 | M6A4 | M6A6 | M6A8 |
| 8 | M8A2 | M8A4 | M8A6 | M8A8 |
| 10 | M10A2 | M10A4 | M10A6 | M10A8 |
| 12 | M12A2 | M12A4 | M12A6 | M12A8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zha, Q.; Li, S.; Cai, G.; Wu, D.; Zhai, C. Experimental Study on the Mechanical Properties and Microstructure of Metakaolin-Based Geopolymer Modified Clay. Molecules 2022, 27, 4805. https://doi.org/10.3390/molecules27154805
Shi X, Zha Q, Li S, Cai G, Wu D, Zhai C. Experimental Study on the Mechanical Properties and Microstructure of Metakaolin-Based Geopolymer Modified Clay. Molecules. 2022; 27(15):4805. https://doi.org/10.3390/molecules27154805
Chicago/Turabian StyleShi, Xianzeng, Qingkun Zha, Shuqing Li, Guojun Cai, Dun Wu, and Chaojiao Zhai. 2022. "Experimental Study on the Mechanical Properties and Microstructure of Metakaolin-Based Geopolymer Modified Clay" Molecules 27, no. 15: 4805. https://doi.org/10.3390/molecules27154805
APA StyleShi, X., Zha, Q., Li, S., Cai, G., Wu, D., & Zhai, C. (2022). Experimental Study on the Mechanical Properties and Microstructure of Metakaolin-Based Geopolymer Modified Clay. Molecules, 27(15), 4805. https://doi.org/10.3390/molecules27154805






