Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy
Abstract
1. Introduction
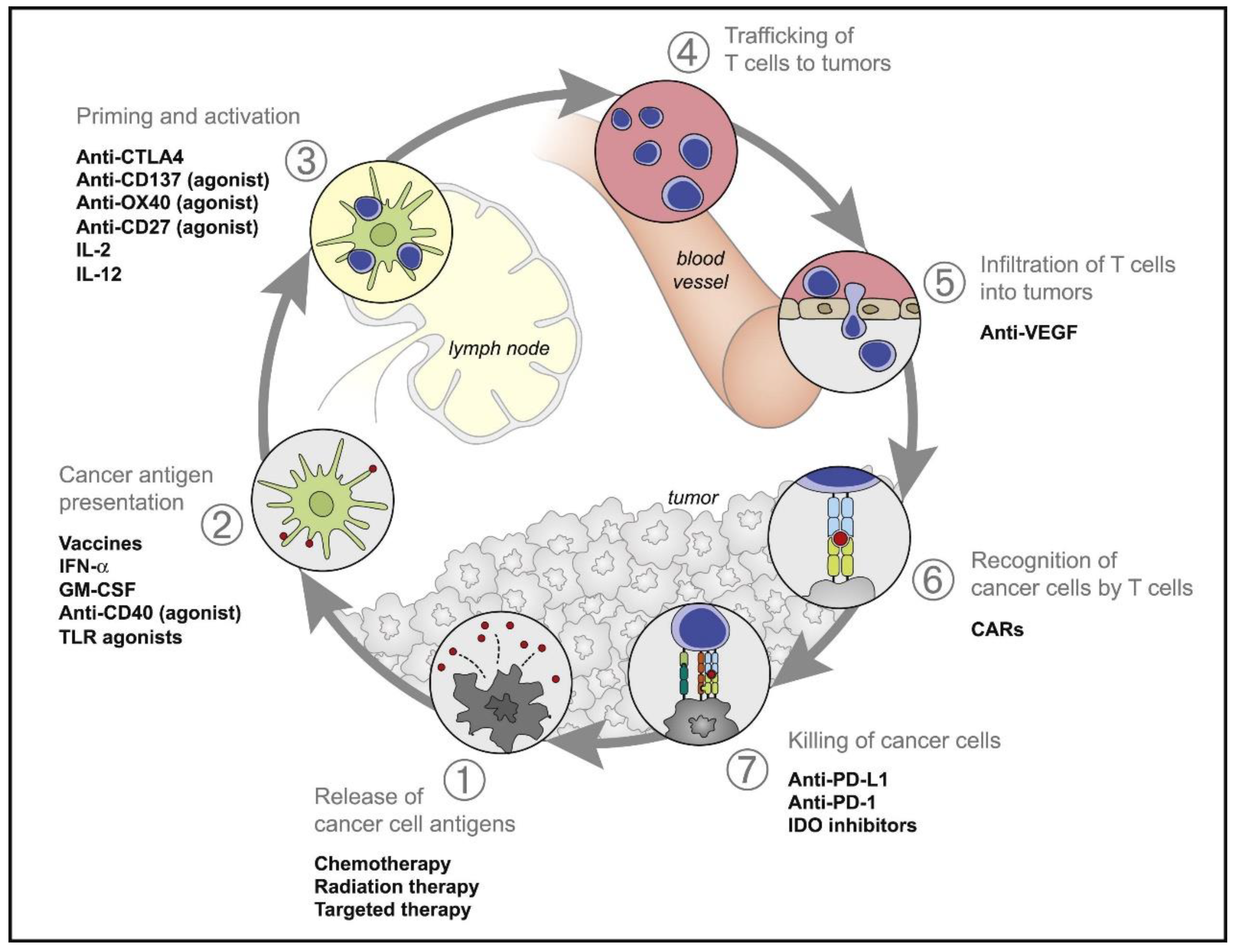
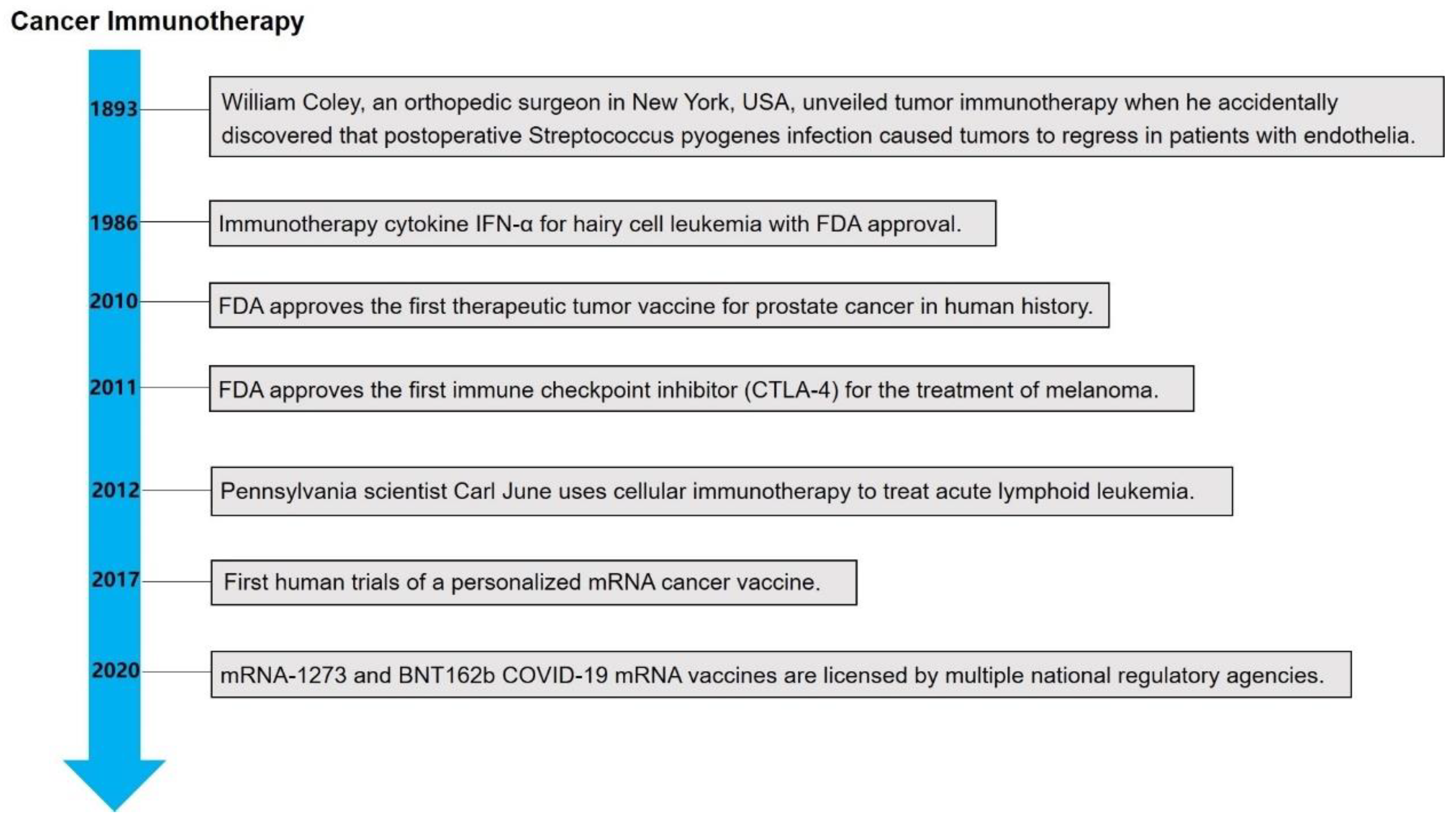
2. The Emerging of Cancer Immunotherapy
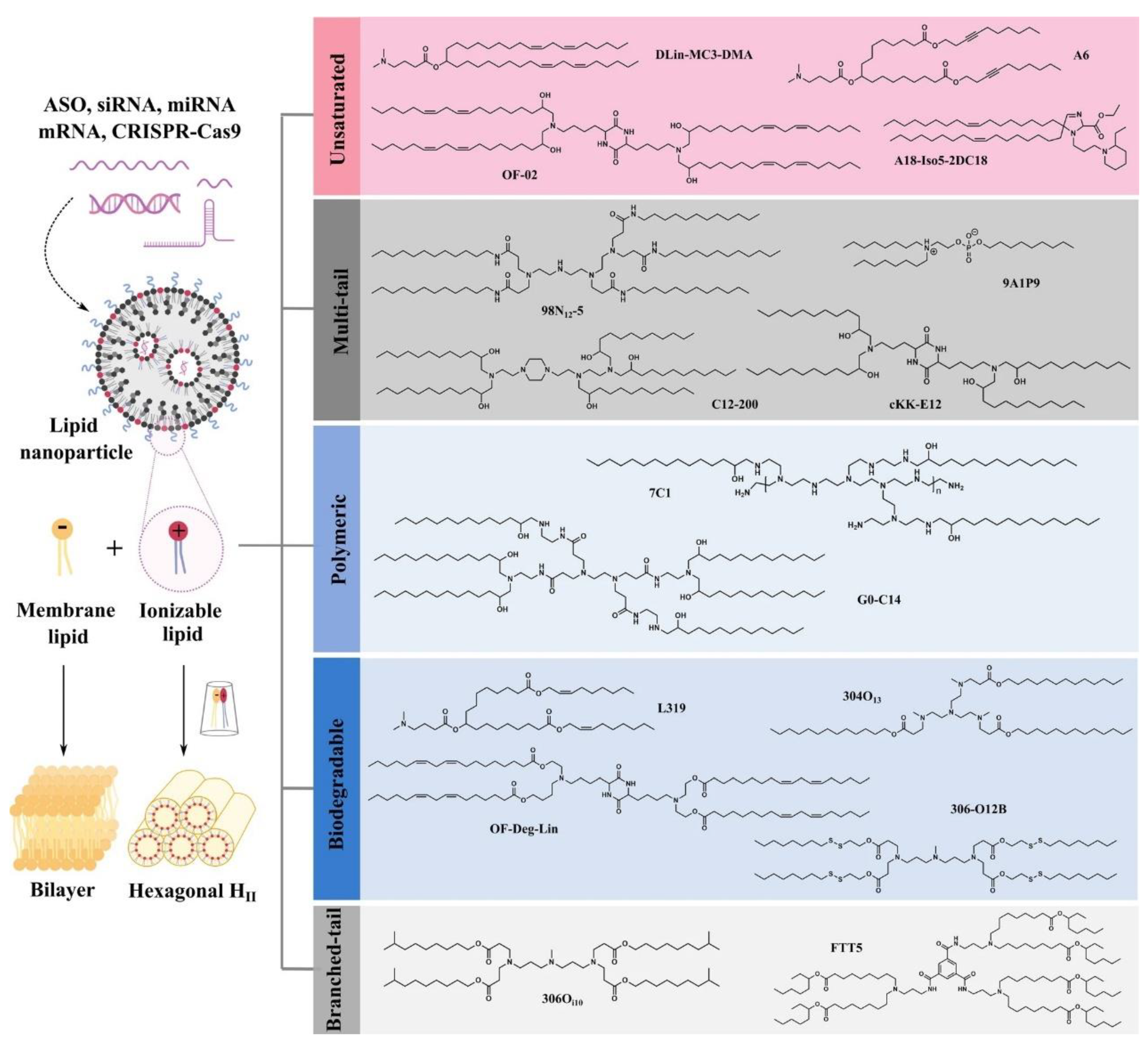
3. Development of Lipids for mRNA Delivery
3.1. Cationic Lipids
3.2. Ionizable Lipids
3.3. Other Types of Lipids
4. Lipid Nanoparticles for mRNA Delivery in Cancer Immunotherapy
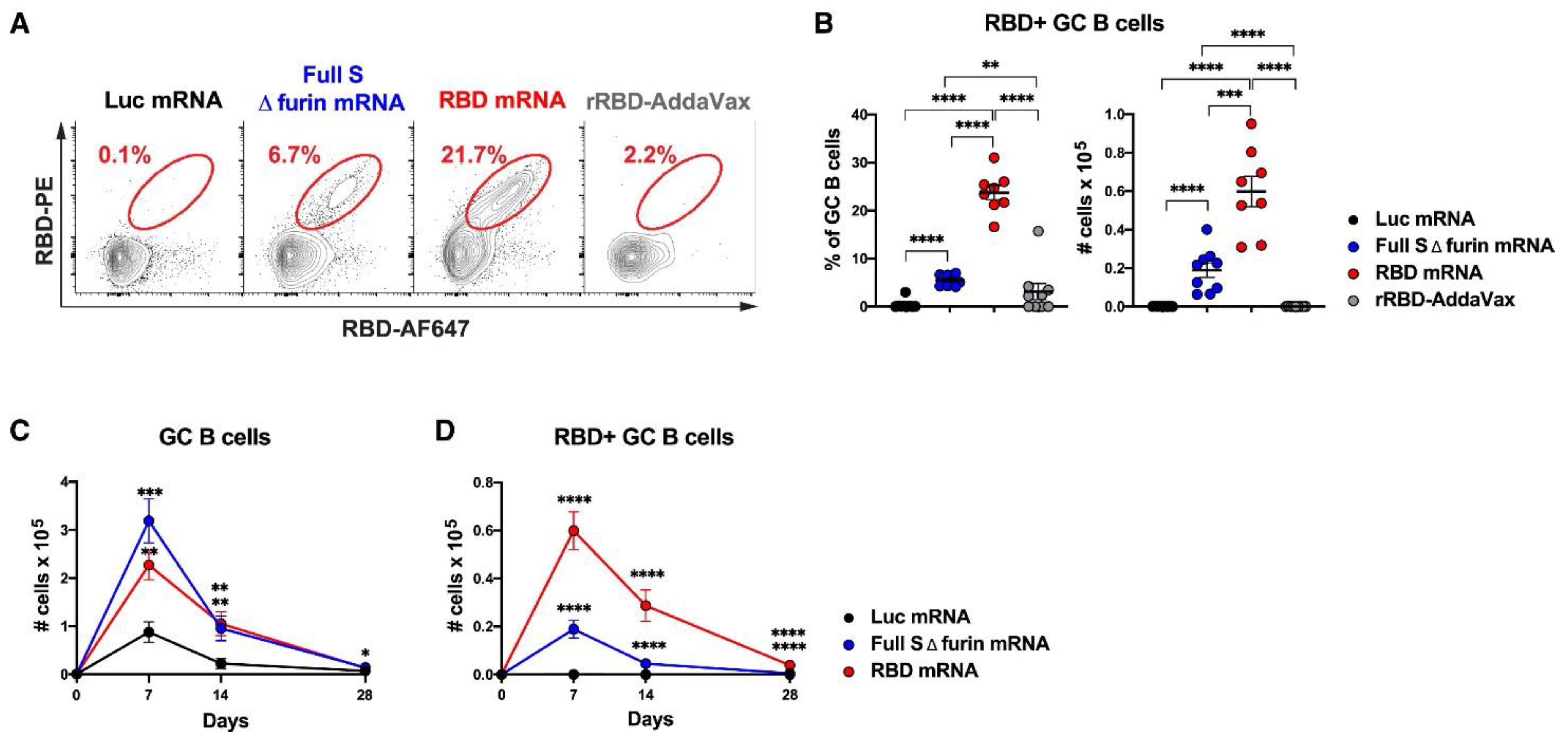
4.1. Liposomes
4.2. Nanodiscs
4.3. Lipid–Polymer Hybrid Nanoparticles
4.4. Micelles
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Miller, K.; Siegel, R.; Lin, C.; Mariotto, A.; Kramer, J.; Rowland, J.; Stein, K.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.; Wu, C. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Luo, M.; Wang, H.; Wang, Z.; Cai, H.; Lu, Z.; Li, Y.; Du, M.; Huang, G.; Wang, C.; Chen, X.; et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 648–654. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.; Bahjat, K.; Schwendeman, A.; Moon, J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef]
- Stadtmauer, E.; Fraietta, J.; Davis, M.; Cohen, A.; Weber, K.; Lancaster, E.; Mangan, P.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.; Brentjens, R. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Ma, L.; Dichwalkar, T.; Chang, J.; Cossette, B.; Garafola, D.; Zhang, A.; Fichter, M.; Wang, C.; Liang, S.; Silva, M.; et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019, 365, 162–168. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, J.; Ruan, H.; Zhang, X.; Ye, Y.; Shen, S.; Wang, C.; Lu, W.; Cheng, K.; et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat. Biomed. Eng. 2018, 2, 831–840. [Google Scholar] [CrossRef]
- Chambers, C.; Kuhns, M.; Egen, J.; Allison, J. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hu, Q.; Bellotti, A.; Gu, Z. Tailoring biomaterials for cancer immunotherapy: Emerging trends and future outlook. Adv. Mater. 2017, 29, 1606036. [Google Scholar] [CrossRef]
- Popovic, A.; Jaffee, E.; Zaidi, N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J. Clin. Investig. 2018, 128, 3209–3218. [Google Scholar] [CrossRef]
- Cremolini, C.; Vitale, E.; Rastaldo, R.; Giachino, C. Advanced Nanotechnology for Enhancing Immune Checkpoint Blockade Therapy. Nanomaterials 2021, 11, 661. [Google Scholar] [CrossRef]
- Gubin, M.; Zhang, X.; Schuster, H.; Caron, E.; Ward, J.; Noguchi, T.; Ivanova, Y.; Hundal, J.; Arthur, C.; Krebber, W.; et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014, 515, 577–581. [Google Scholar] [CrossRef]
- Weinmann, H. Cancer immunotherapy: Selected targets and small-molecule modulators. ChemMedChem 2016, 11, 450–466. [Google Scholar] [CrossRef]
- Yousefi, H.; Yuan, J.; Keshavarz-Fathi, M.; Murphy, J.; Rezaei, N. Immunotherapy of cancers comes of age. Expert Rev. Clin. Immunol. 2017, 13, 1001–1015. [Google Scholar] [CrossRef]
- Chen, D.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Francis, D.; Thomas, S. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv. Drug Deliver. Rev. 2017, 114, 33–42. [Google Scholar] [CrossRef]
- Beck, J.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef]
- Frankiw, L.; Baltimore, D.; Li, G. Alternative mRNA splicing in cancer immunotherapy. Nat. Rev. Immunol. 2019, 19, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Verbeke, R.; Dewitte, H.; Lentacker, I.; Vermaelen, K.; Breckpot, K.; Van Lint, S. mRNA in cancer immunotherapy: Beyond a source of antigen. Mol. Cancer 2021, 20, 48. [Google Scholar] [CrossRef]
- Oberli, M.; Reichmuth, A.; Dorkin, J.; Mitchell, M.; Fenton, O.; Jaklenec, A.; Anderson, D.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Persano, F.; Persano, S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem. 2020, 8, 589959. [Google Scholar] [CrossRef]
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the function of tumor-associated macrophages by siRNA-loaded lipid nanoparticles for cancer immunotherapy. J. Control. Release 2020, 325, 235–248. [Google Scholar] [CrossRef]
- Flandrin, G.; Sigaux, F.; Castaigne, S.; Billard, C.; Aguet, M.; Boiron, M.; Falcoff, E.; Degos, L. Treatment of hairy cell leukemia with recombinant alpha interferon: I. quantitative study of bone marrow changes during the first months of treatment. Blood 1986, 67, 817–820. [Google Scholar] [CrossRef]
- Rosenberg, S. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug. Disc. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef]
- Chen, D.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Freeman-Cook, K.; Hoffman, R.; Miller, N.; Almaden, J.; Chionis, J.; Zhang, Q.; Eisele, K.; Liu, C.; Zhang, C.; Huser, N.; et al. Expanding control of the tumor cell cycle with a CDK2/4/6 inhibitor. Cancer Cell 2021, 39, 1404–1421.e1411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; He, Y.; Li, M.; Wang, X. Cell cycle activity correlates with increased anti-tumor immunity in diverse cancers. Clin. Transl. Immunol. 2020, 10, e98. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stanger, B. Cell cycle regulation meets tumor immunosuppression. Trends Immunol. 2020, 41, 859–863. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Ahmed, Y.; Feng, D.; Li, N.; Ribeiro, M.; Lafdil, F.; Kisseleva, T.; Szabo, G.; Gao, B. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell. Mol. Immunol. 2021, 18, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Dubnika, A.; Manoukian, M.; Mohammadi, M.; Parekh, M.; Gurjarpadhye, A.; Inayathullah, M.; Dubniks, V.; Lakey, J.; Rajadas, J. Cytokines as therapeutic agents and targets in heart disease. Cytokine Growth Factor Rev. 2018, 43, 54–68. [Google Scholar] [CrossRef]
- Shourian, M.; Beltra, J.; Bourdin, B.; Decaluwe, H. Common gamma chain cytokines and CD8 T cells in cancer. Semin. Immunol. 2019, 42, 101307. [Google Scholar] [CrossRef]
- Au, L.; Fendler, A.; Shepherd, S.; Rzeniewicz, K.; Cerrone, M.; Byrne, F.; Carlyle, E.; Edmonds, K.; Del Rosario, L.; Shon, J.; et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat. Med. 2021, 27, 1362–1366. [Google Scholar] [CrossRef]
- Fajgenbaum, D.; June, C. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Meng, Q.; Tian, R.; Long, H.; Wu, X.; Lai, J.; Zharkova, O.; Wang, J.; Chen, X.; Rao, L. Capturing cytokines with advanced materials: A potential strategy to tackle COVID-19 cytokine storm. Adv. Mater. 2021, 33, e2100012. [Google Scholar] [CrossRef]
- De Virgiliis, F.; Di Giovanni, S. Lung innervation in the eye of a cytokine storm: Neuroimmune interactions and COVID-19. Nat. Rev. Neurol. 2020, 16, 645–652. [Google Scholar] [CrossRef]
- Kaufman, H.; Kohlhapp, F.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug. Disc. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Dharmadhikari, N.; Mehnert, J.; Kaufman, H. Oncolytic virus immunotherapy for melanoma. Curr. Treat Option Oncol. 2015, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.; Shettigar, M.; Kaufman, H. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef]
- Viswanath, D.; Liu, H.; Huston, D.; Chua, C.; Grattoni, A. Emerging biomaterial-based strategies for personalized therapeutic in situ cancer vaccines. Biomaterials 2022, 280, 121297. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef]
- Sankaranarayanan, R. HPV vaccination: The most pragmatic cervical cancer primary prevention strategy. Int. J. Gynaecol. Obstet. 2015, 131, S33–S35. [Google Scholar] [CrossRef] [PubMed]
- Taghinezhad, -S.S.; Keyvani, H.; Bermúdez-Humarán, L.; Donders, G.; Fu, X.; Mohseni, A. Twenty years of research on HPV vaccines based on genetically modified lactic acid bacteria: An overview on the gut-vagina axis. Cell. Mol. Life Sci. 2021, 78, 1191–1206. [Google Scholar] [CrossRef]
- Salomon, N.; Selmi, A.; Grunwitz, C.; Kong, A.; Stanganello, E.; Neumaier, J.; Petschenka, J.; Diken, M.; Kreiter, S.; Türeci, Ö.; et al. Local radiotherapy and E7 RNA-LPX vaccination show enhanced therapeutic efficacy in preclinical models of HPV16 cancer. Cancer Immunol. Immunother. 2021, 71, 1975–1988. [Google Scholar] [CrossRef]
- Rossi, I.; Spagnoli, G.; Buttini, F.; Sonvico, F.; Stellari, F.; Cavazzini, D.; Chen, Q.; Müller, M.; Bolchi, A.; Ottonello, S.; et al. A respirable HPV-L2 dry-powder vaccine with GLA as amphiphilic lubricant and immune-adjuvant. J. Control. Release 2021, 340, 209–220. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Rossignol, M.; Koné-Paut, I.; Krivitzky, A.; Lebrun-Frenay, C.; Clet, J.; Brassat, D.; Papeix, C.; Nicolino, M.; Benhamou, P.; et al. Risk of autoimmune diseases and human papilloma virus (HPV) vaccines: Six years of case-referent surveillance. J. Autoimmun. 2017, 79, 84–90. [Google Scholar] [CrossRef]
- Kojic, E.; Conley, L.; Bush, T.; Cu-Uvin, S.; Unger, E.; Henry, K.; Hammer, J.; Escota, G.; Darragh, T.; Palefsky, J.; et al. Prevalence and incidence of anal and cervical high-risk human papillomavirus (HPV) types covered by current HPV vaccines among HIV-infected women in the SUN study. J. Infect. Dis. 2018, 217, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, J.; Kang, T.; Shin, H.; Cheng, M.; Farmer, E.; Wu, T.; Hung, C. Endoplasmic reticulum stress enhances the antigen-specific T cell immune responses and therapeutic antitumor effects generated by therapeutic HPV vaccines. J. Biomed. Sci. 2019, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, L.; Meek, J.; Brackney, M.; Hadler, J.; Sosa, L.; Weinberger, D. Declines in human papillomavirus (HPV)-associated high-grade cervical lesions after introduction of HPV vaccines in connecticut, United States, 2008–2015. Clin. Infect. Dis. 2017, 65, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.; Schiller, J. Human papillomavirus vaccines. J. Infect. Dis. 2021, 224, S367–S378. [Google Scholar] [CrossRef]
- Bogani, G.; Leone Roberti Maggiore, U.; Signorelli, M.; Martinelli, F.; Ditto, A.; Sabatucci, I.; Mosca, L.; Lorusso, D.; Raspagliesi, F. The role of human papillomavirus vaccines in cervical cancer: Prevention and treatment. Crit. Rev. Oncol. Hematol. 2018, 122, 92–97. [Google Scholar] [CrossRef]
- Lehtinen, M.; Dillner, J. Clinical trials of human papillomavirus vaccines and beyond. Nat. Rev. Clin. Oncol. 2013, 10, 400–410. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Delany-Moretlwe, S. HPV vaccines can be the hallmark of cancer prevention. Lancet 2019, 394, 450–451. [Google Scholar] [CrossRef]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef]
- van der Sande, M.; van der Loeff, M. Human papillomavirus vaccinations matter! Lancet Infect. Dis. 2021, 21, 1341–1342. [Google Scholar] [CrossRef]
- Castle, P.; Einstein, M.; Sahasrabuddhe, V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef]
- Zandberg, D.; Bhargava, R.; Badin, S.; Cullen, K. The role of human papillomavirus in nongenital cancers. CA Cancer J. Clin. 2013, 63, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.; Melief, C.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Hollingsworth, R.; Jansen, K. Turning the corner on therapeutic cancer vaccines. npj Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Angioli, R.; Lopez, S.; Aloisi, A.; Terranova, C.; De Cicco, C.; Scaletta, G.; Capriglione, S.; Miranda, A.; Luvero, D.; Ricciardi, R.; et al. Ten years of HPV vaccines: State of art and controversies. Crit. Rev. Oncol. Hematol. 2016, 102, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Zimet, G.; Tatar, O.; Stupiansky, N.; Fisher, W.; Rosberger, Z. Human papillomavirus vaccines: Successes and future challenges. Drugs 2018, 78, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Badiga, S.; Chambers, M.; Huh, W.; Eltoum, I.; Piyathilake, C. Expression of p16 in cervical precancerous lesions that is unlikely to be preventable by human papillomavirus vaccines. Cancers 2016, 122, 3615–3623. [Google Scholar] [CrossRef]
- Jørgensen, L.; Doshi, P.; Gøtzsche, P.; Jefferson, T. Challenges of independent assessment of potential harms of HPV vaccines. BMJ 2018, 362, k3694. [Google Scholar] [CrossRef]
- Roden, R.; Stern, P. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Graubard, B.; Broutian, T.; Pickard, R.; Tong, Z.; Xiao, W.; Kahle, L.; Gillison, M. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef]
- Brotherton, J. HPV vaccines: So much learnt, so many more lessons to come. Lancet Oncol. 2016, 17, 8–9. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.; Stanley, M.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.M.; van Zandvoort, K.; Brisson, M.; Jit, M. Effects of updated demography, disability weights, and cervical cancer burden on estimates of human papillomavirus vaccination impact at the global, regional, and national levels: A PRIME modelling study. Lancet Global Health 2020, 8, e536–e544. [Google Scholar] [CrossRef]
- Markowitz, L. HPV vaccines prophylactic, not therapeutic. JAMA 2007, 298, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. Therapeutic HPV vaccine holds promise. Nat. Rev. Clin. Oncol. 2015, 12, 686. [Google Scholar] [CrossRef]
- Pan, X.; Li, R.; Pan, A.; Larson, H. Human papillomavirus vaccine approval in China: A major step forward but challenges ahead. Lancet Infect. Dis. 2016, 16, 1322–1323. [Google Scholar] [CrossRef]
- Martínez-Lavín, M. HPV vaccine: Adverse event signals were minimised or ignored. BMJ 2019, 366, l4508. [Google Scholar] [CrossRef]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef]
- Chow, S.; Berek, J.; Dorigo, O. Development of therapeutic vaccines for ovarian cancer. Vaccine 2020, 8, 657. [Google Scholar] [CrossRef]
- Corti, C.; Giachetti, P.; Eggermont, A.; Delaloge, S.; Curigliano, G. Therapeutic vaccines for breast cancer: Has the time finally come? Eur. J. Cancer 2022, 160, 150–174. [Google Scholar] [CrossRef]
- Ferber, S.; Gonzalez, R.; Cryer, A.; von Andrian, U.; Artzi, N. Immunology-guided biomaterial design for mucosal cancer vaccines. Adv. Mater. 2020, 32, e1903847. [Google Scholar] [CrossRef]
- Reuven, E.; Leviatan Ben-Arye, S.; Yu, H.; Duchi, R.; Perota, A.; Conchon, S.; Bachar Abramovitch, S.; Soulillou, J.; Galli, C.; Chen, X.; et al. Biomimetic glyconanoparticle vaccine for cancer immunotherapy. ACS Nano 2019, 13, 2936–2947. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.; Jiang, A.; Zhang, P.; Wooster, R.; Anderson, D. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.; Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.; Porter, F.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug. Disc. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, W.; Alvarez, J.; Jia, K.; Shi, L.; Wang, Q.; Zou, N.; He, K.; Zhu, H. Cancer immune checkpoint blockade therapy and its associated autoimmune cardiotoxicity. Acta Pharmacol. Sin. 2018, 39, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, L.; Ceresoli, G.; D’Incecco, A.; Scherpereel, A.; Aerts, J.; Maio, M. Immune checkpoint therapy of mesothelioma: Pre-clinical bases and clinical evidences. Cytokine Growth Factor Rev. 2017, 36, 25–31. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.; Dholaria, B.; Soyano, A.; Knutson, K.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef]
- Wieder, T.; Eigentler, T.; Brenner, E.; Röcken, M. Immune checkpoint blockade therapy. J. Allergy Clin. Immunol. 2018, 142, 1403–1414. [Google Scholar] [CrossRef]
- Topalian, S.; Drake, C.; Pardoll, D. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Postow, M.; Callahan, M.; Wolchok, J. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhao, Y.; Dong, C.; Liu, L.; Pan, Y.; Lai, J.; Liu, Z.; Yu, G.; Chen, X.; Rao, L. Genetically programmable fusion cellular vesicles for cancer immunotherapy. Angew. Chem. Int. Ed. 2021, 60, 26320. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.; Szilagyi, K.; Barclay, N.; van den Berg, T. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020, 588, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Blocking PCSK9 enhances immune checkpoint therapy. Nat. Rev. Drug. Disc. 2021, 20, 20. [Google Scholar] [CrossRef]
- Corsello, S.; Barnabei, A.; Marchetti, P.; De Vecchis, L.; Salvatori, R.; Torino, F. Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab. 2013, 98, 1361–1375. [Google Scholar] [CrossRef]
- Spallarossa, P.; Meliota, G.; Brunelli, C.; Arboscello, E.; Ameri, P.; Dessalvi, C.; Grossi, F.; Deidda, M.; Mele, D.; Sarocchi, M.; et al. Potential cardiac risk of immune-checkpoint blockade as anticancer treatment: What we know, what we do not know, and what we can do to prevent adverse effects. Med. Res. Rev. 2018, 38, 1447–1468. [Google Scholar] [CrossRef]
- Park, Y.; Kuen, D.; Chung, Y. Future prospects of immune checkpoint blockade in cancer: From response prediction to overcoming resistance. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar]
- Byun, D.; Wolchok, J.; Rosenberg, L.; Girotra, M. Cancer immunotherapy—Immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z.; et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 758. [Google Scholar] [CrossRef]
- Xin, G.; Khatun, A.; Topchyan, P.; Zander, R.; Volberding, P.; Chen, Y.; Shen, J.; Fu, C.; Jiang, A.; See, W.; et al. Pathogen-boosted adoptive cell transfer therapy induces endogenous antitumor immunity through antigen spreading. Cancer Immunol. Res. 2020, 8, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Mandriani, B.; Pelle’, E.; Pezzicoli, G.; Strosberg, J.; Abate-Daga, D.; Guarini, A.; Cives, M.; Porta, C. Adoptive T-cell immunotherapy in digestive tract malignancies: Current challenges and future perspectives. Cancer Treat. Rev. 2021, 100, 102288. [Google Scholar] [CrossRef] [PubMed]
- Marotte, L.; Capitao, M.; Deleine, C.; Beauvais, T.; Cadiou, G.; Perrin, J.; Chérel, M.; Scotet, E.; Guilloux, Y.; Bruchertseifer, F.; et al. Anti-tumor efficacy of a combination therapy with PD-L1 targeted alpha therapy and adoptive cell transfer of PD-1 deficient melanoma-specific human T-lymphocytes. Oncoimmunology 2021, 10, 1940676. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Sen, S.; Tatar, A.; Mahvi, D.; Meyers, J.; Srinand, P.; Suresh, M.; Cho, C. Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice. Cancer Immunol. Immunother. 2016, 65, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Leisegang, M.; Xia, M.; Kiyotani, K.; Li, N.; Zeng, C.; Deng, C.; Jiang, J.; Harada, M.; Agrawal, N.; et al. Generation of neoantigen-specific T cells for adoptive cell transfer for treating head and neck squamous cell carcinoma. Oncoimmunology 2021, 10, 1929726. [Google Scholar] [CrossRef]
- Han, F.; Dellacecca, E.; Barse, L.; Cosgrove, C.; Henning, S.; Ankney, C.; Jaishankar, D.; Yemelyanov, A.; Krymskaya, V.; Dilling, D.; et al. Adoptive T-Cell transfer to treat lymphangioleiomyomatosis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 793–804. [Google Scholar] [CrossRef]
- Houot, R.; Schultz, L.; Marabelle, A.; Kohrt, H. T-cell-based Immunotherapy: Adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 2015, 3, 1115–1122. [Google Scholar] [CrossRef]
- Sukari, A.; Abdallah, N.; Nagasaka, M. Unleash the power of the mighty T cells-basis of adoptive cellular therapy. Crit. Rev. Oncol. Hematol. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Xin, G.; Schauder, D.; Jing, W.; Jiang, A.; Joshi, N.; Johnson, B.; Cui, W. Pathogen boosted adoptive cell transfer immunotherapy to treat solid tumors. Proc. Natl. Acad. Sci. USA 2017, 114, 740–745. [Google Scholar] [CrossRef]
- Aspord, C.; Laurin, D.; Richard, M.J.; Vie, H.; Chaperot, L.; Plumas, J. Induction of antiviral cytotoxic T cells by plasmacytoid dendritic cells for adoptive immunotherapy of posttransplant diseases. Am. J. Transplant. 2011, 11, 2613–2626. [Google Scholar] [CrossRef]
- Fan, J.; Shang, D.; Han, B.; Song, J.; Chen, H.; Yang, J. Adoptive cell transfer: Is it a promising immunotherapy for colorectal cancer? Theranostics 2018, 8, 5784–5800. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Kalos, M. Adoptive immunotherapy for cancer. Immunol. Rev. 2014, 257, 14–38. [Google Scholar] [CrossRef]
- Lee, D. Cellular therapy: Adoptive immunotherapy with expanded natural killer cells. Immunol. Rev. 2019, 290, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Yee, C. The use of endogenous T cells for adoptive transfer. Immunol. Rev. 2014, 257, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Maus, M.; June, C.; Sampson, J. Immunotherapy for glioblastoma: Adoptive T-cell strategies. Clin. Cancer Res. 2019, 25, 2042–2048. [Google Scholar] [CrossRef]
- Hammerl, D.; Rieder, D.; Martens, J.; Trajanoski, Z.; Debets, R. Adoptive T cell therapy: New avenues leading to safe targets and powerful allies. Trends Immunol. 2018, 39, 921–936. [Google Scholar] [CrossRef]
- Flores, C.; Wildes, T.; Dean, B.; Moore, G.; Drake, J.; Abraham, R.; Gil, J.; Yegorov, O.; Yang, C.; Dean, J.; et al. Massive clonal expansion of medulloblastoma-specific T cells during adoptive cellular therapy. Sci. Adv. 2019, 5, eaav9879. [Google Scholar] [CrossRef]
- Lövgren, T.; Wolodarski, M.; Wickström, S.; Edbäck, U.; Wallin, M.; Martell, E.; Markland, K.; Blomberg, P.; Nyström, M.; Lundqvist, A.; et al. Complete and long-lasting clinical responses in immune checkpoint inhibitor-resistant, metastasized melanoma treated with adoptive T cell transfer combined with DC vaccination. Oncoimmunology 2020, 9, 1792058. [Google Scholar] [CrossRef]
- Dréno, B.; Khammari, A.; Fortun, A.; Vignard, V.; Saiagh, S.; Beauvais, T.; Jouand, N.; Bercegay, S.; Simon, S.; Lang, F.; et al. Phase I/II clinical trial of adoptive cell transfer of sorted specific T cells for metastatic melanoma patients. Cancer Immunol. Immunother. 2021, 70, 3015–3030. [Google Scholar] [CrossRef]
- Mazzarella, T.; Cambiaghi, V.; Rizzo, N.; Pilla, L.; Parolini, D.; Orsenigo, E.; Colucci, A.; Modorati, G.; Doglioni, C.; Parmiani, G.; et al. Ex vivo enrichment of circulating anti-tumor T cells from both cutaneous and ocular melanoma patients: Clinical implications for adoptive cell transfer therapy. Cancer Immunol. Immunother. 2012, 61, 1169–1182. [Google Scholar] [CrossRef][Green Version]
- Andersen, R.; Donia, M.; Ellebaek, E.; Borch, T.; Kongsted, P.; Iversen, T.; Hölmich, L.; Hendel, H.; Met, Ö.; Andersen, M.; et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin. Cancer Res. 2016, 22, 3734–3745. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Mardiana, S.; House, I.; Sek, K.; Henderson, M.; Giuffrida, L.; Chen, A.; Todd, K.; Petley, E.; Chan, J.; et al. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat. Immunol. 2020, 21, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, I.; Bolaños, E.; Quetglas, J.; Gros, A.; Villanueva, A.; Palomero, J.; Sánchez-Paulete, A.; Piulats, J.; Matias-Guiu, X.; Olivera, I.; et al. Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8 T cells. Cancer Cell 2019, 36, 613–629.e617. [Google Scholar] [CrossRef] [PubMed]
- Kah, J.; Koh, S.; Volz, T.; Ceccarello, E.; Allweiss, L.; Lütgehetmann, M.; Bertoletti, A.; Dandri, M. Lymphocytes transiently expressing virus-specific T cell receptors reduce hepatitis B virus infection. J. Clin. Investig. 2017, 127, 3177–3188. [Google Scholar] [CrossRef]
- Beatty, G.; Haas, A.; Maus, M.; Torigian, D.; Soulen, M.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.; Albelda, S.; et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014, 2, 112–120. [Google Scholar] [CrossRef]
- Wilgenhof, S.; Corthals, J.; Heirman, C.; van Baren, N.; Lucas, S.; Kvistborg, P.; Thielemans, K.; Neyns, B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol. 2016, 34, 1330–1338. [Google Scholar] [CrossRef]
- Janelle, V.; Delisle, J. T-Cell dysfunction as a limitation of adoptive immunotherapy: Current concepts and mitigation strategies. Cancers 2021, 13, 598. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug. Disc. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Morris, V.; Kopetz, S. Don’t blame the messenger: Lessons learned for cancer mRNA vaccines during the COVID-19 pandemic. Nat. Rev. Cancer 2022, 22, 317–318. [Google Scholar] [CrossRef]
- Wardell, C.; Levings, M. mRNA vaccines take on immune tolerance. Nat. Biotechnol. 2021, 39, 419–421. [Google Scholar] [CrossRef]
- El Sahly, H.; Baden, L.; Essink, B.; Doblecki-Lewis, S.; Martin, J.; Anderson, E.; Campbell, T.; Clark, J.; Jackson, L.; Fichtenbaum, C.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.; El Sahly, H.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.; Rouphael, N.; Creech, C.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Painter, M.; Apostolidis, S.; Mathew, D.; Meng, W.; Rosenfeld, A.; Lundgreen, K.; Reynaldi, A.; Khoury, D.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin, T.; Wang, S.; Manzoni, T.; Muramatsu, H.; Hogan, M.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 2020, 53, 1281–1295. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug. Disc. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Meng, Z.; O’Keeffe-Ahern, J.; Lyu, J.; Pierucci, L.; Zhou, D.; Wang, W. A new developing class of gene delivery: Messenger RNA-based therapeutics. Biomater. Sci. 2017, 5, 2381–2392. [Google Scholar] [CrossRef]
- Granot, Y.; Peer, D. Delivering the right message: Challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics-an innate immune system standpoint. Semin. Immunopathol. 2017, 34, 68–77. [Google Scholar] [CrossRef]
- Reichmuth, A.; Oberli, M.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control. Release 2005, 107, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Kumarasinghe, E.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef]
- Guevara, M.; Persano, S.; Persano, F. Lipid-based vectors for therapeutic mRNA-based anti-cancer vaccines. Curr. Pharm. Des. 2019, 25, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.; Rudra, A.; Miao, L.; Anderson, D. Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Li, C.; Yang, T.; Hu, B.; Zhang, M.; Guo, S.; Xiao, H.; Liang, X.; Huang, Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020, 40, 107534. [Google Scholar] [CrossRef]
- Zhao, W.; Hou, X.; Vick, O.; Dong, Y. RNA delivery biomaterials for the treatment of genetic and rare diseases. Biomaterials 2019, 217, 119291. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Dong, Y. Nanoscale platforms for messenger RNA delivery. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1530. [Google Scholar] [CrossRef]
- Sato, Y.; Okabe, N.; Note, Y.; Hashiba, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomater. 2020, 102, 341–350. [Google Scholar] [CrossRef]
- Wölk, C.; Janich, C.; Bakowsky, U.; Langner, A.; Brezesinski, G. Malonic acid based cationic lipids—The way to highly efficient DNA-carriers. Adv. Colloid Interface Sci. 2017, 248, 20–34. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.; Torchilin, V. Recent advancements in liposome technology. Adv. Drug Deliver. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Blakney, A.; McKay, P.; Yus, B.; Aldon, Y.; Shattock, R. Inside out: Optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019, 26, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Anderluzzi, G.; Schmidt, S.; Woods, S.; Gallorini, S.; Brazzoli, M.; Giusti, F.; Ferlenghi, I.; Johnson, R.; Roberts, C.; et al. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: The impact of cationic lipid selection. J. Control. Release 2020, 325, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.; Chan, M.; Shaw, C.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.; Beard, C.; et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Chiarot, E.; Giovani, C.; Buccato, S.; Bonacci, S.; Frigimelica, E.; Margarit, I.; Geall, A.; Bensi, G.; Maione, D. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine 2017, 35, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Baeza Garcia, A.; Siu, E.; Sun, T.; Exler, V.; Brito, L.; Hekele, A.; Otten, G.; Augustijn, K.; Janse, C.; Ulmer, J.; et al. Neutralization of the plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat. Commun. 2018, 9, 2714. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Settanni, G.; Brill, W.; Haas, H.; Schmid, F. pH-dependent behavior of ionizable cationic lipids in mRNA-carrying lipoplexes investigated by molecular dynamics simulations. Macromol. Rapid Commun. 2021, 43, e2100683. [Google Scholar] [CrossRef]
- Tam, Y.; Chen, S.; Cullis, P. Advances in lipid nanoparticles for siRNA delivery. Pharmaceutics 2013, 5, 498–507. [Google Scholar] [CrossRef]
- Evers, M.; Du, W.; Yang, Q.; Kooijmans, S.; Vink, A.; van Steenbergen, M.; Vader, P.; de Jager, S.; Fuchs, S.; Mastrobattista, E.; et al. Delivery of modified mRNA to damaged myocardium by systemic administration of lipid nanoparticles. J. Control. Release 2022, 343, 207–216. [Google Scholar] [CrossRef]
- Cui, L.; Pereira, S.; Sonzini, S.; van Pelt, S.; Romanelli, S.; Liang, L.; Ulkoski, D.; Krishnamurthy, V.; Brannigan, E.; Brankin, C.; et al. Development of a high-throughput platform for screening lipid nanoparticles for mRNA delivery. Nanoscale 2022, 14, 1480–1491. [Google Scholar] [CrossRef]
- Anderluzzi, G.; Lou, G.; Woods, S.; Schmidt, S.; Gallorini, S.; Brazzoli, M.; Johnson, R.; Roberts, C.; O’Hagan, D.; Baudner, B.; et al. The role of nanoparticle format and route of administration on self-amplifying mRNA vaccine potency. J. Control. Release. 2021, 342, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, Q.; Zhao, Y.; Zhi, D.; Chen, H.; Wang, R.; Ju, B.; Zhang, S. Structure-activity relationships of pH-responsive and ionizable lipids for gene delivery. Int. J. Pharm. 2022, 617, 121596. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Bao, Y.; Sacchetti, C.; Brady, J.; Gillard, K.; Yu, H.; Roberts, S.; Rajappan, K.; Tanis, S.; Perez-Garcia, C.; et al. Synthesis and bioactivity of readily hydrolysable novel cationic lipids for potential lung delivery application of mRNAs. Chem. Phys. Lipids 2022, 243, 105178. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Butowska, K.; Swingle, K.; Alameh, M.; Weissman, D.; Mitchell, M. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Tian, H.; Chen, X. Opportunities and challenges for mRNA delivery nanoplatforms. J. Phys. Chem. Lett. 2022, 13, 1314–1322. [Google Scholar] [CrossRef]
- Koltover, I.; Salditt, T.; Rädler, J.; Safinya, C. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science 1998, 281, 78–81. [Google Scholar] [CrossRef]
- Li, W.; Szoka, F. Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 2007, 24, 438–449. [Google Scholar] [CrossRef]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliver. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.; Hou, S.; Esposito, A.; Ketova, T.; Welsher, K.; et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tao, W.; Liu, D.; Wu, J.; Guo, Z.; Ji, X.; Bharwani, Z.; Zhao, L.; Zhao, X.; Farokhzad, O.; et al. Surface de-PEGylation controls nanoparticle-mediated siRNA delivery in vitro and in vivo. Theranostics 2017, 7, 1990–2002. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.; Tam, Y.; Madden, T.; Hope, M.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Shields, C.; Wang, L.; Evans, M.; Mitragotri, S. Materials for immunotherapy. Adv. Mater. 2020, 32, e1901633. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliver. Rev. 2020, 154, 102–122. [Google Scholar] [CrossRef]
- Eloy, J.; Petrilli, R.; Trevizan, L.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef]
- . Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Nguyen, G.; Zhang, C.; Zeng, C.; Yan, J.; Du, S.; Hou, X.; Li, W.; Jiang, J.; et al. Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Sci. Adv. 2020, 6, eabc2315. [Google Scholar] [CrossRef]
- Ahmed, K.; Hussein, S.; Ali, A.; Korma, S.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef] [PubMed]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef]
- Kogure, K.; Moriguchi, R.; Sasaki, K.; Ueno, M.; Futaki, S.; Harashima, H. Development of a non-viral multifunctional envelope-type nano device by a novel lipid film hydration method. J. Control. Release 2004, 98, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Delaney, J.; Schubert, U.; Fahr, A. Fast high-throughput screening of temoporfin-loaded liposomal formulations prepared by ethanol injection method. J. Liposome Res. 2012, 22, 31–41. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D. Stimuli-responsive liposomes for drug delivery. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
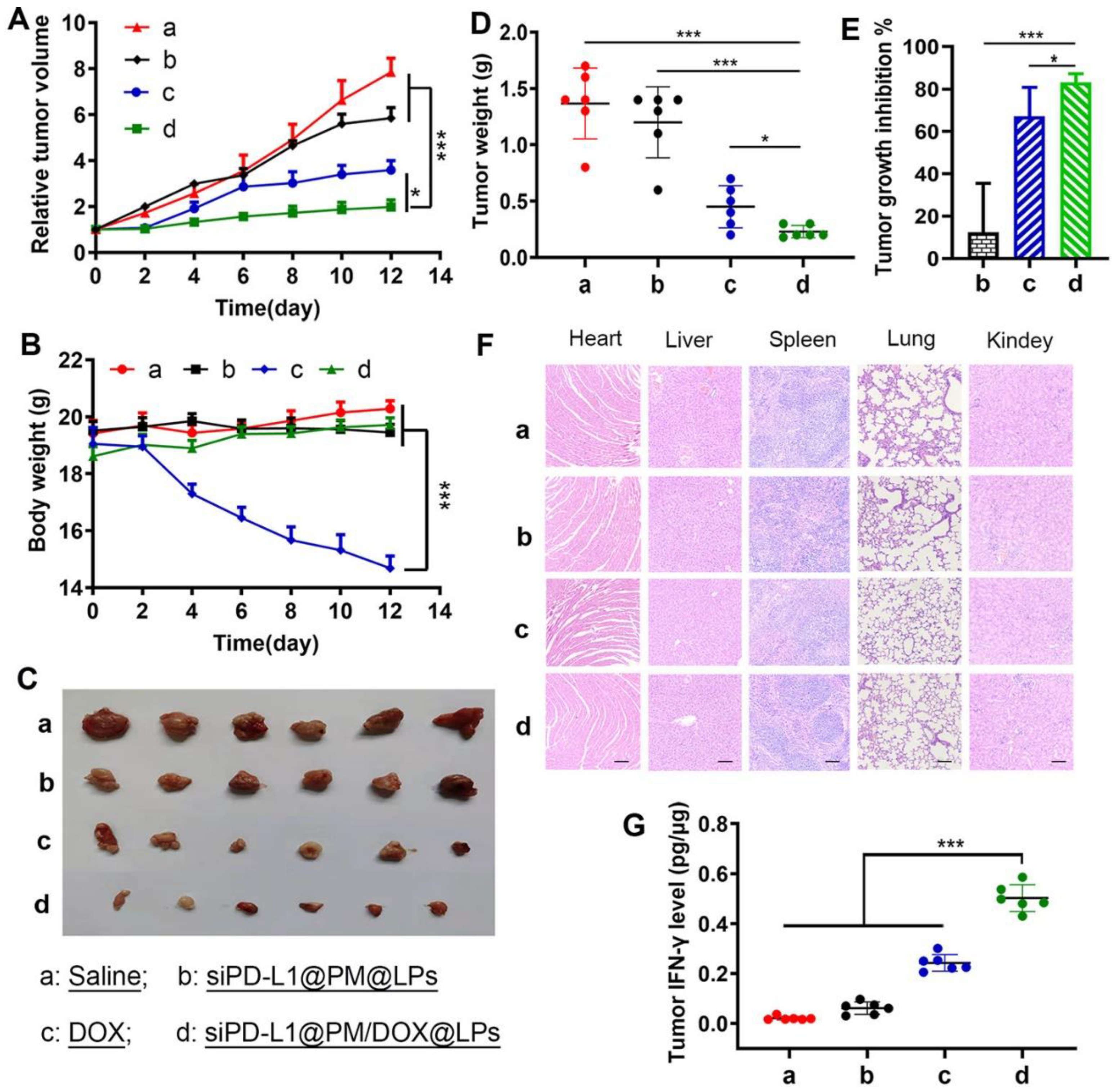
- Gu, Z.; Wang, Q.; Shi, Y.; Huang, Y.; Zhang, J.; Zhang, X.; Lin, G. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Control. Release 2018, 286, 369–380. [Google Scholar] [CrossRef]
- Dhaliwal, H.; Fan, Y.; Kim, J.; Amiji, M. Intranasal delivery and transfection of mRNA therapeutics in the brain using cationic liposomes. Mol. Pharm. 2020, 17, 1996–2005. [Google Scholar] [CrossRef]
- Hollebecque, A.; Chung, H.; de Miguel, M.; Italiano, A.; Machiels, J.; Lin, C.; Dhani, N.; Peeters, M.; Moreno, V.; Su, W.; et al. Safety and antitumor activity of α-PD-L1 antibody as monotherapy or in combination with α-TIM-3 antibody in patients with microsatellite instability-high/mismatch repair-deficient tumors. Clin. Cancer Res. 2021, 27, 6393–6404. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Harb, W.; Peer, C.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-human phase I study of envafolimab, a novel subcutaneous single-domain anti-PD-L1 antibody, in patients with advanced solid tumors. Oncologist 2021, 26, e1514–e1525. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.; Brandi, G. Durvalumab: An investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin. Investig. Drugs 2021, 30, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Yap, T.; Chung, H.; de Miguel, M.; Bang, Y.; Lin, C.; Su, W.; Italiano, A.; Chow, K.; Szpurka, A.; et al. Safety and clinical activity of a new anti-PD-L1 antibody as monotherapy or combined with targeted therapy in advanced solid tumors: The PACT phase Ia/Ib trial. Clin. Cancer Res. 2021, 27, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Heery, C.; Thomas, A.; Mammen, A.; Perry, S.; O’Sullivan Coyne, G.; Guha, U.; Berman, A.; Szabo, E.; Madan, R.; et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (avelumab) treatment in advanced thymoma. J. Immunother. Cancer 2019, 7, 269. [Google Scholar] [CrossRef]
- Ni, X.; Xing, Y.; Sun, X.; Suo, J. The safety and efficacy of anti-PD-1/anti-PD-L1 antibody therapy in the treatment of previously treated, advanced gastric or gastro-oesophageal junction cancer: A meta-analysis of prospective clinical trials. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Arkenau, H.; Lee, J.; Rha, S.; Oh, D.; Wyrwicz, L.; Kang, Y.; Lee, K.; Infante, J.; Lee, S.; et al. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: Phase 1b results from the JAVELIN solid tumor trial. J. Control. Release 2019, 7, 30. [Google Scholar] [CrossRef]
- Doi, T.; Iwasa, S.; Muro, K.; Satoh, T.; Hironaka, S.; Esaki, T.; Nishina, T.; Hara, H.; Machida, N.; Komatsu, Y.; et al. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: The JAVELIN solid tumor JPN trial. Gastric Cancer 2019, 22, 817–827. [Google Scholar] [CrossRef]
- Dirix, L.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.; Forero-Torres, A.; Boccia, R.; Lippman, M.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN solid tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef]
- Brahmer, J.; Tykodi, S.; Chow, L.; Hwu, W.; Topalian, S.; Hwu, P.; Drake, C.; Camacho, L.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Hu, Q.; Yao, J.; Wang, X.; Wang, Y.; Fu, X.; Ma, J.; Lin, H.; Xu, J.; Shen, L.; Yu, X. Combinational chemoimmunotherapy for breast cancer by codelivery of doxorubicin and PD-L1 siRNA using a PAMAM-incorporated liposomal nanoplatform. ACS Appl. Mater. Interfaces 2022, 14, 8782–8792. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.; Zhou, Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Fan, Y.; Moon, J. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccine 2015, 3, 662–685. [Google Scholar] [CrossRef] [PubMed]
- Lizotte, P.; Wen, A.; Sheen, M.; Fields, J.; Rojanasopondist, P.; Steinmetz, N.; Fiering, S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016, 11, 295–303. [Google Scholar] [CrossRef]
- Vasseur, L.; Cens, T.; Wagner, R.; Saint, N.; Kugler, V.; Chavanieu, A.; Ouvry, C.; Dupré, C.; Ferry, G.; Boutin, J. Importance of the choice of a recombinant system to produce large amounts of functional membrane protein hERG. Int. J. Mol. Sci. 2019, 20, 3181. [Google Scholar] [CrossRef] [PubMed]
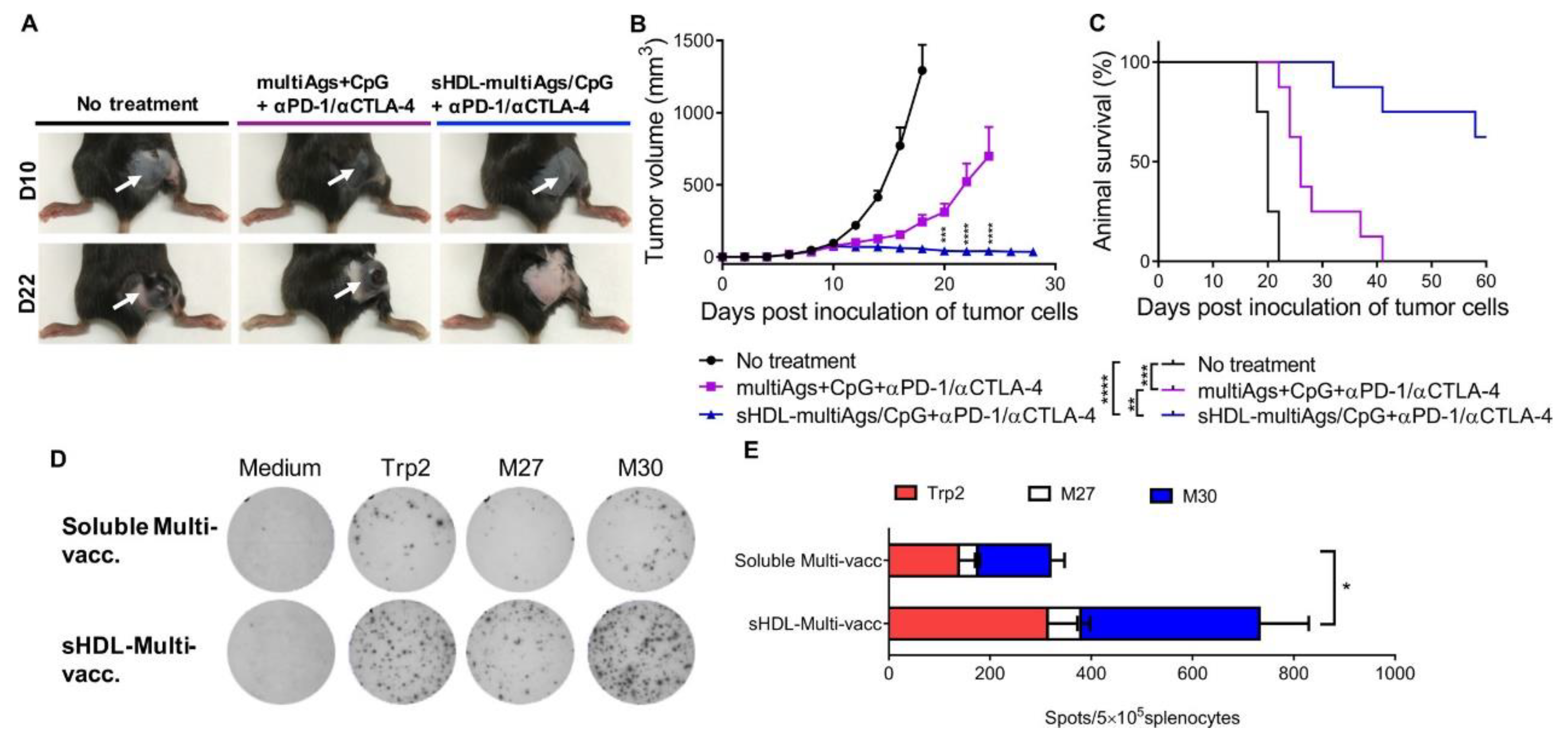
- Kuai, R.; Sun, X.; Yuan, W.; Xu, Y.; Schwendeman, A.; Moon, J. Subcutaneous nanodisc vaccination with neoantigens for combination cancer immunotherapy. Bioconjugate Chem. 2018, 29, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Dehaini, D.; Fang, R.; Luk, B.; Pang, Z.; Hu, C.; Kroll, A.; Yu, C.; Gao, W.; Zhang, L. Ultra-small lipid-polymer hybrid nanoparticles for tumor-penetrating drug delivery. Nanoscale 2016, 8, 14411–14419. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Kalinowski, D.; Richardson, D. Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacol. Res. 2016, 68, 701–787. [Google Scholar] [CrossRef]
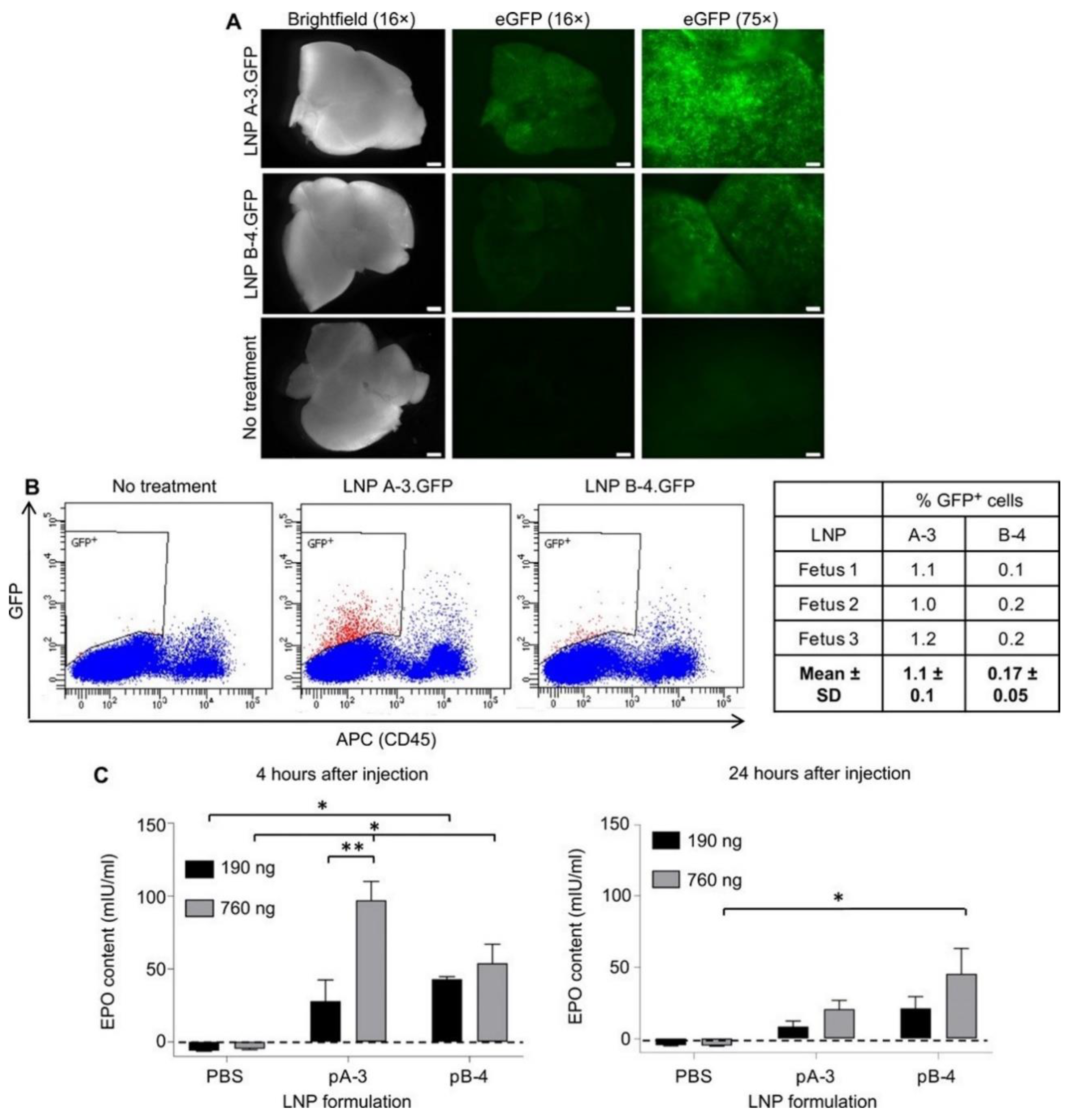
- Riley, R.; Kashyap, M.; Billingsley, M.; White, B.; Alameh, M.; Bose, S.; Zoltick, P.; Li, H.; Zhang, R.; Cheng, A.; et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 2021, 7, eaba1028. [Google Scholar] [CrossRef]
- Allen, T.; Cullis, P. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliver. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Burnett, J.; Rossi, J. RNA-based therapeutics: Current progress and future prospects. Cell Chem. Bio. 2012, 19, 60–71. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Kowalski, P.; Anderson, D. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccine 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Humanes, A.; Coiffier, C.; Gigmes, D.; Verrier, B.; Trimaille, T. Polylactide-based reactive micelles as a robust platform for mRNA delivery. Pharm. Res. 2020, 37, 30. [Google Scholar] [CrossRef] [PubMed]
- Kichler, A.; Leborgne, C.; März, J.; Danos, O.; Bechinger, B. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2003, 100, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Liang, W.; Lan, Y.; Chaudhuri, P.; Chow, M.; Witt, K.; Kudsiova, L.; Mason, A. Effective endogenous gene silencing mediated by pH responsive peptides proceeds via multiple pathways. J. Control. Release 2012, 158, 293–303. [Google Scholar] [CrossRef]
- McCarthy, H.; McCaffrey, J.; McCrudden, C.; Zholobenko, A.; Ali, A.; McBride, J.; Massey, A.; Pentlavalli, S.; Chen, K.; Cole, G.; et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control. Release 2014, 189, 141–149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-L.; Wang, Z.-G.; Liu, S.-L. Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules 2022, 27, 5607. https://doi.org/10.3390/molecules27175607
Wang H-L, Wang Z-G, Liu S-L. Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules. 2022; 27(17):5607. https://doi.org/10.3390/molecules27175607
Chicago/Turabian StyleWang, Hong-Li, Zhi-Gang Wang, and Shu-Lin Liu. 2022. "Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy" Molecules 27, no. 17: 5607. https://doi.org/10.3390/molecules27175607
APA StyleWang, H.-L., Wang, Z.-G., & Liu, S.-L. (2022). Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules, 27(17), 5607. https://doi.org/10.3390/molecules27175607






