The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection
Abstract
:1. Introduction
2. Thromboxane A2 Biosynthesis
3. Thromboxane A2 Receptor Isoforms and Signaling
| Receptor Property | TPα | TPβ |
|---|---|---|
| Length of cytoplasmic tail | 15 amino acids [22] | 79 amino acids [37] |
| Specific 2nd messengers | Gαh [34], Gαs, stimulates cAMP [35]. | Gαi, Inhibits cAMP [35] |
| Response to Stimulation | ||
| Acute | Phosphorylated, not internalized [38,39] | Internalized in a GRK, and arrestin dependent manner [39] |
| Chronic | Decreased expression [38] | Enhanced expression [38] |
| Desensitization | ||
| Phosphorylation Sites | Ser329 [40,41] and Ser331 [42,43] | Thr399 [44] |
| Kinases or agonists involved | Ser329: PGD2 [40], PGI2 [41,42], PGE2 [43], and cAMP/PKA [45] Ser331: NO/PKG [42,43] | PKC-α [38] and G-protein receptor kinases 2, 5 and 6 [39] |
| Resensitization to ligand | Dephosphorylation phosphatases PP1 and PP2A [46] | Recycling of the receptor to the surface after lysosomal-mediated ligand degradation [39] |
4. TXA2 Signaling in Cancer
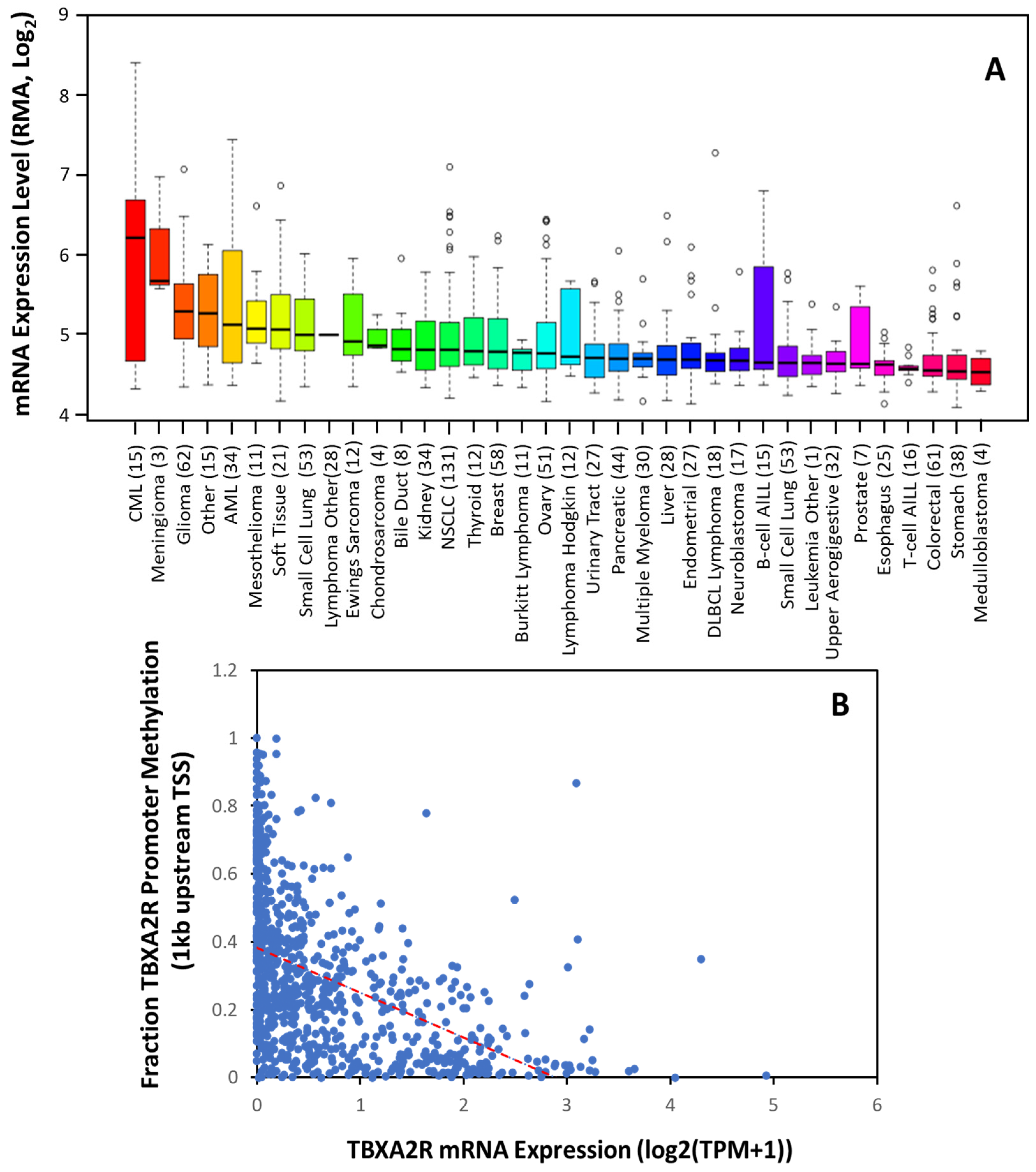
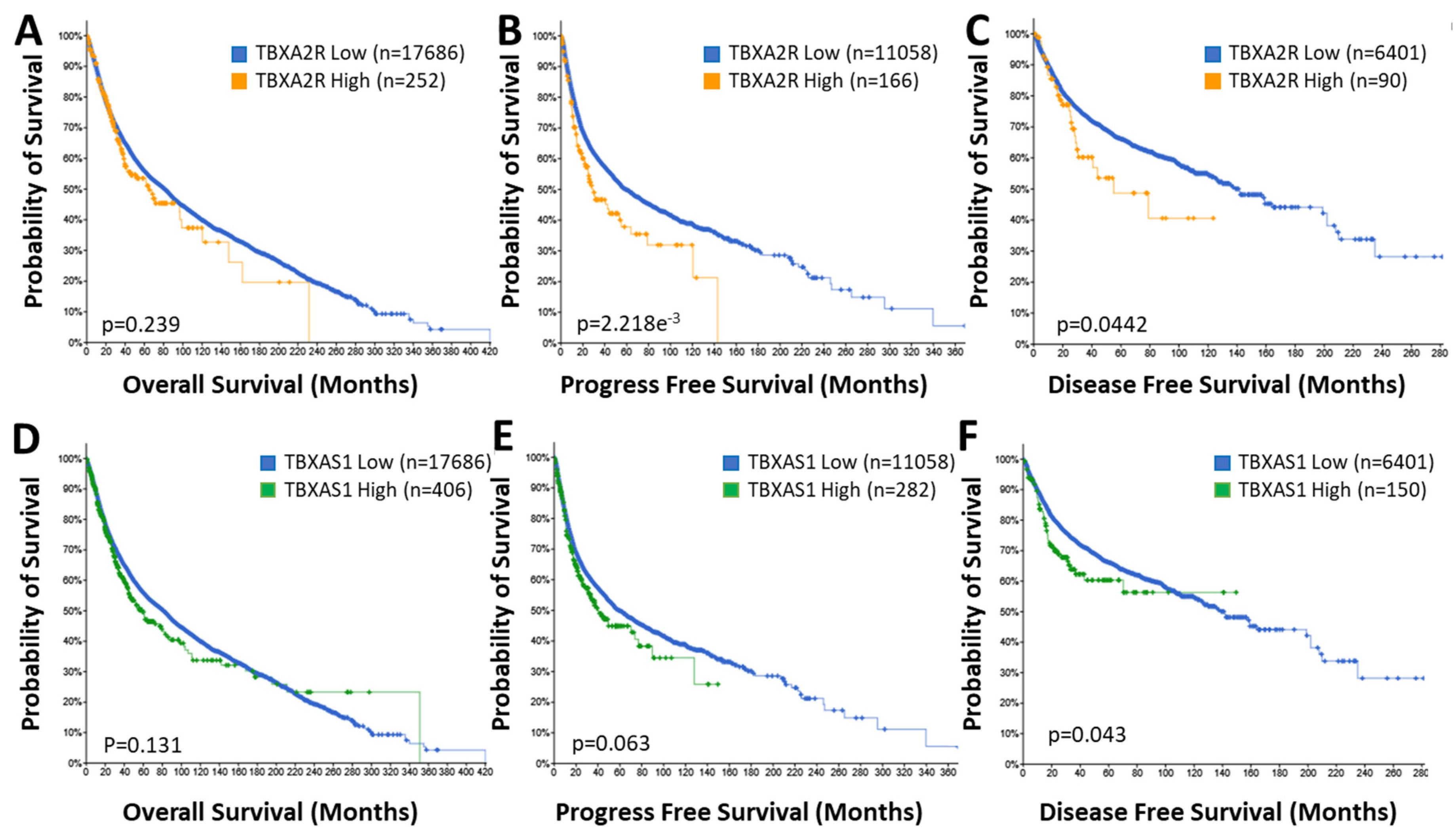
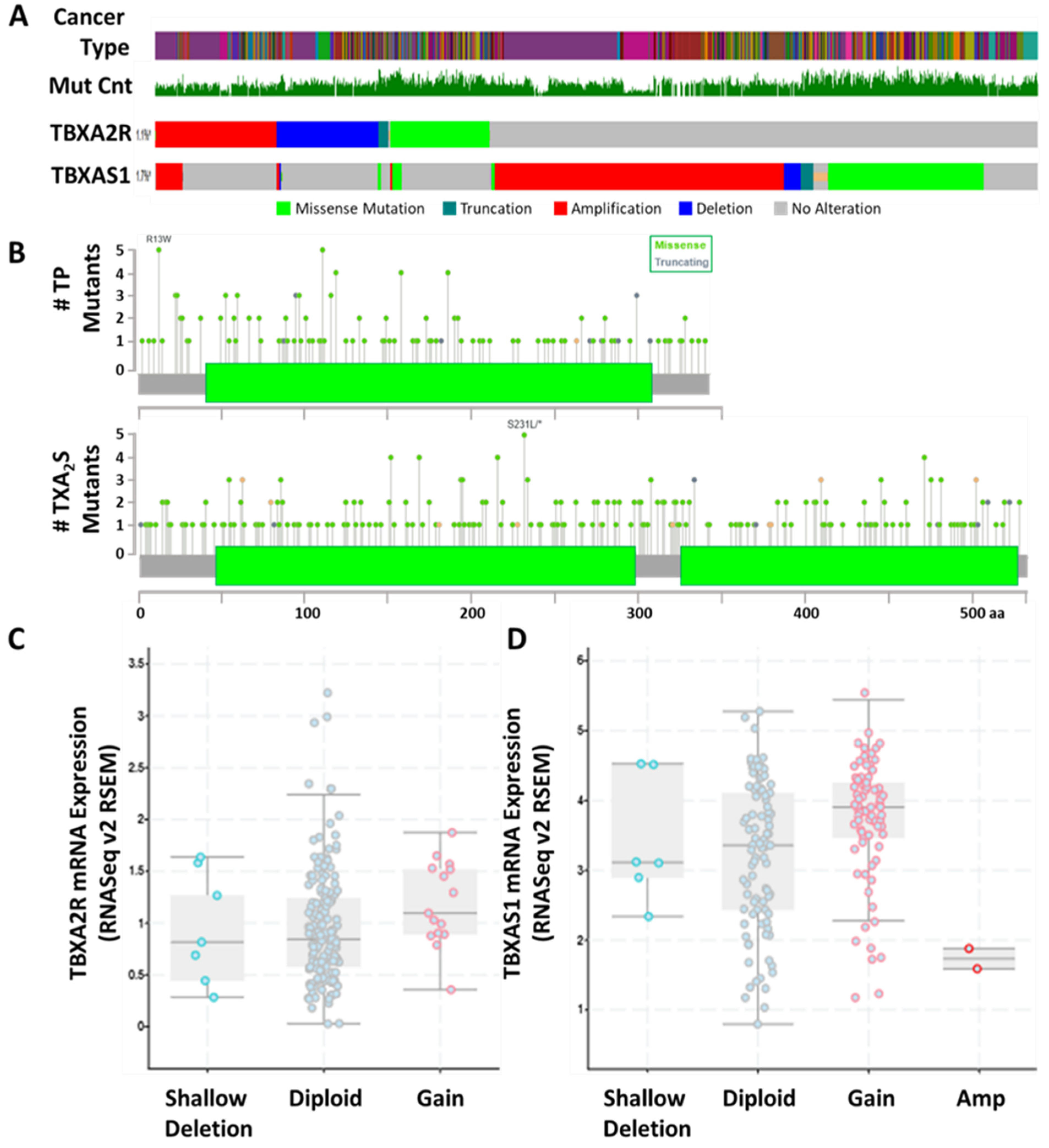
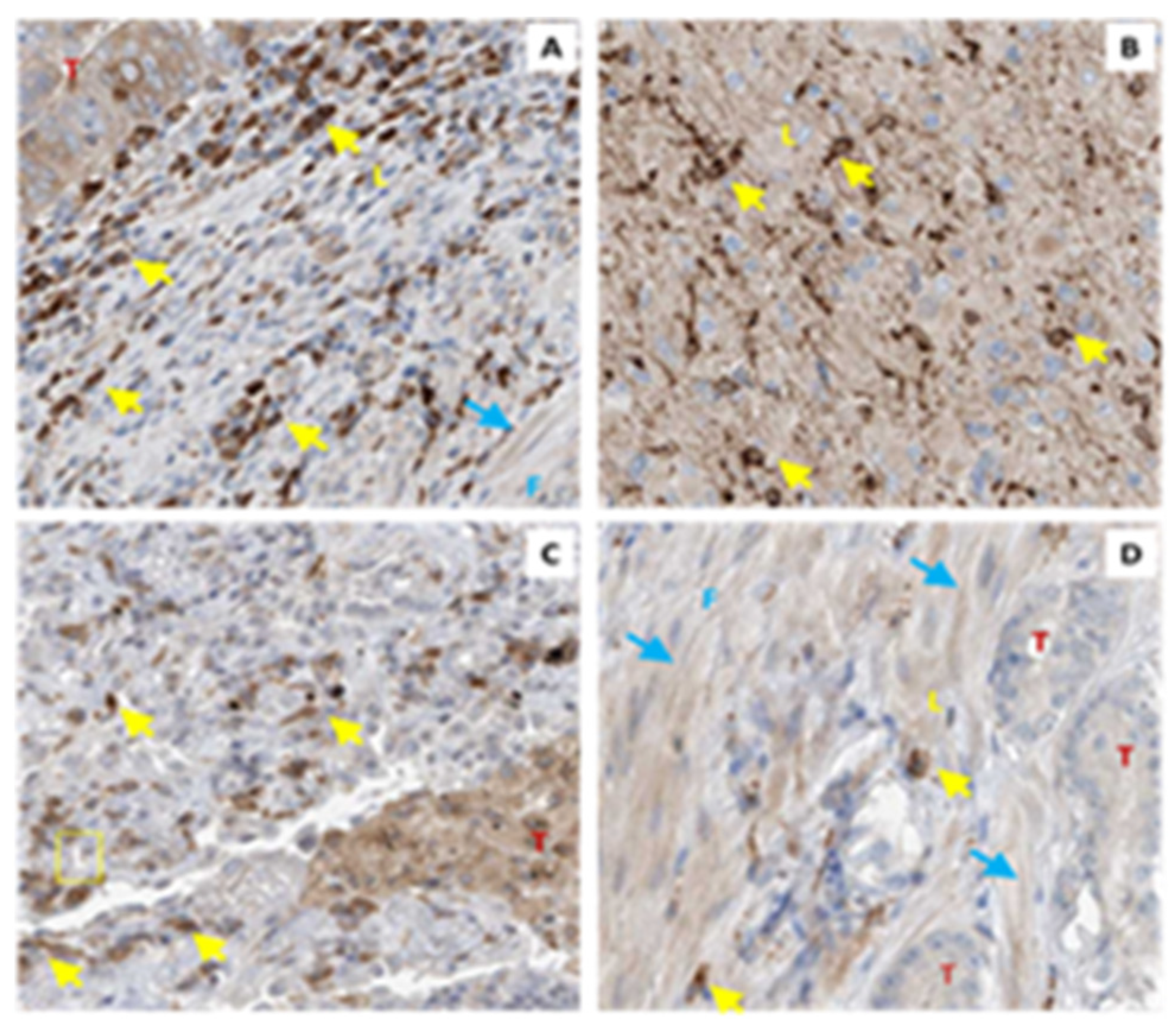
4.1. TP Isoforms in Cancer
4.2. TBXAS1 in Cancer
5. Trojan Horses: The By-Products of Txa2 Biosynthesis in Cancer
6. TXA2/TP Antagonists as Adjuvant Therapies
7. Sleight of Hand: Looking beyond the Tumor Epithelium to Determine the Role of TXA2 in Cancer
8. Immune Modulation by TXA2 Signaling in Cancer
9. Role of TXA2 Signaling in Priming Sites of Metastatic Spread
10. Role and Regulation of Endothelial Cell Migration and Angiogenesis by TXA2
11. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Wang, M.T.; Honn, K.V.; Nie, D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007, 26, 525–534. [Google Scholar] [CrossRef]
- Ornelas, A.; Zacharias-Millward, N.; Menter, D.G.; Davis, J.S.; Lichtenberger, L.; Hawke, D.; Hawk, E.; Vilar, E.; Bhattacharya, P.; Millward, S. Beyond COX-1: The effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017, 36, 289–303. [Google Scholar] [CrossRef]
- Toloczko-Iwaniuk, N.; Dziemianczyk-Pakiela, D.; Nowaszewska, B.K.; Celinska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention—Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef]
- Ko, C.J.; Lan, S.W.; Lu, Y.C.; Cheng, T.S.; Lai, P.F.; Tsai, C.H.; Hsu, T.W.; Lin, H.Y.; Shyu, H.Y.; Wu, S.R.; et al. Inhibition of cyclooxygenase-2-mediated matriptase activation contributes to the suppression of prostate cancer cell motility and metastasis. Oncogene 2017, 36, 4597–4609. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, K.; Jiang, T.; Wang, L.; Shen, S.; Luo, Z.; Tuo, Y.; Liu, X.; Hu, Y.; Pang, Z. Celecoxib normalizes the tumor microenvironment and enhances small nanotherapeutics delivery to A549 tumors in nude mice. Sci. Rep. 2017, 7, 10071. [Google Scholar] [CrossRef]
- Brasky, T.M.; Moysich, K.B.; Cohn, D.E.; White, E. Non-steroidal anti-inflammatory drugs and endometrial cancer risk in the VITamins And Lifestyle (VITAL) cohort. Gynecol. Oncol. 2013, 128, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Neill, A.S.; Nagle, C.M.; Protani, M.M.; Obermair, A.; Spurdle, A.B.; Webb, P.M.; Australian National Endometrial Cancer Study, G. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: A case-control study, systematic review and meta-analysis. Int. J. Cancer 2013, 132, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Llobat, P.; Dovizio, M.; Bruno, A.; Ricciotti, E.; Cufino, V.; Sacco, A.; Grande, R.; Alberti, S.; Arena, V.; Cirillo, M.; et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget 2016, 7, 32462–32477. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Svensson, J.; Samuelsson, B. Thromboxanes: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Natl. Acad. Sci. USA 1975, 72, 2994–2998. [Google Scholar] [CrossRef]
- Miyata, A.; Yokoyama, C.; Ihara, H.; Bandoh, S.; Takeda, O.; Takahashi, E.; Tanabe, T. Characterization of the human gene (TBXAS1) encoding thromboxane synthase. Eur. J. Biochem. 1994, 224, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Halushka, P.V. Thromboxane A(2) recept.tors: Where have you gone? Prostaglandins Other Lipid Mediat. 2000, 60, 175–189. [Google Scholar] [CrossRef]
- Miggin, S.M.; Kinsella, B.T. Expression and tissue distribution of the mRNAs encoding the human thromboxane A2 receptor (TP) alpha and beta isoforms. Biochim. Biophys. Acta 1998, 1425, 543–559. [Google Scholar] [CrossRef]
- Hecker, M.; Ullrich, V. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J. Biol. Chem. 1989, 264, 141–150. [Google Scholar] [CrossRef]
- Diczfalusy, U.; Hammarstrom, S. A structural requirement for the conversion of prostaglandin endoperoxides to thromboxanes. FEBS Lett. 1979, 105, 291–295. [Google Scholar] [CrossRef]
- Okuno, T.; Yokomizo, T. Biological functions of 12(S)-hydroxyheptadecatrienoic acid as a ligand of leukotriene B4 receptor 2. Inflamm. Regen. 2018, 38, 29. [Google Scholar] [CrossRef]
- Ahmed, O.S.; Galano, J.M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: Where are we now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef]
- Wilson, S.J.; McGinley, K.; Huang, A.J.; Smyth, E.M. Heterodimerization of the alpha and beta isoforms of the human thromboxane receptor enhances isoprostane signaling. Biochem. Biophys. Res. Commun. 2007, 352, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Hayashi, Y.; Ushikubi, F.; Yokota, Y.; Kageyama, R.; Nakanishi, S.; Narumiya, S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 1991, 349, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, B.T. Thromboxane A2 signalling in humans: A ‘Tail’ of two receptors. Biochem. Soc. Trans. 2001, 29, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, N. Thromboxane A2: Physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol. Ther. 2008, 118, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Nüsing, R.M.; Hirata, M.; Kakizuka, A.; Eki, T.; Ozawa, K.; Narumiya, S. Characterization and chromosomal mapping of the human thromboxane A2 receptor gene. J. Biol. Chem. 1993, 268, 25253–25259. [Google Scholar] [CrossRef]
- Coyle, A.T.; Kinsella, B.T. Characterization of promoter 3 of the human thromboxane A receptor gene. A functional AP-1 and octamer motif are required for basal promoter activity. Febs J. 2005, 272, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Coyle, A.T.; Miggin, S.M.; Kinsella, B.T. Characterization of the 5’ untranslated region of alpha and beta isoforms of the human thromboxane A2 receptor (TP). Differential promoter utilization by the TP isoforms. Eur. J. Biochem. 2002, 269, 4058–4073. [Google Scholar] [CrossRef]
- Habib, A.; FitzGerald, G.A.; Maclouf, J. Phosphorylation of the thromboxane receptor alpha, the predominant isoform expressed in human platelets. J. Biol. Chem. 1999, 274, 2645–2651. [Google Scholar] [CrossRef]
- Bambi-Nyanguile, S.M.; Hanson, J.; Ooms, A.; Alpan, L.; Kolh, P.; Dogne, J.M.; Pirotte, B. Synthesis and pharmacological evaluation of 2-aryloxy/arylamino-5-cyanobenzenesulfonylureas as novel thromboxane A(2) receptor antagonists. Eur. J. Med. Chem. 2013, 65, 32–40. [Google Scholar] [CrossRef]
- Qiao, N.; Reynaud, D.; Demin, P.; Halushka, P.V.; Pace-Asciak, C.R. The thromboxane receptor antagonist PBT-3, a hepoxilin stable analog, selectively antagonizes the TPalpha isoform in transfected COS-7 cells. J. Pharmacol. Exp. Ther. 2003, 307, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Hanson, J.; Reynaud, D.; Qiao, N.; Devel, P.; Moray, A.L.; Renard, J.F.; Kelley, L.P.; Winum, J.Y.; Montero, J.L.; Kinsella, B.T.; et al. Synthesis and pharmacological evaluation of novel nitrobenzenic thromboxane modulators as antiplatelet agents acting on both the alpha and beta isoforms of the human thromboxane receptor. J. Med. Chem. 2006, 49, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Dogné, J.M.; Ghiotto, J.; Moray, A.L.; Kinsella, B.T.; Pirotte, B. Design, synthesis, and SAR study of a series of N-alkyl-N’-[2-(aryloxy)-5-nitrobenzenesulfonyl]ureas and -cyanoguanidine as selective antagonists of the TPalpha and TPbeta isoforms of the human thromboxane A2 receptor. J. Med. Chem. 2007, 50, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; DiLizio, C.; Kim, D.; Smyth, E.M.; Manning, D.R. The G12 family of G proteins as a reporter of thromboxane A2 receptor activity. Mol. Pharmacol. 2006, 69, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Vezza, R.; Habib, A.; FitzGerald, G.A. Differential signaling by the thromboxane receptor isoforms via the novel GTP-binding protein, Gh. J. Biol. Chem. 1999, 274, 12774–12779. [Google Scholar] [CrossRef]
- Hirata, T.; Ushikubi, F.; Kakizuka, A.; Okuma, M.; Narumiya, S. Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J. Clin. Investig. 1996, 97, 949–956. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, S.; Zhou, S.; Ware, J.A. The thromboxane A2 receptor activates mitogen-activated protein kinase via protein kinase C-dependent Gi coupling and Src-dependent phosphorylation of the epidermal growth factor receptor. J. Pharmacol. Exp. Ther. 2001, 296, 426–433. [Google Scholar]
- Raychowdhury, M.K.; Yukawa, M.; Collins, L.J.; McGrail, S.H.; Kent, K.C.; Ware, J.A. Alternative splicing produces a divergent cytoplasmic tail in the human endothelial thromboxane A2 receptor. J. Biol. Chem. 1995, 270, 7011. [Google Scholar] [CrossRef]
- Yukawa, M.; Yokota, R.; Eberhardt, R.T.; von Andrian, L.; Ware, J.A. Differential desensitization of thromboxane A2 receptor subtypes. Circ. Res. 1997, 80, 551–556. [Google Scholar] [CrossRef]
- Parent, J.L.; Labrecque, P.; Orsini, M.J.; Benovic, J.L. Internalization of the TXA2 receptor alpha and beta isoforms. Role of the differentially spliced cooh terminus in agonist-promoted receptor internalization. J. Biol. Chem. 1999, 274, 8941–8948. [Google Scholar] [CrossRef]
- Foley, J.F.; Kelley, L.P.; Kinsella, B.T. Prostaglandin D(2) receptor-mediated desensitization of the alpha isoform of the human thromboxane A(2) receptor. Biochem. Pharmacol. 2001, 62, 229–239. [Google Scholar] [CrossRef]
- Walsh, M.T.; Foley, J.F.; Kinsella, B.T. The alpha, but not the beta, isoform of the human thromboxane A2 receptor is a target for prostacyclin-mediated desensitization. J. Biol. Chem. 2000, 275, 20412–20423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Yan, F.; Zhou, H.; Tai, H.H. Serine 331 is the major site of receptor phosphorylation induced by agents that activate protein kinase G in HEK 293 cells overexpressing thromboxane receptor alpha. Arch. Biochem. Biophys. 2001, 393, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.R.; Zhu, Y.; Halushka, P.V.; Lincoln, T.M.; Mendelsohn, M.E. Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 4888–4893. [Google Scholar] [CrossRef] [PubMed]
- Kelley-Hickie, L.P.; Kinsella, B.T. EP1- and FP-mediated cross-desensitization of the alpha (a) and beta (b) isoforms of the human thromboxane A2 receptor. Br. J. Pharmacol. 2004, 142, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.T.; Kinsella, B.T. Regulation of the human prostanoid TPalpha and TPbeta receptor isoforms mediated through activation of the EP(1) and IP receptors. Br. J. Pharmacol. 2000, 131, 601–609. [Google Scholar] [CrossRef]
- Spurney, R.F. Regulation of thromboxane receptor (TP) phosphorylation by protein phosphatase 1 (PP1) and PP2A. J. Pharmacol. Exp. Ther. 2001, 296, 592–599. [Google Scholar]
- Reid, H.M.; Kinsella, B.T. Palmitoylation of the TPbeta isoform of the human thromboxane A2 receptor. Modulation of G protein: Effector coupling and modes of receptor internalization. Cell Signal. 2007, 19, 1056–1070. [Google Scholar] [CrossRef]
- Hamelin, E.; Thériault, C.; Laroche, G.; Parent, J.L. The intracellular trafficking of the G protein-coupled receptor TPbeta depends on a direct interaction with Rab11. J. Biol. Chem. 2005, 280, 36195–36205. [Google Scholar] [CrossRef]
- Rochdi, M.D.; Laroche, G.; Dupré, E.; Giguère, P.; Lebel, A.; Watier, V.; Hamelin, E.; Lépine, M.C.; Dupuis, G.; Parent, J.L. Nm23-H2 interacts with a G protein-coupled receptor to regulate its endocytosis through an Rac1-dependent mechanism. J. Biol. Chem. 2004, 279, 18981–18989. [Google Scholar] [CrossRef]
- Ekambaram, P.; Lambiv, W.; Cazzolli, R.; Ashton, A.W.; Honn, K.V. The thromboxane synthase and receptor signaling pathway in cancer: An emerging paradigm in cancer progression and metastasis. Cancer Metastasis Rev. 2011, 30, 397–408. [Google Scholar] [CrossRef]
- Gannon, A.M.; Kinsella, B.T. Regulation of the human thromboxane A2 receptor gene by Sp1, Egr1, NF-E2, GATA-1, and Ets-1 in megakaryocytes. J. Lipid Res. 2008, 49, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Orr, K.; Buckley, N.E.; Haddock, P.; James, C.; Parent, J.L.; McQuaid, S.; Mullan, P.B. Thromboxane A2 receptor (TBXA2R) is a potent survival factor for triple negative breast cancers (TNBCs). Oncotarget 2016, 7, 55458–55472. [Google Scholar] [CrossRef] [Green Version]
- Keating, G.L.; Reid, H.M.; Eivers, S.B.; Mulvaney, E.P.; Kinsella, B.T. Transcriptional regulation of the human thromboxane A2 receptor gene by Wilms’ tumor (WT)1 and hypermethylated in cancer (HIC) 1 in prostate and breast cancers. Biochim. Biophys. Acta 2014, 1839, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, E.P.; Shilling, C.; Eivers, S.B.; Perry, A.S.; Bjartell, A.; Kay, E.W.; Watson, R.W.; Kinsella, B.T. Expression of the TPα and TPβ isoforms of the thromboxane prostanoid receptor (TP) in prostate cancer: Clinical significance and diagnostic potential. Oncotarget 2016, 7, 73171–73187. [Google Scholar] [CrossRef]
- Kanwal, R.; Gupta, S. Epigenetic modifications in cancer. Clin. Genet. 2012, 81, 303–311. [Google Scholar] [CrossRef]
- Moussa, O.; Ashton, A.W.; Fraig, M.; Garrett-Mayer, E.; Ghoneim, M.A.; Halushka, P.V.; Watson, D.K. Novel role of thromboxane receptors beta isoform in bladder cancer pathogenesis. Cancer Res. 2008, 68, 4097–4104. [Google Scholar] [CrossRef]
- Honma, S.; Saika, M.; Ohkubo, S.; Kurose, H.; Nakahata, N. Thromboxane A2 receptor-mediated G12/13-dependent glial morphological change. Eur. J. Pharmacol. 2006, 545, 100–108. [Google Scholar] [CrossRef]
- Nie, D.; Guo, Y.; Yang, D.; Tang, Y.; Chen, Y.; Wang, M.T.; Zacharek, A.; Qiao, Y.; Che, M.; Honn, K.V. Thromboxane A2 receptors in prostate carcinoma: Expression and its role in regulating cell motility via small GTPase Rho. Cancer Res. 2008, 68, 115–121. [Google Scholar] [CrossRef]
- Kelly, P.; Stemmle, L.N.; Madden, J.F.; Fields, T.A.; Daaka, Y.; Casey, P.J. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J. Biol. Chem. 2006, 281, 26483–26490. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, H. Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Sobolesky, P.M.; Halushka, P.V.; Garrett-Mayer, E.; Smith, M.T.; Moussa, O. Regulation of the tumor suppressor FOXO3 by the thromboxane-A2 receptors in urothelial cancer. PLoS ONE 2014, 9, e107530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.Y.; Li, M.Y.; Hsin, M.K.; Underwood, M.J.; Ma, L.T.; Mok, T.S.; Warner, T.D.; Chen, G.G. 4-Methylnitrosamino-1-3-pyridyl-1-butanone (NNK) promotes lung cancer cell survival by stimulating thromboxane A2 and its receptor. Oncogene 2011, 30, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tai, H.H. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis 2009, 30, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.G.; Mulvaney, E.P.; Hyland, P.B.; Kinsella, B.T. Protein kinase C-related kinase 1 and 2 play an essential role in thromboxane-mediated neoplastic responses in prostate cancer. Oncotarget 2015, 6, 26437–26456. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.C.; Kavanagh, D.J.; Mulvaney, E.P.; McLean, C.; Wikström, K.; Reid, H.M.; Kinsella, B.T. Identification of an interaction between the TPalpha and TPbeta isoforms of the human thromboxane A2 receptor with protein kinase C-related kinase (PRK) 1: Implications for prostate cancer. J. Biol. Chem. 2011, 286, 15440–15457. [Google Scholar] [CrossRef]
- Huang, R.Y.; Li, M.Y.; Ng, C.S.; Wan, I.Y.; Kong, A.W.; Du, J.; Long, X.; Underwood, M.J.; Mok, T.S.; Chen, G.G. Thromboxane A2 receptor α promotes tumor growth through an autoregulatory feedback pathway. J. Mol. Cell Biol. 2013, 5, 380–390. [Google Scholar] [CrossRef]
- Watkins, G.; Douglas-Jones, A.; Mansel, R.E.; Jiang, W.G. Expression of thromboxane synthase, TBXAS1 and the thromboxane A2 receptor, TBXA2R, in human breast cancer. Int. Semin. Surg. Oncol. 2005, 2, 23. [Google Scholar] [CrossRef]
- Li, H.; Lee, M.H.; Liu, K.; Wang, T.; Song, M.; Han, Y.; Yao, K.; Xie, H.; Zhu, F.; Grossmann, M.; et al. Inhibiting breast cancer by targeting the thromboxane A(2) pathway. NPJ Precis. Oncol. 2017, 1, 8. [Google Scholar] [CrossRef]
- Gustafsson, A.; Hansson, E.; Kressner, U.; Nordgren, S.; Andersson, M.; Lönnroth, C.; Lundholm, K. Prostanoid receptor expression in colorectal cancer related to tumor stage, differentiation and progression. Acta Oncol. 2007, 46, 1107–1112. [Google Scholar] [CrossRef]
- Dassesse, T.; de Leval, X.; de Leval, L.; Pirotte, B.; Castronovo, V.; Waltregny, D. Activation of the thromboxane A2 pathway in human prostate cancer correlates with tumor Gleason score and pathologic stage. Eur. Urol. 2006, 50, 1021–1031, discussion 1031. [Google Scholar] [CrossRef]
- Coyle, A.T.; O’Keeffe, M.B.; Kinsella, B.T. 15-deoxy Delta12,14-prostaglandin J2 suppresses transcription by promoter 3 of the human thromboxane A2 receptor gene through peroxisome proliferator-activated receptor gamma in human erythroleukemia cells. Febs J. 2005, 272, 4754–4773. [Google Scholar] [CrossRef]
- Coyle, A.T.; Kinsella, B.T. Synthetic peroxisome proliferator-activated receptor gamma agonists rosiglitazone and troglitazone suppress transcription by promoter 3 of the human thromboxane A2 receptor gene in human erythroleukemia cells. Biochem. Pharmacol. 2006, 71, 1308–1323. [Google Scholar] [CrossRef]
- Valentin, F.; Field, M.C.; Tippins, J.R. The mechanism of oxidative stress stabilization of the thromboxane receptor in COS-7 cells. J. Biol. Chem. 2004, 279, 8316–8324. [Google Scholar] [CrossRef]
- Valentin, F.; Tippins, J.R.; Field, M.C. The role of alternative splicing and C-terminal amino acids in thromboxane receptor stabilization. Biochem. Biophys. Res. Commun. 2005, 329, 898–904. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Werfel, T.A.; Hicks, D.J.; Rahman, B.; Bendeman, W.E.; Duvernay, M.T.; Maeng, J.G.; Hamm, H.; Lavieri, R.R.; Joly, M.M.; Pulley, J.M.; et al. Repurposing of a Thromboxane Receptor Inhibitor Based on a Novel Role in Metastasis Identified by Phenome-Wide Association Study. Mol. Cancer Ther. 2020, 19, 2454–2464. [Google Scholar] [CrossRef]
- Chen, G.G.; Lee, T.W.; Yip, J.H.; Xu, H.; Lee, I.K.; Mok, T.S.; Warner, T.D.; Yim, A.P. Increased thromboxane B(2) levels are associated with lipid peroxidation and Bcl-2 expression in human lung carcinoma. Cancer Lett. 2006, 234, 193–198. [Google Scholar] [CrossRef]
- Karmali, R.A.; Welt, S.; Thaler, H.T.; Lefevre, F. Prostaglandins in breast cancer: Relationship to disease stage and hormone status. Br. J. Cancer 1983, 48, 689–696. [Google Scholar] [CrossRef]
- Moussa, O.; Ciupek, A.; Watson, D.K.; Halushka, P.V. Urinary thromboxane B2 and thromboxane receptors in bladder cancer: Opportunity for detection and monitoring. Prostaglandins Other Lipid Mediat. 2011, 96, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Kajita, S.; Ruebel, K.H.; Casey, M.B.; Nakamura, N.; Lloyd, R.V. Role of COX-2, thromboxane A2 synthase, and prostaglandin I2 synthase in papillary thyroid carcinoma growth. Mod. Pathol. 2005, 18, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Suzuki, T.; Takahashi, Y.; Ukai, M.; Tauchi, K.; Fujii, T.; Horikawa, N.; Minamimura, T.; Tabuchi, Y.; Morii, M.; et al. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Lett. 2006, 580, 3368–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussa, O.; Yordy, J.S.; Abol-Enein, H.; Sinha, D.; Bissada, N.K.; Halushka, P.V.; Ghoneim, M.A.; Watson, D.K. Prognostic and functional significance of thromboxane synthase gene overexpression in invasive bladder cancer. Cancer Res. 2005, 65, 11581–11587. [Google Scholar] [CrossRef]
- Kuo, H.L.; Lien, J.C.; Chung, C.H.; Chang, C.H.; Lo, S.C.; Tsai, I.C.; Peng, H.C.; Kuo, S.C.; Huang, T.F. NP-184[2-(5-methyl-2-furyl) benzimidazole], a novel orally active antithrombotic agent with dual antiplatelet and anticoagulant activities. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 495–505. [Google Scholar] [CrossRef]
- Cathcart, M.C.; Gately, K.; Cummins, R.; Drakeford, C.; Kay, E.W.; O’Byrne, K.J.; Pidgeon, G.P. Thromboxane synthase expression and correlation with VEGF and angiogenesis in non-small cell lung cancer. Biochim. Biophys. Acta 2014, 1842, 747–755. [Google Scholar] [CrossRef]
- Huang, R.Y.; Chu, Y.L.; Jiang, Z.B.; Chen, X.M.; Zhang, X.; Zeng, X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell Physiol. Biochem. 2014, 33, 375–388. [Google Scholar] [CrossRef]
- Kurzel, F.; Hagel, C.; Zapf, S.; Meissner, H.; Westphal, M.; Giese, A. Cyclo-oxygenase inhibitors and thromboxane synthase inhibitors differentially regulate migration arrest, growth inhibition and apoptosis in human glioma cells. Acta Neurochir. 2002, 144, 71–87. [Google Scholar] [CrossRef]
- Leung, K.C.; Hsin, M.K.; Chan, J.S.; Yip, J.H.; Li, M.; Leung, B.C.; Mok, T.S.; Warner, T.D.; Underwood, M.J.; Chen, G.G. Inhibition of thromboxane synthase induces lung cancer cell death via increasing the nuclear p27. Exp. Cell Res. 2009, 315, 2974–2981. [Google Scholar] [CrossRef]
- Leung, K.C.; Li, M.Y.; Leung, B.C.; Hsin, M.K.; Mok, T.S.; Underwood, M.J.; Chen, G.G. Thromboxane synthase suppression induces lung cancer cell apoptosis via inhibiting NF-kappaB. Exp. Cell Res. 2010, 316, 3468–3477. [Google Scholar] [CrossRef]
- Liu, Q.; Tao, B.; Liu, G.; Chen, G.; Zhu, Q.; Yu, Y.; Yu, Y.; Xiong, H. Thromboxane A2 Receptor Inhibition Suppresses Multiple Myeloma Cell Proliferation by Inducing p38/c-Jun N-terminal Kinase (JNK) Mitogen-activated Protein Kinase (MAPK)-mediated G2/M Progression Delay and Cell Apoptosis. J. Biol. Chem. 2016, 291, 4779–4792. [Google Scholar] [CrossRef]
- Li, X.; Tai, H.H. Increased expression of matrix metalloproteinases mediates thromboxane A2-induced invasion in lung cancer cells. Curr. Cancer Drug Targets 2012, 12, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tai, H.H. Activation of thromboxane A2 receptor (TP) increases the expression of monocyte chemoattractant protein -1 (MCP-1)/chemokine (C-C motif) ligand 2 (CCL2) and recruits macrophages to promote invasion of lung cancer cells. PLoS ONE 2013, 8, e54073. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Amano, H.; Ito, Y.; Eshima, K.; Suzuki, T.; Ogawa, F.; Iyoda, A.; Satoh, Y.; Kato, S.; Nakamura, M.; et al. Thromboxane A₂ receptor signaling facilitates tumor colonization through P-selectin-mediated interaction of tumor cells with platelets and endothelial cells. Cancer Sci. 2012, 103, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yan, Y.; Zheng, X.; Zhang, H.; Chen, W.; Li, H.; Dong, Z. Aspirin suppresses breast cancer metastasis to lung by targeting anoikis resistance. Carcinogenesis 2022, 43, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.; Hagel, C.; Kim, E.L.; Zapf, S.; Djawaheri, J.; Berens, M.E.; Westphal, M. Thromboxane synthase regulates the migratory phenotype of human glioma cells. Neuro. Oncol. 1999, 1, 3–13. [Google Scholar] [CrossRef]
- Schauff, A.K.; Kim, E.L.; Leppert, J.; Nadrowitz, R.; Wuestenberg, R.; Brockmann, M.A.; Giese, A. Inhibition of invasion-associated thromboxane synthase sensitizes experimental gliomas to gamma-radiation. J. Neuro. Oncol. 2009, 91, 241–249. [Google Scholar] [CrossRef]
- Abraham, J.E.; Harrington, P.; Driver, K.E.; Tyrer, J.; Easton, D.F.; Dunning, A.M.; Pharoah, P.D. Common polymorphisms in the prostaglandin pathway genes and their association with breast cancer susceptibility and survival. Clin. Cancer Res. 2009, 15, 2181–2191. [Google Scholar] [CrossRef]
- Lee, K.D.; Baek, S.J.; Shen, R.F. Multiple factors regulating the expression of human thromboxane synthase gene. Biochem. J. 1996, 319 Pt 3, 783–791. [Google Scholar] [CrossRef]
- Basu, A.K.; Marnett, L.J. Unequivocal demonstration that malondialdehyde is a mutagen. Carcinogenesis 1983, 4, 331–333. [Google Scholar] [CrossRef]
- Shao, B.; Pennathur, S.; Pagani, I.; Oda, M.N.; Witztum, J.L.; Oram, J.F.; Heinecke, J.W. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J. Biol. Chem. 2010, 285, 18473–18484. [Google Scholar] [CrossRef]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Zagol-Ikapite, I.; Sosa, I.R.; Oram, D.; Judd, A.; Amarnath, K.; Amarnath, V.; Stec, D.; Oates, J.A.; Boutaud, O. Modification of platelet proteins by malondialdehyde: Prevention by dicarbonyl scavengers. J. Lipid Res. 2015, 56, 2196–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Sierra, C.; Laranjeira, P.; Domingues, M.R.; Paiva, A. Lipoxidation and cancer immunity. Redox Biol. 2019, 23, 101103. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.S.; Lin, S.R.; Huang, C.C.; Tseng, H.Y.; Huang, P.H.; Shi, G.Y.; Wu, H.L.; Tang, C.L.; Chu, P.H.; Wang, L.H.; et al. TXAS-deleted mice exhibit normal thrombopoiesis, defective hemostasis, and resistance to arachidonate-induced death. Blood 2004, 104, 135–142. [Google Scholar] [CrossRef]
- Saeki, K.; Yokomizo, T. Identification, signaling, and functions of LTB4 receptors. Semin. Immunol. 2017, 33, 30–36. [Google Scholar] [CrossRef]
- Cho, N.K.; Joo, Y.C.; Wei, J.D.; Park, J.I.; Kim, J.H. BLT2 is a pro-tumorigenic mediator during cancer progression and a therapeutic target for anti-cancer drug development. Am. J. Cancer Res. 2013, 3, 347–355. [Google Scholar]
- Park, J.; Jang, J.H.; Park, G.S.; Chung, Y.; You, H.J.; Kim, J.H. BLT2, a leukotriene B4 receptor 2, as a novel prognostic biomarker of triple-negative breast cancer. BMB Rep. 2018, 51, 373–377. [Google Scholar] [CrossRef]
- Kim, E.Y.; Seo, J.M.; Kim, C.; Lee, J.E.; Lee, K.M.; Kim, J.H. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic Biol. Med. 2010, 49, 1072–1081. [Google Scholar] [CrossRef]
- Yoo, M.H.; Song, H.; Woo, C.H.; Kim, H.; Kim, J.H. Role of the BLT2, a leukotriene B4 receptor, in Ras transformation. Oncogene 2004, 23, 9259–9268. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.A.; Park, G.S.; Kim, J.H. BLT2 up-regulates interleukin-8 production and promotes the invasiveness of breast cancer cells. PLoS ONE 2012, 7, e49186. [Google Scholar] [CrossRef]
- Park, G.S.; Kim, J.H. Myeloid differentiation primary response gene 88-leukotriene B4 receptor 2 cascade mediates lipopolysaccharide-potentiated invasiveness of breast cancer cells. Oncotarget 2015, 6, 5749–5759. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Fujimura, M.; Kasahara, K.; Shirasaki, H.; Heki, U.; Iwasa, K.; Ueda, A.; Matsuda, T. Up-regulation of ICH-1L protein by thromboxane A2 antagonists enhances cisplatin-induced apoptosis in non-small-cell lung-cancer cell lines. J. Cancer Res. Clin. Oncol. 1999, 125, 389–394. [Google Scholar] [CrossRef]
- Nie, D.; Che, M.; Zacharek, A.; Qiao, Y.; Li, L.; Li, X.; Lamberti, M.; Tang, K.; Cai, Y.; Guo, Y.; et al. Differential expression of thromboxane synthase in prostate carcinoma: Role in tumor cell motility. Am. J. Pathol. 2004, 164, 429–439. [Google Scholar] [CrossRef]
- Nie, D.; Lamberti, M.; Zacharek, A.; Li, L.; Szekeres, K.; Tang, K.; Chen, Y.; Honn, K.V. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem. Biophys. Res. Commun. 2000, 267, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.O.; Ziu, M.; Cargioli, T.; Westphal, M.; Giese, A.; Black, P.M.; Carroll, R.S. Inhibition of thromboxane synthase activity improves glioblastoma response to alkylation chemotherapy. Transl. Oncol. 2010, 3, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, L.; Grover, G.J.; Stier, C.T., Jr. Ifetroban sodium: An effective TxA2/PGH2 receptor antagonist. Cardiovasc. Drug Rev. 2001, 19, 97–115. [Google Scholar] [CrossRef]
- Wei, J.; Yan, W.; Li, X.; Ding, Y.; Tai, H.H. Thromboxane receptor alpha mediates tumor growth and angiogenesis via induction of vascular endothelial growth factor expression in human lung cancer cells. Lung Cancer 2010, 69, 26–32. [Google Scholar] [CrossRef]
- Li, B.H.; Xu, S.B.; Li, F.; Zou, X.G.; Saimaiti, A.; Simayi, D.; Wang, Y.H.; Zhang, Y.; Yuan, J.; Zhang, W.J. Stat6 activity-related Th2 cytokine profile and tumor growth advantage of human colorectal cancer cells in vitro and in vivo. Cell Signal. 2012, 24, 718–725. [Google Scholar] [CrossRef]
- Ushikubi, F.; Aiba, Y.; Nakamura, K.; Namba, T.; Hirata, M.; Mazda, O.; Katsura, Y.; Narumiya, S. Thromboxane A2 receptor is highly expressed in mouse immature thymocytes and mediates DNA fragmentation and apoptosis. J. Exp. Med. 1993, 178, 1825–1830. [Google Scholar] [CrossRef]
- Rocha, P.N.; Plumb, T.J.; Robinson, L.A.; Spurney, R.; Pisetsky, D.; Koller, B.H.; Coffman, T.M. Role of thromboxane A2 in the induction of apoptosis of immature thymocytes by lipopolysaccharide. Clin. Diagn Lab. Immunol. 2005, 12, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Murata, T.; Tanaka, H.; Matsuoka, T.; Sakata, D.; Yoshida, N.; Katagiri, K.; Kinashi, T.; Tanaka, T.; Miyasaka, M.; et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nat. Immunol. 2003, 4, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Moalli, F.; Cupovic, J.; Thelen, F.; Halbherr, P.; Fukui, Y.; Narumiya, S.; Ludewig, B.; Stein, J.V. Thromboxane A2 acts as tonic immunoregulator by preferential disruption of low-avidity CD4+ T cell-dendritic cell interactions. J. Exp. Med. 2014, 211, 2507–2517. [Google Scholar] [CrossRef] [Green Version]
- Konishi, Y.; Ichise, H.; Watabe, T.; Oki, C.; Tsukiji, S.; Hamazaki, Y.; Murakawa, Y.; Takaori-Kondo, A.; Terai, K.; Matsuda, M. Intravital Imaging Identifies the VEGF-TXA2 Axis as a Critical Promoter of PGE2 Secretion from Tumor Cells and Immune Evasion. Cancer Res. 2021, 81, 4124–4132. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Metastasis: Quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2’-deoxyuridine. J. Natl. Cancer Inst. 1970, 45, 773–782. [Google Scholar] [PubMed]
- Wong, C.W.; Lee, A.; Shientag, L.; Yu, J.; Dong, Y.; Kao, G.; Al-Mehdi, A.B.; Bernhard, E.J.; Muschel, R.J. Apoptosis: An early event in metastatic inefficiency. Cancer Res. 2001, 61, 333–338. [Google Scholar]
- Ruiz, P.; Rey, L.; Spurney, R.; Coffman, T.; Viciana, A. Thromboxane augmentation of alloreactive T cell function. Transplantation 1992, 54, 498–505. [Google Scholar] [CrossRef]
- Rola-Pleszczynski, M.; Gagnon, L.; Bolduc, D.; LeBreton, G. Evidence for the involvement of the thromboxane synthase pathway in human natural cytotoxic cell activity. J. Immunol. 1985, 135, 4114–4119. [Google Scholar]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef]
- Campbell, P.B.; Tolson, T.A. Modulation of human monocyte leukotactic responsiveness by thromboxane A2 and 12-hydroxyheptadecatrienoic acid (12-HHT). J. Leeeukoc. Biol. 1988, 43, 117–124. [Google Scholar] [CrossRef]
- Bayat, H.; Schroder, K.; Pimentel, D.R.; Brandes, R.P.; Verbeuren, T.J.; Cohen, R.A.; Jiang, B. Activation of thromboxane receptor modulates interleukin-1beta-induced monocyte adhesion--a novel role of Nox1. Free Radic. Biol. Med. 2012, 52, 1760–1766. [Google Scholar] [CrossRef]
- Carestia, A.; Mena, H.A.; Olexen, C.M.; Ortiz Wilczynski, J.M.; Negrotto, S.; Errasti, A.E.; Gomez, R.M.; Jenne, C.N.; Carrera Silva, E.A.; Schattner, M. Platelets Promote Macrophage Polarization toward Pro-inflammatory Phenotype and Increase Survival of Septic Mice. Cell Rep. 2019, 28, 896–908.e895. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Bae, S.K.; Park, H.J.; Kim, M.K.; Kim, K.; Park, S.Y.; Jang, H.O.; Yun, I.; Kim, Y.J.; Yoo, M.A.; et al. Thromboxane A(2) increases endothelial permeability through upregulation of interleukin-8. Biochem. Biophys. Res. Commun. 2010, 397, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.W.; Ware, G.M.; Kaul, D.K.; Ware, J.A. Inhibition of tumor necrosis factor alpha-mediated NFkappaB activation and leukocyte adhesion, with enhanced endothelial apoptosis, by G protein-linked receptor (TP) ligands. J. Biol. Chem. 2003, 278, 11858–11866. [Google Scholar] [CrossRef] [Green Version]
- Lucotti, S.; Cerutti, C.; Soyer, M.; Gil-Bernabe, A.M.; Gomes, A.L.; Allen, P.D.; Smart, S.; Markelc, B.; Watson, K.; Armstrong, P.C.; et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J. Clin. Investig. 2019, 129, 1845–1862. [Google Scholar] [CrossRef]
- Nanji, A.A. Thromboxane synthase and organ preference for metastases. N. Engl. J. Med. 1993, 329, 138–139. [Google Scholar] [CrossRef]
- Suzuki, T.; Kropski, J.A.; Chen, J.; Carrier, E.J.; Chen, X.; Sherrill, T.P.; Winters, N.I.; Camarata, J.E.; Polosukhin, V.V.; Han, W.; et al. Thromboxane-Prostanoid Receptor Signaling Drives Persistent Fibroblast Activation in Pulmonary Fibrosis. Am. J. Respir. Crit Care Med. 2022. [Google Scholar] [CrossRef]
- Feinmark, S.J.; Bailey, J.M. Lipid metabolism in cultured cells. Activators of endogenous thromboxane A2 synthesis in cultured lung fibroblasts. J. Biol. Chem. 1982, 257, 2816–2821. [Google Scholar]
- Cruz-Gervis, R.; Stecenko, A.A.; Dworski, R.; Lane, K.B.; Loyd, J.E.; Pierson, R.; King, G.; Brigham, K.L. Altered prostanoid production by fibroblasts cultured from the lungs of human subjects with idiopathic pulmonary fibrosis. Respir. Res. 2002, 3, 17. [Google Scholar] [CrossRef]
- Yaekashiwa, M.; Wang, L.H. Nrf2 regulates thromboxane synthase gene expression in human lung cells. DNA Cell Biol. 2003, 22, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, M.; Zheng, D.; Zhang, H.; Lv, Y.; Zhang, L.; Tan, H.S.; Zhou, H.; Lao, Y.Z.; Xu, H.X. Garcinol inhibits esophageal cancer metastasis by suppressing the p300 and TGF-beta1 signaling pathways. Acta Pharmacol. Sin. 2020, 41, 82–92. [Google Scholar] [CrossRef]
- Murota, S.I.; Morita, I.; Abe, M. The effects of thromboxane B2 and 6-ketoprostaglandin F1alpha on cultured fibroblasts. Biochim. Biophys. Acta 1977, 479, 122–125. [Google Scholar] [CrossRef]
- Bruggeman, L.A.; Horigan, E.A.; Horikoshi, S.; Ray, P.E.; Klotman, P.E. Thromboxane stimulates synthesis of extracellular matrix proteins in vitro. Am. J. Physiol. 1991, 261, F488–F494. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, H.J.; Hwang, S.J.; Min, H.Y.; Kang, H.N.; Park, A.Y.; Hyun, S.Y.; Sim, J.Y.; Lee, H.J.; Jang, H.J.; et al. The Interplay between Slow-Cycling, Chemoresistant Cancer Cells and Fibroblasts Creates a Proinflammatory Niche for Tumor Progression. Cancer Res. 2020, 80, 2257–2272. [Google Scholar] [CrossRef] [PubMed]
- Polgar, P.; Taylor, L. Alterations in prostaglandin synthesis during senescence of human lung fibroblasts. Mech. Ageing Dev. 1980, 12, 305–310. [Google Scholar] [CrossRef]
- Kabir, T.D.; Leigh, R.J.; Tasena, H.; Mellone, M.; Coletta, R.D.; Parkinson, E.K.; Prime, S.S.; Thomas, G.J.; Paterson, I.C.; Zhou, D.; et al. A miR-335/COX-2/PTEN axis regulates the secretory phenotype of senescent cancer-associated fibroblasts. Aging 2016, 8, 1608–1635. [Google Scholar] [CrossRef]
- Ionescu, C.; Oprea, B.; Ciobanu, G.; Georgescu, M.; Bica, R.; Mateescu, G.O.; Huseynova, F.; Barragan-Montero, V. The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview. Medicina 2022, 58, 903. [Google Scholar] [CrossRef]
- Ashton, A.W.; Yokota, R.; John, G.; Zhao, S.; Suadicani, S.O.; Spray, D.C.; Ware, J.A. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2). J. Biol. Chem. 1999, 274, 35562–35570. [Google Scholar] [CrossRef]
- Ashton, A.W.; Ware, J.A. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ. Res. 2004, 95, 372–379. [Google Scholar] [CrossRef]
- Ashton, A.W.; Cheng, Y.; Helisch, A.; Ware, J.A. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: Role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ. Res. 2004, 94, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yokota, R.; Tang, S.; Ashton, A.W.; Ware, J.A. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2). Circ. Res. 2000, 87, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.H.; Martinez-Bermudez, A.K.; Gobeil, F., Jr.; Marrache, A.M.; Hou, X.; Speranza, G.; Abran, D.; Quiniou, C.; Lachapelle, P.; Roberts, J., 2nd; et al. Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathy. J. Appl. Physiol. 2001, 90, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Shi, C.; Cohen, R.A. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 2002, 51, 198–203. [Google Scholar] [CrossRef]
- De La Cruz, J.P.; Moreno, A.; Ruiz-Ruiz, M.I.; Sánchez De La Cuesta, F. Effect of DT-TX 30, a combined thromboxane synthase inhibitor and thromboxane receptor antagonist, on retinal vascularity in experimental diabetes mellitus. Thromb. Res. 2000, 97, 125–131. [Google Scholar] [CrossRef]
- Kishi, Y.; Numano, F. In vitro study of vascular endothelial injury by activated platelets and its prevention. Atherosclerosis 1989, 76, 95–101. [Google Scholar] [CrossRef]
- Pal, S.; Wu, J.; Murray, J.K.; Gellman, S.H.; Wozniak, M.A.; Keely, P.J.; Boyer, M.E.; Gomez, T.M.; Hasso, S.M.; Fallon, J.F.; et al. An antiangiogenic neurokinin-B/thromboxane A2 regulatory axis. J. Cell Biol. 2006, 174, 1047–1058. [Google Scholar] [CrossRef]
- Benndorf, R.A.; Schwedhelm, E.; Gnann, A.; Taheri, R.; Kom, G.; Didié, M.; Steenpass, A.; Ergün, S.; Böger, R.H. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: A potential link between oxidative stress and impaired angiogenesis. Circ. Res. 2008, 103, 1037–1046. [Google Scholar] [CrossRef]
- Pradono, P.; Tazawa, R.; Maemondo, M.; Tanaka, M.; Usui, K.; Saijo, Y.; Hagiwara, K.; Nukiwa, T. Gene transfer of thromboxane A(2) synthase and prostaglandin I(2) synthase antithetically altered tumor angiogenesis and tumor growth. Cancer Res. 2002, 62, 63–66. [Google Scholar]
- Daniel, T.O.; Liu, H.; Morrow, J.D.; Crews, B.C.; Marnett, L.J. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999, 59, 4574–4577. [Google Scholar]
- De Leval, X.; Dassesse, T.; Dogné, J.M.; Waltregny, D.; Bellahcène, A.; Benoit, V.; Pirotte, B.; Castronovo, V. Evaluation of original dual thromboxane A2 modulators as antiangiogenic agents. J. Pharmacol. Exp. Ther. 2006, 318, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Tamura, K.; Kogo, H. Stimulatory effects of eicosanoids on ovarian angiogenesis in early luteal phase in cyclooxygenase-2 inhibitor-treated rats. Eur. J. Pharmacol. 2005, 516, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Båtshake, B.; Nilsson, C.; Sundelin, J. Structure and expression of the murine thromboxane A2 receptor gene. Biochem. Biophys. Res. Commun. 1999, 256, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Rocca, B.; Loeb, A.L.; Strauss, J.F., 3rd; Vezza, R.; Habib, A.; Li, H.; FitzGerald, G.A. Directed vascular expression of the thromboxane A2 receptor results in intrauterine growth retardation. Nat. Med. 2000, 6, 219–221. [Google Scholar] [CrossRef]
- Reid, H.M.; Wikstrom, K.; Kavanagh, D.J.; Mulvaney, E.P.; Kinsella, B.T. Interaction of angio-associated migratory cell protein with the TPalpha and TPbeta isoforms of the human thromboxane A(2) receptor. Cell Signal. 2011, 23, 700–717. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashton, A.W.; Zhang, Y.; Cazzolli, R.; Honn, K.V. The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection. Molecules 2022, 27, 6234. https://doi.org/10.3390/molecules27196234
Ashton AW, Zhang Y, Cazzolli R, Honn KV. The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection. Molecules. 2022; 27(19):6234. https://doi.org/10.3390/molecules27196234
Chicago/Turabian StyleAshton, Anthony W., Yunjia Zhang, Rosanna Cazzolli, and Kenneth V. Honn. 2022. "The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection" Molecules 27, no. 19: 6234. https://doi.org/10.3390/molecules27196234
APA StyleAshton, A. W., Zhang, Y., Cazzolli, R., & Honn, K. V. (2022). The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection. Molecules, 27(19), 6234. https://doi.org/10.3390/molecules27196234








