Thiocoumarins: From the Synthesis to the Biological Applications
Abstract
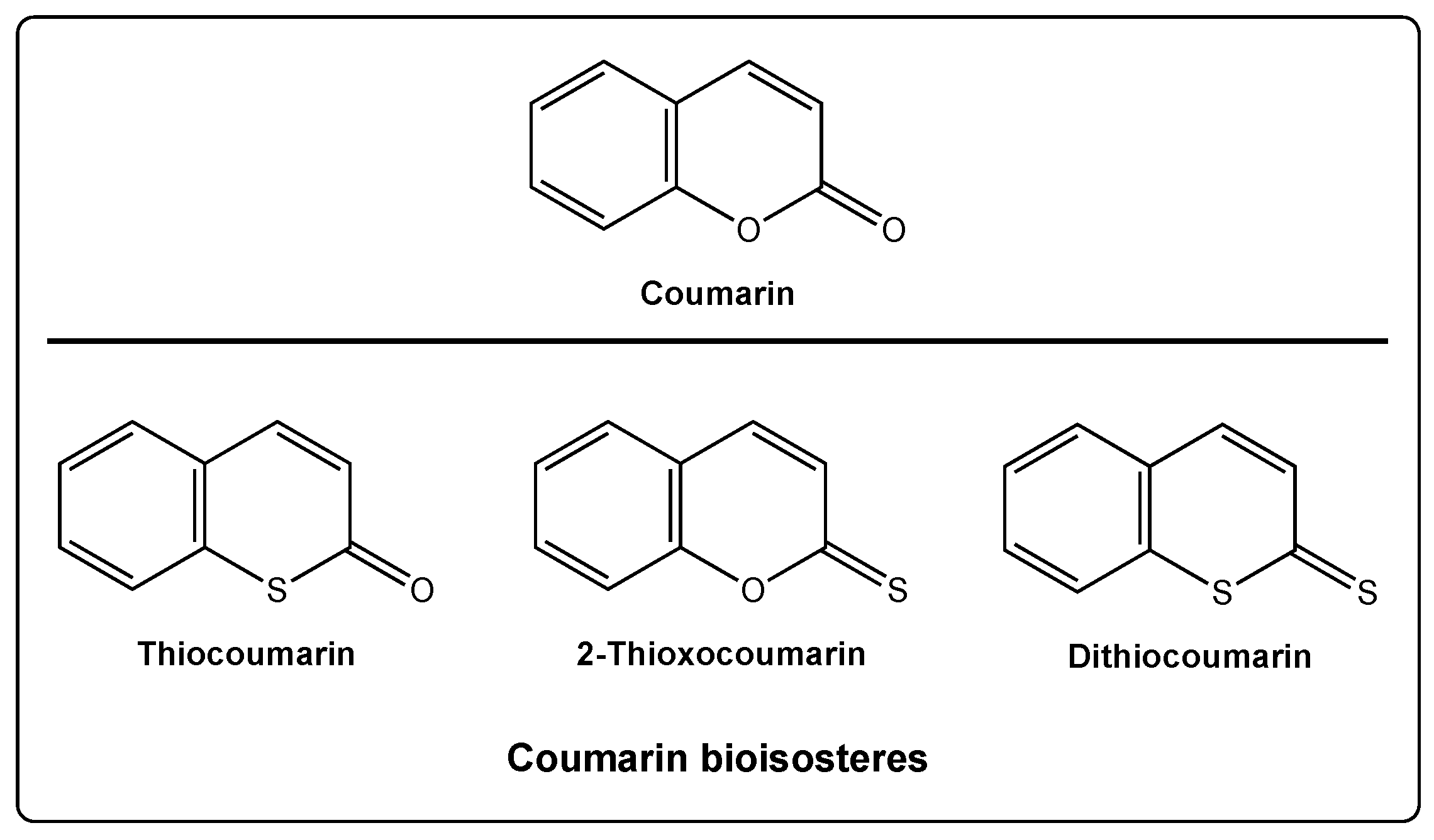
:1. Introduction
2. Discussion
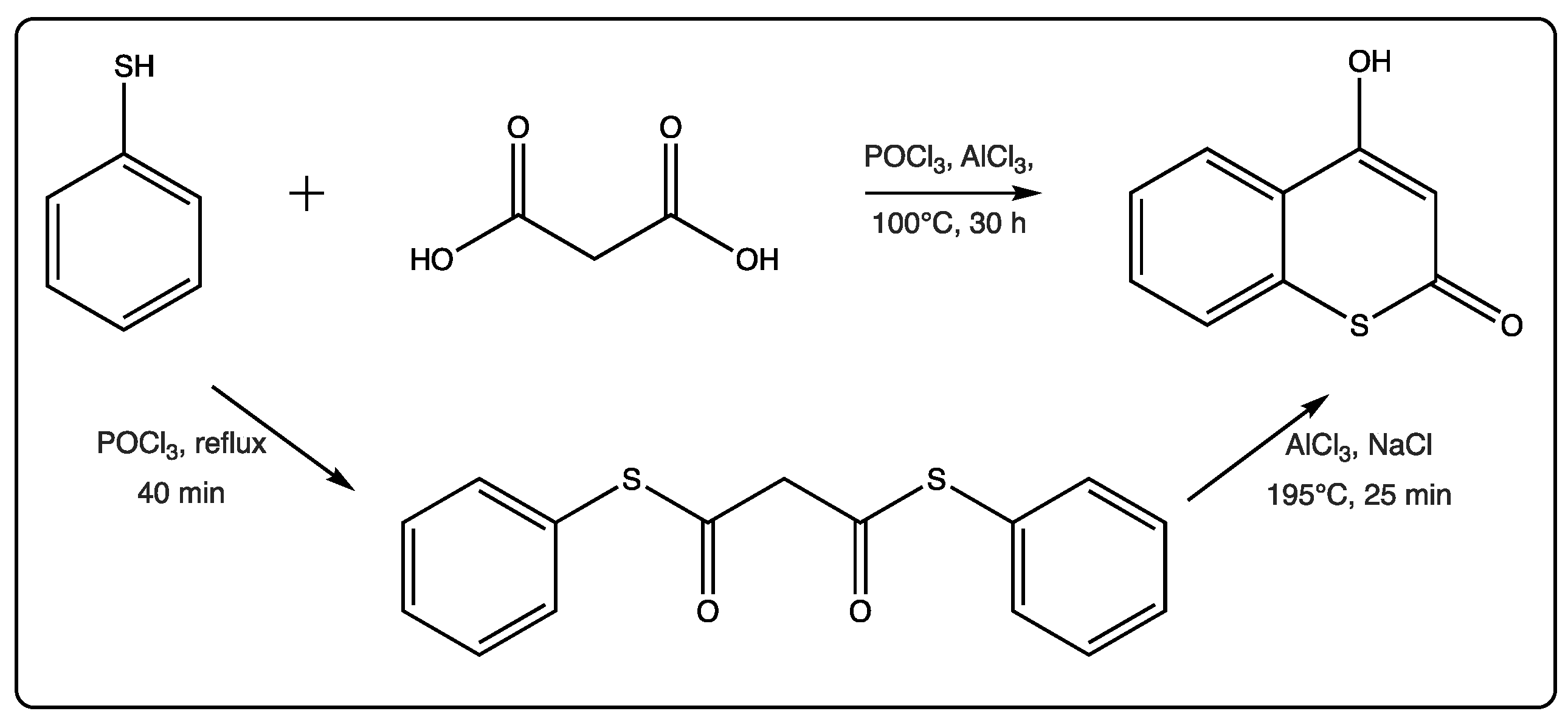
2.1. Thiocoumarins
2.2. 2-Thioxocoumarins
2.3. Dithiocoumarins
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Beno, B.R.; Yeung, K.-S.; Bartberger, M.D.; Pennington, L.D.; Meanwell, N.A. A survey of the role of noncovalent sulfur interactions in drug design. J. Med. Chem. 2015, 58, 4383–4438. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I. Replacement of oxygen by sulfur in small organic molecules. 3. Theoretical studies on the tautomeric equilibria of the 2OH and 4OH-substituted oxazole and thiazole and the 3OH and 4OH-substituted isoxazole and isothiazole in the isolated state and in solution. Int. J. Mol. Sci. 2016, 17, 1094. [Google Scholar]
- Chaudhary, D.; Pramanik, T.; Santra, S. Thiocoumarins and dithiocoumarins: Advances in Synthesis and Pharmacological Activity. Curr. Org. Chem. 2020, 24, 1793–1814. [Google Scholar] [CrossRef]
- Meth-Cohn, O.; Tarnowski, B. Thiocoumarins. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 1980; pp. 115–133. [Google Scholar]
- Fourel, I.; Berlioz-Barbier, A.; Benoit, E. Mass spectrometry characterization of anticoagulant rodenticides and hydroxyl metabolites. Rapid Commun. Mass Spectrom. 2020, 34, e8871. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.A.; Rapoport, H. Synthesis of thiocoumarins from acrylic and propiolic ortho esters and benzenethiols. J. Org. Chem. 1982, 47, 2626–2628. [Google Scholar] [CrossRef]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T. Carbonic anhydrase inhibition/activation: Trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr. Pharm. Des. 2010, 16, 3233–3245. [Google Scholar] [CrossRef]
- Ferraroni, M.; Carta, F.; Scozzafava, A.; Supuran, C.T. Thioxocoumarins show an alternative carbonic anhydrase inhibition mechanism compared to coumarins. J. Med. Chem. 2016, 59, 462–473. [Google Scholar] [CrossRef]
- Zalubovskis, R. In a search for selective inhibitors of carbonic anhydrases: Coumarin and its bioisosteres—Synthesis and derivatization. Chem. Heterocycl. Compd. 2015, 51, 607–612. [Google Scholar] [CrossRef]
- Monti, S.M.; Supuran, C.T.; De Simone, G. Anticancer carbonic anhydrase inhibitors: A patent review (2008–2013). Expert Opin. Ther. Pat. 2013, 23, 737–749. [Google Scholar] [CrossRef]
- Haywood, R.D.; Franks, M.A. Synthetic design of coumarin and thiocoumarin derivatives to inhibit carcinogénesis. In Proceedings of the 245th ACS National Meeting & Exposition, New Orleans, LA, USA, 18 March 2013. [Google Scholar]
- Barraja, P.; Sciabica, L.; Diana, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G.; Disaro, S.; Basso, G.; et al. Synthesis and photochemotherapeutic activity of thiopyrano[2,3-e]indol-2-ones. Bioorgan. Med. Chem. Lett. 2005, 15, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.A.; Sahni, S.; Lee, L.; Luo, Y.; Nieduzak, T.R.; Liang, G.; Chiang, Y.; Collar, N.; Fink, D.; He, W.; et al. Design, synthesis and characterization of a novel class of coumarin-based inhibitors of inducible nitric oxide synthase. Bioorgan. Med. Chem. 2005, 13, 2723–2739. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.M. Utility of 4-hydroxythiocoumarin in organic synthesis. Arab. J. Chem. 2017, 10, S3955–S3961. [Google Scholar] [CrossRef] [Green Version]
- Shinde, R.G.; Khan, A.A.; Barik, A. Formation of two centre three electron bond by hydroxyl radical induced reaction of thiocoumarin: Evidence from experimental and theoretical studies. Free Radic. Res. 2019, 53, 629–640. [Google Scholar] [CrossRef]
- Mokrov, G.V.; Voronina, T.A.; Litvinova, S.A.; Kovalev, I.G.; Nerobkova, L.N.; Durnev, A.D.; Gudasheva, T.A.; Seredenin, S.B. Synthesis and anticonvulsant activity of 4-amino-3-nitro-1-thiocoumarins and 4-amino-3-nitroquinolin-2-ones. Pharm. Chem. J. 2019, 53, 194–200. [Google Scholar] [CrossRef]
- Mokrov, G.V.; Litvinova, S.A.; Voronina, T.A.; Nerobkova, L.N.; Kutepova, I.S.; Kovalev, I.G.; Gudasheva, T.A.; Durnev, A.D. Design, synthesis, and anticonvulsant evaluation of 4-GABA-3-nitrocoumarines, 1-thiocoumarines, quinolone-2-ones, and their derivatives. Med. Chem. Res. 2019, 28, 1901–1911. [Google Scholar] [CrossRef]
- He, X.; Wu, Y.; Zuo, Y.; Xie, M.; Li, R.; Shang, Y. Transition metal- and oxidant-free sulfonylation of 1-sulfonyl-1H-1,2,3-triazoles to enols for the synthesis of sulfonate derivatives. Synth. Commun. 2019, 49, 959–972. [Google Scholar] [CrossRef]
- Jamkhandi, P.; Rajagopal, S. The chemistry of anticoagulants. Synthesis of aryl-substituted 4-hydroxythiocoumarin. Monatsh. Chem. 1963, 94, 1271–1273. [Google Scholar] [CrossRef]
- Lee, S.-C.; Liao, H.-H.; Chatupheeraphat, A.; Rueping, M. Nickel-catalyzed C-S bond formation via decarbonylative thioetherification of esters, amides and intramolecular recombination fragment coupling of thioesters. Chem. Eur. J. 2018, 24, 3608–3612. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-M.; Reddy, G.M.; Liu, M.-H.; Wang, T.-P.; Yu, J.-K.; Lin, W. Diastereoselective synthesis of Rauhut-Currier-type adducts via an unexpected α-addition of α,β-unsaturated γ-butyrolactams to coumarin derivatives. J. Org. Chem. 2017, 82, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ando, A.; Nakagawa, M. Synthesis of 4-acylcoumarins by NHC-catalyzed nucleophilic substitution. Tetrahedron Lett. 2018, 59, 4276–4278. [Google Scholar] [CrossRef]
- Chopade, A.U.; Chopade, M.U.; Chanda, B.M.; Sawaikar, D.D.; Sonawane, K.B.; Gurjar, M.K. A synthesis of (±)-thia-calanolide A, its resolution and in vitro biological evaluation. Arab. J. Chem. 2016, 9, S1597–S1602. [Google Scholar] [CrossRef] [Green Version]
- Onkara, P.; Kumar, A.S.; Kanakaraju, S.; Prasanna, B.; Pydisetty, Y.; Chandramouli, G.V.P. Molecular docking studies, synthesis and anti-bacterial properties of new Mannich bases. Int. J. Pharm. Biol. Sci. 2013, 4, 263–270. [Google Scholar]
- Kucherenko, A.S.; Siyutkin, D.E.; Nigmatov, A.G.; Chizhov, A.O.; Zlotin, S.G. Chiral primary amine tagged to ionic group as reusable organocatalyst for asymmetric Michael reactions of C-nucleophiles with α,β-unsaturated ketones. Adv. Synth. Catal. 2012, 354, 3078–3086. [Google Scholar] [CrossRef]
- Valente, S.; Kirsch, G. Facile synthesis of 4-acetyl-coumarins, -thiocoumarin and -quinolin-2(1H)-one via very high α-regioselective Heck coupling on tosylates. Tetrahedron Lett. 2011, 52, 3429–3432. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Pal, A.K. Regioselective synthesis of pentacyclic heterocycles by the thermal and Lewis acid catalyzed Claisen rearrangement. J. Sulfur Chem. 2009, 30, 481–489. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Maji, P.K.; Pal, A.K. Sequential thermal and catalyzed Claisen rearrangements toward the synthesis of thiocoumarin-annulated furopyrans. Lett. Org. Chem. 2008, 5, 218–223. [Google Scholar] [CrossRef]
- Majumdar, K.; Chattopadhyay, S.; Mukhopadhyay, P. Studies on amine oxide rearrangement: Synthesis of pyrrolo[3,2-c][1]benzothiopyran-4-one. Synth. Commun. 2006, 36, 1291–1297. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Chattopadhyay, S.K.; Ghosh, M. Regioselective synthesis of 3-(aryloxyacetyl)-2,3-dihydrothieno[3,2-c][1]benzothiopyran-4-ones. A tandem [2,3] and [3,3]sigmatropic rearrangement approach. Lett. Org. Chem. 2005, 2, 231–234. [Google Scholar] [CrossRef]
- Park, H.-J.; Lee, K.-I. Three-component synthesis of 3-aminomethylene-thiochroman-2,4-diones from 4-hydroxythiocoumarin. Synth. Commun. 2004, 34, 2053–2061. [Google Scholar] [CrossRef]
- Reddy, P.V.K.; Kumar, P.N.; Chandramouli, G.V.P. Synthesis and antimicrobial activity of 6,6’-arylidene-bis-[5-hydroxy-9-methyl-2,3-diarylthieno[3,2-g]thiocoumarins]. J. Heterocycl. Chem. 2005, 42, 283–286. [Google Scholar] [CrossRef]
- Vedaldi, D.; Dolmella, A.; Moro, S.; Miolo, G.; Viola, G.; Caffieri, S.; Dall’Acqua, F. Thioangelicin: Crystal structure, computer-aided studies and photobiological activity. Farmaco 2004, 59, 125–132. [Google Scholar] [CrossRef]
- Xia, P.; Yin, Z.-J.; Chen, Y.; Zhang, Q.; Zhang, B.; Xia, Y.; Yang, Z.-Y.; Kilgore, N.; Wild, C.; Morris-Natschke, S.L.; et al. Anti-AIDS agents. Part 58: Synthesis and anti-HIV activity of 1-thia-di-O-(−)-camphanoyl-(+)-cis-khellactone (1-thia-DCK) analogues. Bioorg. Med. Chem. Lett. 2004, 14, 3341–3343. [Google Scholar] [PubMed]
- Majumdar, K.C.; Pal, A.K.; Ghosh, M. Regioselective synthesis of thiopyrano[3,2-c][1]benzothiopyran-5(2H)-one and thieno[3,2-c][1]benzothiopyran-4(2H)-one. Synth. Commun. 2007, 37, 1525–1534. [Google Scholar] [CrossRef]
- Xu, L.; Shao, Z.; Wang, L.; Xiao, J. Tandem sp3 C-H functionalization/decarboxylation of 2-alkylazaarenes with coumarin-3-carboxylic acids. Org. Lett. 2014, 16, 796–799. [Google Scholar] [CrossRef]
- Shao, Z.; Xu, L.; Wang, L.; Wei, H.; Xiao, J. Catalyst-free tandem Michael addition/decarboxylation of (thio)coumarin-3-carboxylic acids with indoles: Facile synthesis of indole-3-substituted 3,4-dihydro(thio)coumarins. Org. Biomol. Chem. 2014, 12, 2185–2188. [Google Scholar] [CrossRef]
- Debnath, M.; Sasmal, S.; Haldar, D. Fabrication of egg shell-like nanovesicles from a thiocoumarin-based ε-amino ester: A potential carrier. J. Mater. Chem. B 2017, 5, 5450–5457. [Google Scholar] [CrossRef]
- Tiwari, M.R.; Patel, N.B. Synthesis and pharmacological activities of oxadiazole and pyrimidine bearing thiocoumarin derivatives. Curr. Microw. Chem. 2021, 8, 33–43. [Google Scholar] [CrossRef]
- Voss, J.; Edler, R.; Adiwidjaja, G. Preparation of new tert-butyl substituted coumarins, thiocoumarins and dithiocoumarins. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1893–1905. [Google Scholar] [CrossRef]
- Xu, Z.Y.; He, X.D.; Han, L.; Wang, X.H.; Huang, S.L.; Chen, J.R.; Xu, L.Q.; Luo, H.Q.; Li, N.B. Engineering of a multifunctional small molecule enables dual-channel fluorescence visualizing of environmental unamiable heavy metal ions as well as photoinactivation-based and ultra-efficient eliminating of human pathogens. Chem. Eng. J. 2022, 444, 136601. [Google Scholar] [CrossRef]
- Cho, M.; Nguyen, V.-N.; Yoon, J. Simultaneous detection of hypochlorite and singlet oxygen by a yhiocoumarin-based ratiometric fluorescent probe. ACS Meas. Sci. Au 2022, 2, 219–223. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Heo, S.; Kim, S.; Swamy, K.M.K.; Ha, J.; Park, S.; Yoon, J. A thiocoumarin-based turn-on fluorescent probe for hypochlorite detection and its application to live-cell imaging. Sens. Actuators B Chem. 2020, 317, 128213. [Google Scholar] [CrossRef]
- Li, D.; Zhao, S.; Zhao, B.; Xia, Y.; Liu, A.; Wang, K.; Hou, R. Fluorescent Probe Containing Coumarin for Selective Recognition of Mercury Ion and Preparation Thereof. CN113666898A, 29 March 2017. (In Chinese). [Google Scholar]
- Nocentini, A.; Angeli, A.; Carta, F.; Winum, J.-Y.; Zalubovskis, R.; Carradori, S.; Capasso, C.; Donald, W.A.; Supuran, C.T. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J. Enzym. Inhib. Med. Chem. 2021, 36, 561–580. [Google Scholar] [CrossRef]
- Ma, J.; Ripp, A.; Wassy, D.; Dürr, T.; Qiu, D.; Häner, M.; Haas, T.; Popp, C.; Bezold, D.; Richert, S.; et al. Thiocoumarin caged nucleotides: Synthetic access and their photophysical properties. Molecules 2020, 25, 5325. [Google Scholar] [CrossRef]
- Kumar, S.; Tyagi, Y.K.; Kumar, M.; Kumar, S. Synthesis of novel 4-methylthiocoumarin and comparison with conventional coumarin derivative as a multi-target-directed ligand in Alzheimer’s disease. 3 Biotech 2020, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A. Product class 9: Benzothiopyranones and benzothiopyranthiones. Sci. Synth. 2003, 14, 787–816. [Google Scholar]
- Anderson-McKay, J.E.; Liepa, A.J. The synthesis of 4-hydroxydithiocoumarins: A case of unusual tautomer stability. Aust. J. Chem. 1987, 40, 1179–1190. [Google Scholar] [CrossRef]
- Mahato, K.; Arora, N.; Bagdi, P.R.; Gattu, R.; Ghosh, S.S.; Khan, A.T. An oxidative cross-coupling reaction of 4-hydroxydithiocoumarin and amines/thiols using a combination of I2 and TBHP: Access to lead molecules for biomedical applications. Chem. Commun. 2018, 54, 1513–1516. [Google Scholar] [CrossRef]
- Mahato, K.; Bagdi, P.R.; Khan, A.T. K2CO3 catalyzed regioselective synthesis of thieno[2,3-b]thiochromen-4-one oximes: Access to the corresponding amine and nitroso derivatives. Org. Biomol. Chem. 2017, 15, 5625–5634. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ponra, S.; Ghosh, T. Green approach to highly functionalized thiopyrano derivatives via domino multi-component reaction in water. RSC Adv. 2012, 2, 144–1152. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Taher, A.; Ponra, S. Green synthesis of benzopyran-annulated thiopyrano[2,3-b]thiochromen-5(4H)-ones by domino Knoevenagel-hetero-Diels-Alder reaction. Synthesis 2010, 23, 4043–4050. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Kiamehr, M.; Khodabakhshi, M.R.; Mirjafary, Z.; Fathi, S.; Saeidian, H. A new domino Knoevenagel-hetero-Diels-Alder reaction: An efficient catalyst-free synthesis of novel thiochromone-annulated thiopyranocoumarin derivatives in aqueous medium. Tetrahedron 2010, 66, 8615–8622. [Google Scholar] [CrossRef]
- Dobelmann-Mara, L.; Riedmueller, S.; Schraub, M. Compounds for optically active devices. WO2017032442A1, 2 March 2017. [Google Scholar]
- Dobelmann-Mara, L.; Riedmueller, S.; Schraub, M. Hydrophilic Compounds for Optically Active Devices. WO 2017032444A1, 2 March 2017. [Google Scholar]
- Supuran, C.; Dedhar, S.; Carta, F.; Winum, J.-Y.; McDonald, P.C. Preparation of Coumarin and Thiocoumarin Glycosides as Carbonic Anhydrase Inhibitors with Antimetastatic Activity. WO2012070024A1, 31 May 2012. [Google Scholar]
- Alvarado, S.I.; Marc, P.A.; Dahlke, B.J.; Reilly-Horch, E.M. Preparation of 4-Phenoxycoumarins as Herbicidal Agents. US5681968A, 28 October 1997. [Google Scholar]
- Alvarado, S.I.; Marc, P.A.; Dahlke, B.J.; Reilly, E.M. Preparation of 4-Phenoxycoumarins and Their Thio Derivatives as Herbicides. EP694257A1, 31 January 1996. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, M.J.; Santana, L.; Uriarte, E.; Borges, F. Thiocoumarins: From the Synthesis to the Biological Applications. Molecules 2022, 27, 4901. https://doi.org/10.3390/molecules27154901
Matos MJ, Santana L, Uriarte E, Borges F. Thiocoumarins: From the Synthesis to the Biological Applications. Molecules. 2022; 27(15):4901. https://doi.org/10.3390/molecules27154901
Chicago/Turabian StyleMatos, Maria J., Lourdes Santana, Eugenio Uriarte, and Fernanda Borges. 2022. "Thiocoumarins: From the Synthesis to the Biological Applications" Molecules 27, no. 15: 4901. https://doi.org/10.3390/molecules27154901
APA StyleMatos, M. J., Santana, L., Uriarte, E., & Borges, F. (2022). Thiocoumarins: From the Synthesis to the Biological Applications. Molecules, 27(15), 4901. https://doi.org/10.3390/molecules27154901







