Authentication of Citrus spp. Cold-Pressed Essential Oils by Their Oxygenated Heterocyclic Components
Abstract
:1. Introduction
2. Results and Discussions
2.1. Method Validation
2.2. Comparison of Citrus EO Coumarin and Furanocoumarin Content
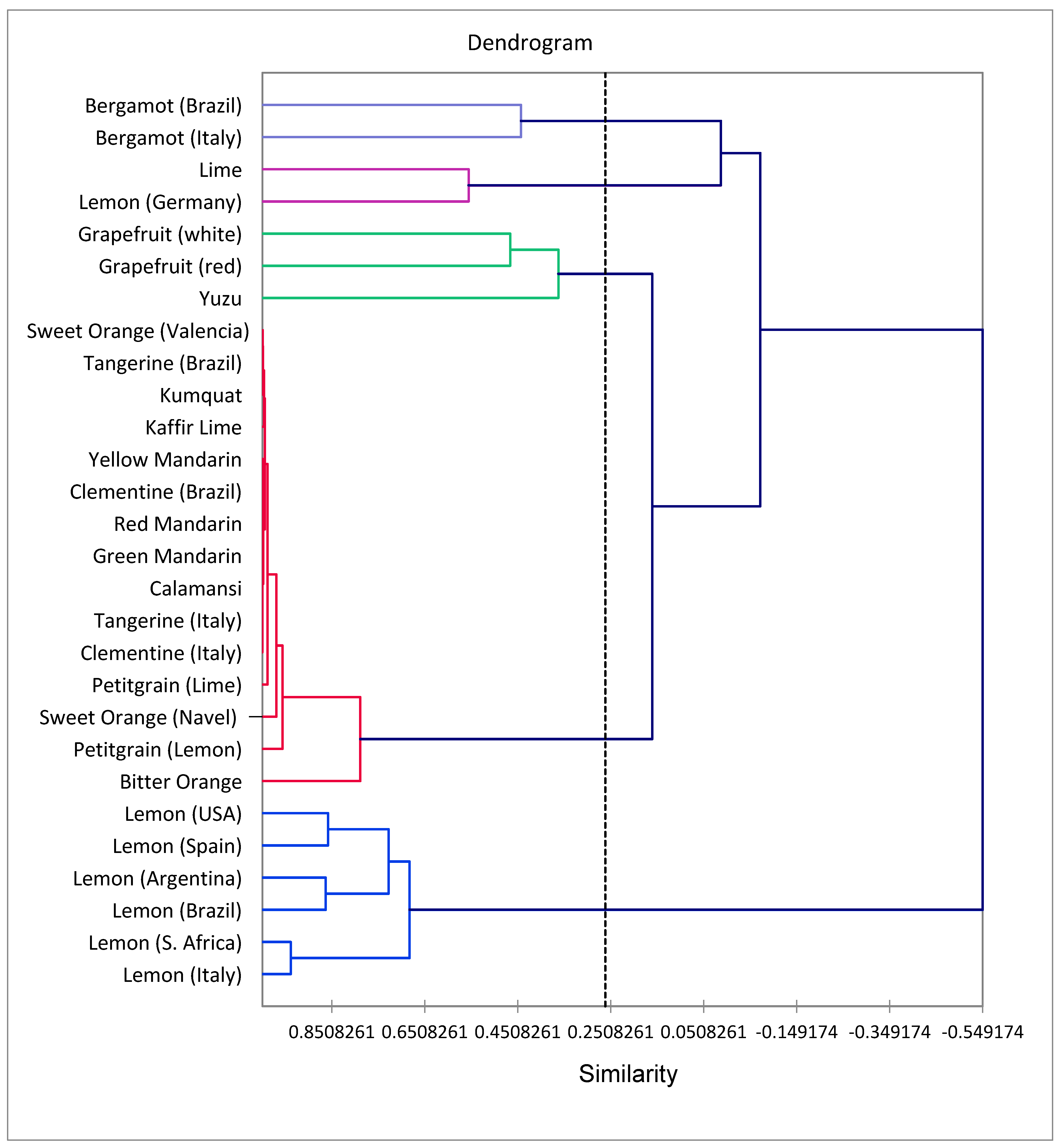
2.3. Multivariate Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Essential Oil Samples
3.3. UPLC-MS/MS Analyses
3.4. Method Validation
3.5. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHC | agglomerative hierarchical cluster analysis |
| DAD | diode array detector |
| ELISA | enzyme-linked immunosorbent assay |
| EO | essential oil |
| FC | furanocoumarin |
| GC-MS | gas chromatography–mass spectroscopy |
| HPLC | high-performance liquid chromatography |
| LC-MS | liquid chromatography- mass spectrometry |
| LOQ | limit of quantification |
| MRM | multiple reaction monitoring mode |
| NMR | nuclear magnetic resonance |
| OTUs | operational taxonomic units |
| PCA | Principal component analysis |
| ppm | parts per million |
| QC | quality control |
| RP-HPLC | reversed-phase-high-performance liquid chromatography |
| UPGMA | unweighted pair group method with arithmetic average |
| UPLC-MS/MS | ultra-performance liquid chromatography-tandem mass spectrometry |
References
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT-Food Sci. Technol. 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Perez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Pathak, M.A.; Daniels, F.; Fitzpatrick, T.B. The presently known distribution of furocoumarins (psoralens) in plants. J. Invest. Dermatol. 1962, 39, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D.H.; Méndez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry, and Biochemistry; Wiley: Chichester, UK, 1982. [Google Scholar]
- Nitao, J.K.; Berhow, M.; Duval, S.M.; Weisleder, D.; Vaughn, S.F.; Zangerl, A.; Berenbaum, M.R. Characterization of furanocoumarin metabolites in parsnip webworm, Depressaria pastinacella. J. Chem. Ecol. 2003, 29, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Ziegler, H.; Spiteller, G. Coumarins and psoralens from Sicilian lemon oil (Citrus limon (L.) Burm. f.). Flavour Fragr. J. 1992, 7, 129–139. [Google Scholar] [CrossRef]
- Saita, T.; Fujito, H.; Mori, M. Screening of furanocoumarin derivatives in Citrus fruits by enzyme-linked immunosorbent assay. Biol. Pharm. Bull. 2004, 27, 974–977. [Google Scholar] [CrossRef]
- Thompson, H.J.; Brown, S.A. Separations of some coumarins of higher plants by liquid chromatography. J. Chromatogr. A 1984, 314, 323–336. [Google Scholar] [CrossRef]
- Kamiński, M.; Kartanowicz, R.; Kamiński, M.M.; Królicka, A.; Sidwa-Gorycka, M.; Łojkowska, E.; Gorzeń, W. HPLC-DAD in identification and quantification of selected coumarins in crude extracts from plant cultures of Ammi majus and Ruta graveolens. J. Sep. Sci. 2003, 26, 1287–1291. [Google Scholar] [CrossRef]
- Frérot, E.; Decorzant, E. Quantification of total furocoumarins in Citrus oils by HPLC coupled with UV, fluorescence and mass detection. J. Agric. Food Chem. 2004, 52, 6879–6886. [Google Scholar] [CrossRef]
- Sommer, J.; Bertram, H.J.; Krammer, G.; Kindel, G.; Kuhnle, T.; Reinders, G.; Reiss, I.; Schmidt, C.O.; Schreiber, K.; Stumpe, W.; et al. HPLC–NMR—a powerful tool for the identification of non-volatiles in lemon peel oils. Perfum. Flavor 2003, 38, 18–34. [Google Scholar]
- Dugrand, A.; Olry, A.; Duval, T.; Hehn, A.; Froelicher, Y.; Bourgaud, F. Coumarin and furanocoumarin quantitation in Citrus peel via ultraperformance liquid chromatography coupled with mass spectrometry (UPLC-MS). J. Agric. Food Chem. 2013, 61, 10677–10684. [Google Scholar] [CrossRef] [PubMed]
- Dugrand-Judek, A.; Olry, A.; Hehn, A.; Costantino, G.; Ollitrault, P.; Froelicher, Y.; Bourgaud, F. The distribution of coumarins and furanocoumarins in Citrus species closely matches Citrus phylogeny and reflects the organization of biosynthetic pathways. PLoS ONE 2015, 10, e0142757. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Mondello, L.; Dugo, L.; Stancanelli, R.; Dugo, G. LC-MS for the identification of oxygen heterocyclic compounds in Citrus essential oils. J. Pharm. Biomed. Anal. 2000, 24, 147–154. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Q.; Simon, J.E.; Lou, S.-N.; Ho, C.-T. Authenticity analysis of Citrus essential oils by HPLC-UV-MS on oxygenated heterocyclic components. J. Food Drug Anal. 2015, 23, 30–39. [Google Scholar] [CrossRef]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef]
- Lawrence, B.M. Progress in Essential Oils. Perfum. Flavorist 2004, 29, 44–59. [Google Scholar]
- Dugo, P.; Mondello, L.; Sebastiani, E.; Ottanà, R.; Errante, G.; Dugo, G. Identification of minor oxygen heterocyclic compounds of Citrus essential oils by liquid chromatography-atmospheric pressure chemical ionisation mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 2991–3005. [Google Scholar] [CrossRef]
- Verzera, A.; la Rosa, G.; Zappala, M.; Cotroneo, A. Essential oil composition of different cultivars of bergamot grown in Sicily. Ital J. Food Sci. 1999, 12, 493–501. [Google Scholar]
- Bonaccorsi, I.; Sciarrone, D.; Cotroneo, A.; Mondello, L.; Dugo, P.; Dugo, G. Enantiomeric distribution of key volatile components of Citrus essential oils. Rev. Bras. Farmacogn. (Brasil J. Pharmacogn.) 2011, 21, 841–849. [Google Scholar] [CrossRef]
- Russo, M.; Torre, G.; Carnovale, C.; Bonaccorsi, I.; Mondello, L.; Dugo, P. A New HPLC method developed for the analysis of oxygen heterocyclic compounds in Citrus essential oils. J. Essent. Oil Res. 2012, 24, 119–129. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Proteggente, A.R.; Cavazza, A.; Dugo, G. Oxygen heterocyclic compounds of bergamot essential oils. Rivista Italiana EPPOS 1999, 27, 31–41. [Google Scholar]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Isolation of furocoumarins from bergamot fruits as HL-60 differentiation-inducing compounds. J. Agric. Food Chem 1999, 47, 4073–4078. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, C.; Postorino, E.; Gionfriddo, F.; Catalfamo, M.; Manganaro, R. Evaluation of the genuineness of cold-pressed bergamot oil. Perfum. Flav. 2009, 34, 26–32. [Google Scholar]
- Costa, R.; Dugo, P.; Navarra, M.; Raymo, V.; Dugo, G.; Mondello, L. Study on the chemical composition variability of some processed bergamot (Citrus bergamia) essential oils. Flav. Fragr. J. 2010, 25, 4–12. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Provenzano, E.; Cindio, B.D.; Menichini, F. In vitro photo-induced cytotoxic activity of Citrus bergamia and C. medica L. cv. diamante peel essential oils and identified active coumarins. Pharm. Biol. 2010, 48, 1059–1065. [Google Scholar] [CrossRef]
- Dugo, G.; Bonaccorsi, I.; Sciarrone, D.; Schipilliti, L.; Russo, M.; Cotroneo, A.; Dugo, P.; Mondello, L.; Raymo, V. Characterization of cold-pressed and processed bergamot oils by using GC-FID, GC-MS, GC-C-IRMS, Enantio-GC, MDGC, HPLC and HPLC-MS-IT-TOF. J. Essent. Oil Res. 2012, 24, 93–117. [Google Scholar] [CrossRef]
- USP <1225> Validation of Compendial Procedures. 2021. Available online: https://latam-edu.usp.org/wp-content/uploads/2021/08/1225.pdf (accessed on 18 September 2022).
- ICH Expert Working Group, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Ich Harmonised Tripartite Guideline Validation of Analytical Procedures: Text and Methodology Q2(R1). 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 18 September 2022).
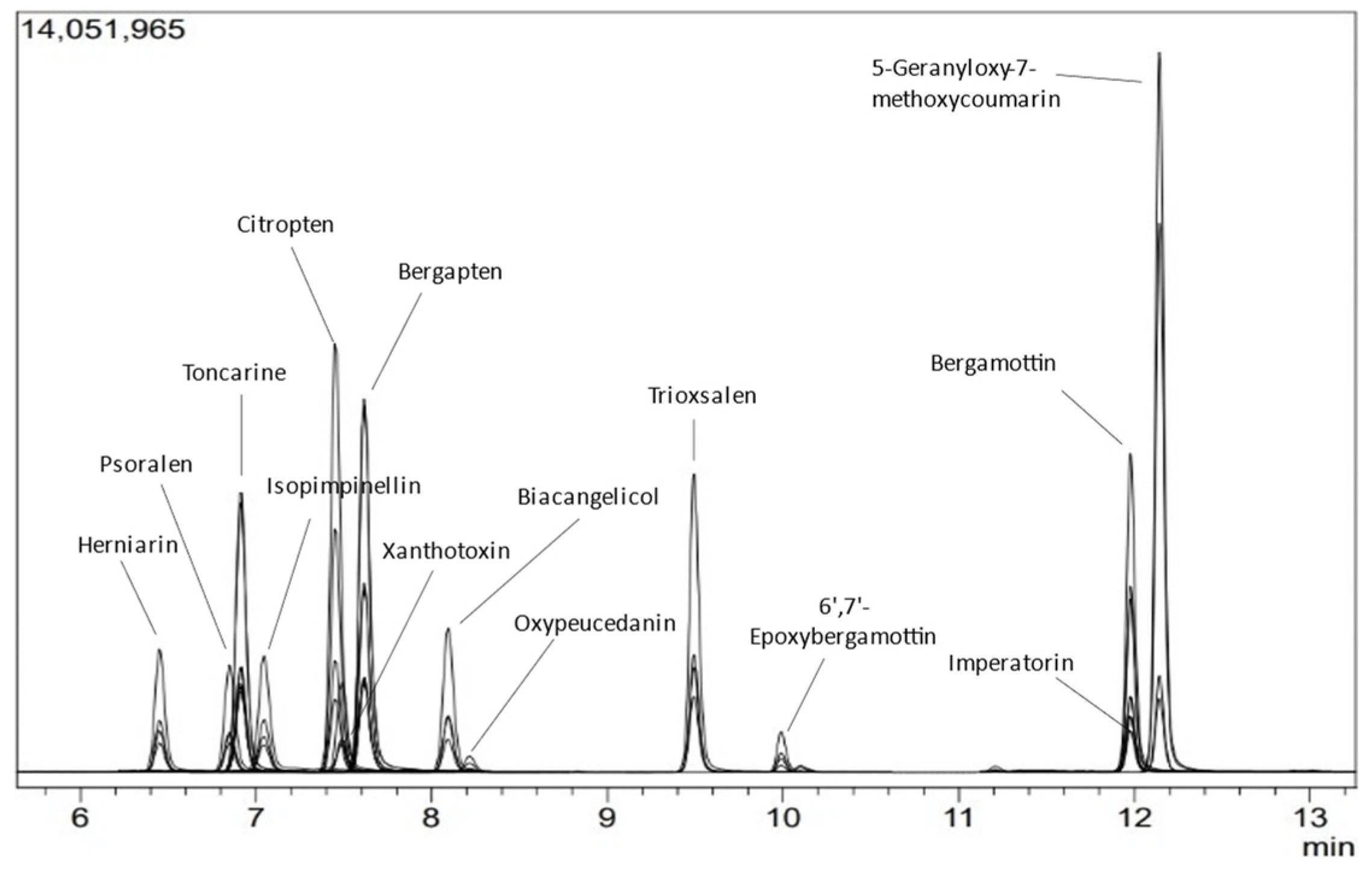

| Compound | Linearity | LOQ (ppm) | Accuracy | Precision | Intermediate Precision | ||
|---|---|---|---|---|---|---|---|
| Linear Range (ppm) | Equation | r2 | Recovery % | RSD% | RSD% | ||
| Coumarins | |||||||
| Citropten | 0.001–0.1 | Y = 0.9847x + 0.0012 | 0.9991 | 0.001 | 98.92–113.80 | 2.60 | 4.44 |
| 5-Geranyloxy-7-methoxycoumarin | 0.0001–0.1 | Y = 0.9976x + 0.0002 | 0.9989 | 0.0001 | 94.07–105.44 | 2.23 | 2.05 |
| Toncarine | 0.005–0.1 | Y = 0.9961x + 0.0003 | 0.9991 | 0.005 | 97.50–114.53 | 1.28 | 2.44 |
| Herniarin | 0.001–0.1 | Y = 0.989x + 0.0008 | 0.9996 | 0.001 | 98.25–113.40 | 2.33 | 4.90 |
| Linear furanocoumarins | |||||||
| 6’,7’-Epoxybergamottin | 0.001–0.1 | Y = 0.9948x + 0.0004 | 0.9990 | 0.001 | 96.83–109.00 | 2.29 | 1.05 |
| Bergamottin | 0.001–0.1 | Y = 0.9975x + 0.0002 | 0.9988 | 0.001 | 95.75–104.56 | 2.26 | 3.05 |
| Bergapten | 0.0001–0.1 | Y = 0.9956x + 0.0003 | 0.9994 | 0.0001 | 96.92–109.33 | 2.50 | 0.46 |
| Biacangelicol | 0.001–0.1 | Y = 0.9923x + 0.0006 | 0.9992 | 0.001 | 97.17–107.27 | 2.18 | 2.32 |
| Imperatorin | 0.001–0.1 | Y = 0.9904x + 0.0007 | 0.9997 | 0.001 | 96.40–106.67 | 2.59 | 2.32 |
| Isopimpinellin | 0.0001–0.1 | Y = 1.0002x + 8x10-6 | 0.9989 | 0.0001 | 95.75–112.13 | 2.89 | 2.26 |
| Oxypeucedanin | 0.005–0.1 | Y = 0.9946x + 0.0004 | 0.9987 | 0.005 | 96.33–107.73 | 2.62 | 1.17 |
| Psoralen | 0.001–0.1 | Y = 0.9936x + 0.0005 | 0.9985 | 0.001 | 97.00–113.00 | 2.38 | 2.94 |
| Trioxsalen | 0.001–0.1 | Y = 0.993x + 0.0005 | 0.9997 | 0.001 | 98.50–106.11 | 2.18 | 2.06 |
| Xanthotoxin | 0.001–0.1 | Y = 0.9874x + 0.001 | 0.9997 | 0.001 | 98.58–112.07 | 3.67 | 2.54 |
| Citrus Oil | Total Coumarin (ppm) | Total FC (ppm) | Coumarin Distribution |
|---|---|---|---|
| Bergamot (Brazil) | 52,473.90 ± 1775.63 | 48,798.90 ± 174.98 | Bergamottin > imperatorin > bergapten > 5-geranyloxy-7-methoxycoumarin > citropten > xanthotoxin > 6’,7’-epoxybergamottin > herniarin > psoralen > oxypeucedanin > isopimpinellin > biacangelicol |
| Bergamot (Italy) | 171,453.11 ± 9227.11 | 167,281.60 ± 1017.74 | Bergamottin > imperatorin > 6’,7’-epoxybergamottin > 5-geranyloxy-7-methoxycoumarin > citropten > xanthotoxin > bergapten > herniarin > oxypeucedanin > isopimpinellin > psoralen > biacangelicol |
| Bitter Orange | 814.95 ± 9.52 | 809.21 ± 1.30 | 6’,7’-Epoxybergamottin > xanthotoxin > bergapten > imperatorin > bergamottin > citropten > psoralen > 5-geranyloxy-7-methoxycoumarin > herniarin > isopimpinellin |
| Calamansi | 0.15 ± 0.02 | 0 | 5-Geranyloxy-7-methoxycoumarin |
| Clementine (Brazil) | 75.26 ± 0.08 | 43.03 ± 0.11 | 5-Geranyloxy-7-methoxycoumarin > bergamottin > imperatorin > citropten > oxypeucedanin > xanthotoxin > bergapten > herniarin > isopimpinellin |
| Clementine (Italy) | 4.69 ± 0.03 | 3.38 ± 0.04 | Oxypeucedanin > bergamottin > citropten > xanthotoxin > biacangelicol > bergapten > 5-geranyloxy-7-methoxycoumarin > psoralen |
| Grapefruit (Red) | 13,099.29 ± 207.97 | 13,013.55 ± 22.87 | 6’,7’-Epoxybergamottin > bergamottin > imperatorin > oxypeucedanin > biacangelicol > xanthotoxin > bergapten > 5-geranyloxy-7-methoxycoumarin > citropten > isopimpinellin > psoralen > herniarin |
| Grapefruit (White) | 9163.08 ± 229.85 | 9027.29 ± 25.14 | 6’,7’-Epoxybergamottin > imperatorin > bergamottin > oxypeucedanin > biacangelicol > xanthotoxin > psoralen > bergapten > herniarin > 5-geranyloxy-7-methoxycoumarin > isopimpinellin > citropten |
| Kaffir Lime | 75.46 ± 5.13 | 43.15 ± 0.38 | Imperatorin > citropten > bergamottin > 5-geranyloxy-7-methoxycoumarin > xanthotoxin > bergapten > oxypeucedanin > herniarin > isopimpinellin > psoralen |
| Kumquat | 169.65 ± 0.72 | 93.19 ± 0.53 | Bergamottin > 5-geranyloxy-7-methoxycoumarin > imperatorin > citropten > xanthotoxin > bergapten > oxypeucedanin > herniarin > isopimpinellin > 6′,7′-epoxybergamottin > biacangelicol |
| Lemon (Argentina) | 5404.76 ± 3.60 | 3861.29 ± 3.41 | Imperatorin > bergamottin > citropten > oxypeucedanin > 5-geranyloxy-7-methoxycoumarin > biacangelicol > 6′,7′-epoxybergamottin > herniarin > xanthotoxin > bergapten > isopimpinellin |
| Lemon (Brazil) | 3321.86 ± 1.84 | 2335.29 ± 1.77 | Imperatorin > bergamottin > citropten > 5-geranyloxy-7-methoxycoumarin > oxypeucedanin> biacangelicol > 6′,7′-epoxybergamottin > xanthotoxin > bergapten > herniarin > isopimpinellin |
| Lemon (Germany) | 3107.99 ± 3.27 | 2029.93 ± 2.61 | Imperatorin > bergamottin > 5-geranyloxy-7-methoxycoumarin > citropten > oxypeucedanin > 6′,7′-epoxybergamottin > biacangelicol > herniarin > xanthotoxin > bergapten > isopimpinellin |
| Lemon (Italy) | 10,874.88 ± 8.28 | 8346.28 ± 9.30 | Bergamottin > imperatorin > oxypeucedanin > 5-geranyloxy-7-methoxycoumarin > citropten > biacangelicol > 6′,7′-epoxybergamottin > xanthotoxin > bergapten > herniarin > isopimpinellin > psoralen |
| Lemon (South Africa) | 4268.48 ± 2.13 | 3185.78 ± 2.81 | Oxypeucedanin > imperatorin > bergamottin > citropten > 5-geranyloxy-7-methoxycoumarin > biacangelicol > 6′,7′-epoxybergamottin > xanthotoxin > bergapten > herniarin > isopimpinellin |
| Lemon (Spain) | 3343.46 ± 4.76 | 2467.31 ± 4.28 | Imperatorin > bergamottin > 5-geranyloxy-7-methoxycoumarin > citropten > oxypeucedanin > 6′,7′-epoxybergamottin > biacangelicol > xanthotoxin > bergapten > herniarin > isopimpinellin |
| Lemon (USA) | 2717.40 ± 4.45 | 1985.52 ± 4.93 | Imperatorin > bergamottin > citropten > 5-geranyloxy-7-methoxycoumarin > oxypeucedanin > biacangelicol > 6′,7′-epoxybergamottin > xanthotoxin > bergapten > herniarin > isopimpinellin > psoralen |
| Lime | 23,795.43 ± 564.22 | 16,725.07 ± 43.80 | Bergamottin > imperatorin > 5-geranyloxy-7-methoxycoumarin > citropten > oxypeucedanin > xanthotoxin > herniarin > bergapten > isopimpinellin > 6′,7′-epoxybergamottin > biacangelicol > psoralen |
| Mandarin (Green) | 32.27 ± 0.35 | 22.77 ± 0.46 | Imperatorin > bergamottin > 5-geranyloxy-7-methoxycoumarin > citropten > xanthotoxin > oxypeucedanin > herniarin > bergapten > isopimpinellin > biacangelicol |
| Mandarin (Red) | 27.42 ± 0.06 | 19.06 ± 0.08 | Imperatorin > bergamottin > 5-geranyloxy-7-methoxycoumarin > citropten > oxypeucedanin > xanthotoxin > bergapten > herniarin > isopimpinellin > biacangelicol |
| Mandarin (Yellow) | 52.89 ± 0.04 | 37.96 ± 0.05 | Imperatorin > bergamottin > 5-geranyloxy-7-methoxycoumarin > citropten > xanthotoxin > bergapten > herniarin > oxypeucedanin > isopimpinellin > biacangelicol |
| Petitgrain (Lemon) | 36.22 ± 2.10 | 20.74 ± 0.18 | Herniarin > imperatorin > citropten > bergapten > xanthotoxin > bergamottin > psoralen > 5-geranyloxy-7-methoxycoumarin > oxypeucedanin > isopimpinellin > biacangelicol |
| Petitgrain (Lime) | 58.93 ± 1.37 | 47.47 ± 0.11 | Imperatorin > xanthotoxin > citropten > bergapten > isopimpinellin > bergamottin > 5-geranyloxy-7-methoxycoumarin > herniarin > oxypeucedanin > psoralen |
| Sweet Orange (Navel) | 179.26 ± 9.94 | 140.13 ± 0.75 | 6′,7′-Epoxybergamottin > bergamottin > oxypeucedanin > imperatorin > 5-geranyloxy-7-methoxycoumarin > citropten > biacangelicol > xanthotoxin > bergapten > isopimpinellin > herniarin > psoralen |
| Sweet Orange (Valencia) | 122.27 ± 2.29 | 68.75 ± 0.19 | 5-Geranyloxy-7-methoxycoumarin > imperatorin > bergamottin > citropten > oxypeucedanin > xanthotoxin > bergapten > herniarin > isopimpinellin > 6′,7′-epoxybergamottin > biacangelicol |
| Tangerine (Brazil) | 149.45 ± 1.58 | 94.31 ± 1.45 | Imperatorin > bergamottin > 5-geranyloxy-7-methoxycoumarin > citropten > xanthotoxin > bergapten > 6′,7′-epoxybergamottin > oxypeucedanin > herniarin > isopimpinellin > biacangelicol > psoralen |
| Tangerine (Italy) | 2.44 ± 0.02 | 1.95 ± 0.03 | Bergamottin > bergapten > xanthotoxin > oxypeucedanin > citropten > 6′,7′-epoxybergamottin > 5-geranyloxy-7-methoxycoumarin |
| Yuzu | 609.06 ± 0.33 | 597.1 ± 0.41 | 6′,7′-Epoxybegamottin > biacangelicol > oxypeucedanin > xanthotoxin > bergapten > imperatorin > citropten > bergamottin > herniarin > 5-Geranyloxy-7-methoxycoumarin > isopimpinellin |
| Citrus Oil | Non-Volatile Components | Reported Amount | Reference(s) |
|---|---|---|---|
| Bitter orange | Bergapten Epoxybergamottin Psoralen | 0.035–0.073% 0.082% 0.007% | [3] |
| Bergamot CP | 5-Geranloxy-7-methoxycoumarin 5-Methoxy-7-geranoxycoumarin Bergamottin Bergaptol Psoralen Bergapten Citropten | 0.08–0.68% 0.04–0.15% 0.68–2.75% 0–0.19% 0–0.0026% 0.11–0.33% 0.01–0.35% | [3] |
| Lemon | 5-Geranloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Byakangelicol Bergapten Citropten Isopimpinellin oxypeucedanin | 0.18–0.28% 0.01–0.045% 0.16–0.54% 0.006–0.16% 0.0001–0.035% 0.05–0.17% 0–0.011% 0.09–0.82% | [3] |
| Lime | 5-Geranloxy-7-methoxycoumarin 5-Geranoxy-8-methoxypsoralen 8-Geranyloxypsoralen 5-Methoxy-7-geranoxycoumarin Bergamottin Bergapten Citropten Isopimpinellin oxypeucedanin | 1.7–3.2% 0.2–0.9% 0.10–0.14% 1.7–5.2% 1.7–3.0% 0.17–0.33% 0.4–2.2% 0.1–1.3% 0.02–0.3% | [3] |
| Grapefruit | Bergamottin Epoxybergamottin Bergapten | <0.11% 0.1126% 0.012–0.19% | [3] |
| Mandarin | Bergamottin Bergapten | 0–0.001% 0–0.0003% | [3] |
| Mandarin CO2 | Bergapten Citropten | 0.07% 0.76% | [20] |
| Lemon (coastal) | Bergapten Citropten Herniarin Isopimpinellin | 0–10 ppm 700–1300 ppm 0–10 ppm 0–5 ppm | [21] |
| Lemon (desert) | Bergapten Citropten Herniarin Isopimpinellin | 50–350 ppm 700–1700 ppm <10 ppm 35–110 ppm | [21] |
| Lemon | 5-Geranoxy-7-methoxycoumarin 5-Isopent-2′-enyloxy-8-(2′,3′-epoxyisopentyloxypsoralen) 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Byakangelicol Citropten Isoimperatorin Oxypeucedanin Oxypeucedanin hydrate | 1800–2500 ppm 190–370 ppm tr 190–360 ppm 1600–1910 ppm 660–1230 ppm 520–1420 ppm tr 890–1570 ppm tr | [21] |
| Lemon | 5-Geranoxy-7-methoxycoumarin 5-Isopent-2′-enyloxy-8-(2′,3′-epoxyisopentyloxypsoralen) 8-Geranyloxypsoralen Bergamottin Byakangelicol Citropten Oxypeucedanin | 2453–2845 ppm 204–324 ppm 399–454 ppm 2635–2973 ppm 555–1640 ppm 659–1495 ppm 863–2200 ppm | [22] |
| Lime oil (Mexican type B) | 5-Geranoxy-7-methoxycoumarin 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Bergapten Byakangelicol Citropten Cnidicin Herniarin Imperatorin Isoimperatorin Isopimpinellin Oxypeucedanin Oxypeucedanin hydrate | 27,770–45,350 ppm 2100–2790 ppm 3800–4540 ppm 25,320–41,590 ppm 2160–3920 ppm 80–1020 ppm 5940–10,950 ppm 70–250 ppm 3350–4670 ppm 380–660 ppm 70–410 ppm 3010–7300 ppm 6660–10,720 ppm 1620–1710 ppm | [23] |
| Lime (type A) | 5-Geranoxy-7-methoxycoumarin 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Bergapten Byakangelicol Citropten Cnidicin Herniarin Imperatorin Isopimpinellin Oxypeucedanin Oxypeucedanin hydrate | 41,550–63,320 ppm 4170–4830 ppm 6520–8100 ppm 37,300–56,130 ppm 2000–3450 ppm 0–90 ppm 7350–11,740 ppm 90–340 ppm 1460–2970 ppm 830–900 ppm 5670–10,210 ppm 0–260 ppm 780–1160 ppm | [23] |
| Key lime CP | 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Bergapten Byakangelicol Citropten Cnidicin Cnidilin Herniarin Imperatorin Isoimperatorin Isopimpinellin Oxypeucedanin Oxypeucedanin hydrate | 2790 ± 15 ppm 4470 ± 28.7 ppm 36,401 ± 150.9 ppm 3000 ± 31.1 ppm 92 ± 9.9 ppm 10,950 ± 92.8 ppm 250 ± 62 ppm 249 ± 7.6 ppm 3880 ± 45.8 ppm 39 ± 10.3 ppm 88 ± 5.9 ppm 7300 ± 46.9 ppm 10,600 ± 85.1 ppm 1690 ± 203 ppm | [24] |
| Key Lime (type A) | 5-Geranoxy-7-methoxycoumarin 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Bergapten Citropten Cnidilin Herniarin Isoimperatorin Isopimpinellin Oxypeucedanin hydrate Xanthotoxin | 306.5–404.5 ppm <0.1 ppm <0.1 ppm 315.7–328.3 ppm 10–12.4 ppm 49.1–63.2 ppm 2.5–3.5 ppm 8.6–9.6 ppm <0.1 ppm 35–36.5 ppm <0.1 ppm <0.1 ppm | [25] |
| Key Lime (type A) | 5-Geranoxy-7-methoxycoumarin 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Bergapten Citropten Cnidilin Herniarin Isoimperatorin Isopimpinellin Oxypeucedanin Oxypeucedanin hydrate Xanthotoxin | 409.3 ppm <0.1 ppm <0.1 ppm 315.4 ppm 8.9 ppm 48.4 ppm 2.4 ppm 7.4 ppm <0.1 ppm 33.1 ppm 14.4 ppm <0.1 ppm <0.1 ppm | [25] |
| Persian Lime | 5-Geranoxy-7-methoxycoumarin 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Bergapten Citropten Cnidilin Herniarin Isoimperatorin Isopimpinellin Oxypeucedanin Oxypeucedanin hydrate Xanthotoxin | 194.3–378 ppm <0.1 ppm <0.1 ppm 222.1–391.8 ppm 15.8–25 ppm 32.6–56.9 ppm 0.5–0.8 ppm 33.9–59.4 ppm <0.1 ppm 16.9–29.3 ppm 21–32.8 ppm <0.1 ppm <0.1 ppm | [25] |
| Bergamot oil (Italian) | 5-Geranoxy-7-methoxycoumarin Bergamottin Bergapten Citropten | 0.14–0.18% 1.37–1.6% 0.18–0.21% 0.18–0.26% | [25] |
| Bergamot CP | 5-Geranoxy-7-methoxycoumarin Bergamottin Bergapten Citropten | 8–27 ppm 100–275 ppm 10–32 ppm 12–35 ppm | [25] |
| Bergamot oil (commercial) | 5-Geranoxy-7-methoxycoumarin 5-Geranyloxy-8-methoxypsoralen 5-Isopentenyl-8-(2′,3′-dihydroxyisopentyloxy)psoralen 5-Isopentenyloxy-7-methoxycoumarin 8-Geranyloxypsoralen Bergamottin Bergapten Citropten Herniarin Isopimpinellin Oxypeucedanin | 18–37 ppm <5 ppm <5 ppm <5 ppm <5 ppm 68–116 ppm 4–10 ppm 10–13 ppm <5 ppm <5 ppm <5 ppm | [25] |
| Bergamot oil | Bergamottin Bergapten Citropten | 96.7 ug/100mg 152.5 ug/100mg 21.7 ug/100mg | [26] |
| Bergamot | Bergamottin Bergapten Epoxybergamottin Oxypeucedanin | 16,312 ppm 8 ppm 70.3 ppm 53.5 ppm | [12] |
| Bergamot | 5-Geranoxy-7-methoxycoumarin Bergamottin Bergapten Citropten | 0.08–0.104% 1.097–1.409% 0.138–0.209% 0.134–0.212% | [27] |
| Bergamot | 5-Geranoxy-7-methoxycoumarin Bergamottin Bergapten Citropten Herniarin | 0–2.827 ppm 0–39.203 ppm 0–4.215 ppm 0–6.134 ppm 0–0.251 ppm | [28] |
| Bergamot | Bergapten Citropten | 1.70% 0.40% | [29] |
| Bergamot | 5-Geranoxy-7-methoxycoumarin Bergamottin Bergapten Citropten | 0–3 ppm 0–37 ppm 0–268 ppm 0–14 ppm | [30] |
| Bergamot | 5-Geranoxy-7-methoxycoumarin Bergamottin Bergapten Citropten Herniarin | 1065 ± 7.5 ppm 19,605 ± 73.2 ppm 2474 ± 28.4 ppm 2232 ± 26.3 ppm 67 ± 3.2 ppm | [24] |
| Bergamot | Bergamottin Bergapten Citropten | 1.14–2.73% 0.06–0.4% 0.1–0.3% | [25] |
| Citrus Oil | Scientific Name | No. of Samples | Origin |
|---|---|---|---|
| Calamansi | Citrus × microcarpa (Bunge) Wijnands | 5 | Philippines |
| Tangerine | Citrus tangerina Hort. Ex Tanaka | 13 | Brazil |
| Kumquat | Citrus japonica Thunb | 3 | Brazil |
| Mandarin | Citrus reticulata Blanco | 33 | Brazil |
| Clementine | Citrus clementina Hort. Ex Tanaka | 6 | Brazil |
| Yuzu or Yuja | Citrus junos Sieb. Ex Tanaka | 16 | Brazil |
| Bitter Orange | Citrus aurantium L. | 6 | Japan |
| Sweet Orange | Citrus sinensis L. | 36 | Brazil |
| Lime | Citrus aurantifolia (Christm.) Swingle | 28 | Brazil |
| Bergamot | Citrus bergamia Risso & Poit | 66 | Italy and Brazil |
| Grapefruit | Citrus × paradisi Macfady | 45 | South Africa and USA |
| Lemon | Citrus limon Osbeck | 97 | Spain, Argentina, Brazil, Italy, USA, South Africa, and Germany |
| Petitgrain | Citrus aurantifolia leaf and Citrus limon leaf | 20 | Paraguay |
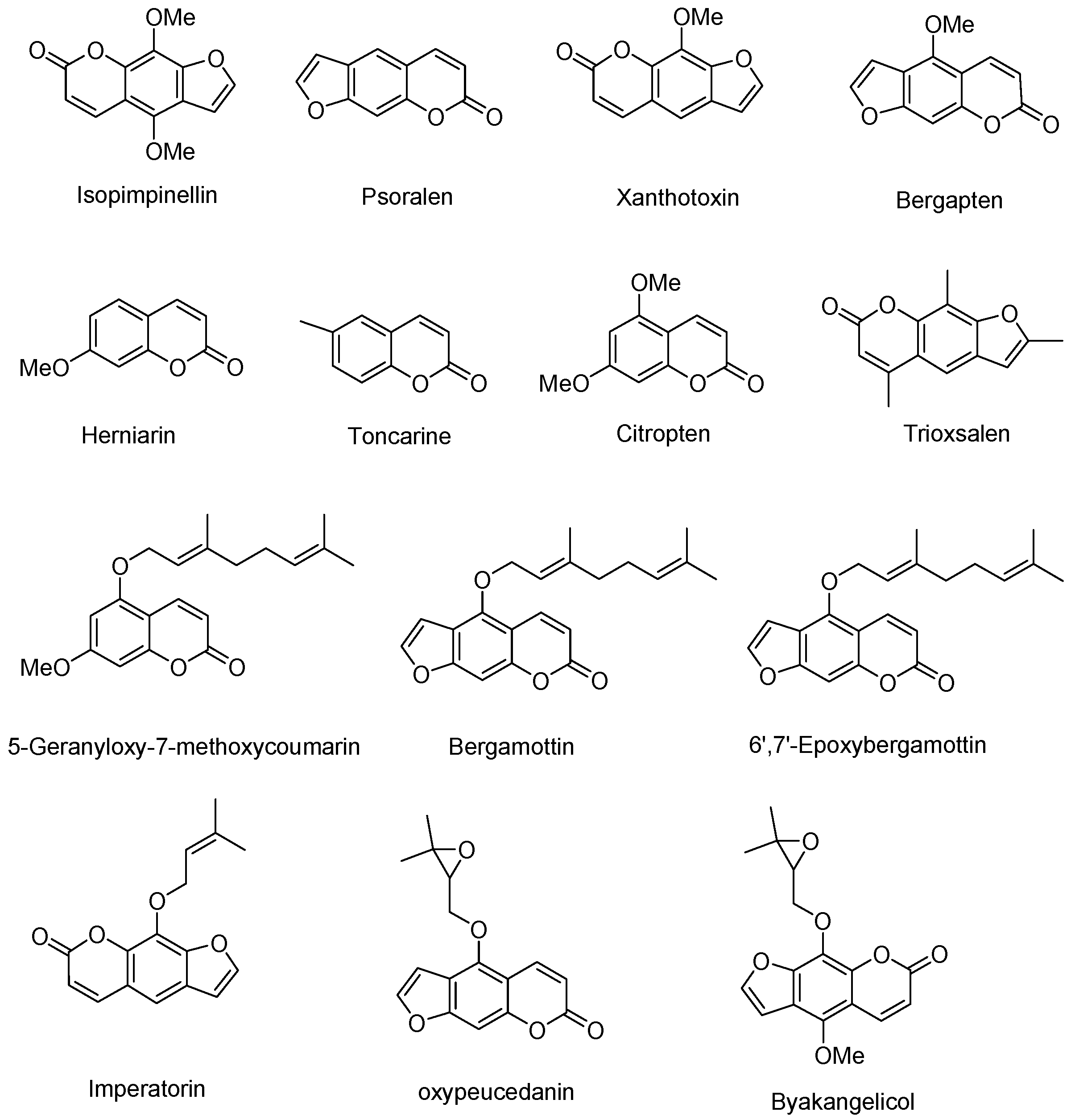
| Name | Other Name(s) | CAS # | Precursor (m/z) | Product 1 (m/z) | Product 2 (m/z) | Product 3 (m/z) | RT (min) |
|---|---|---|---|---|---|---|---|
| Coumarins | |||||||
| Citropten or Limettin | 5,7-dimethoxycoumarin | 487-06-9 | 206.90 | 192.10 | 149.10 | 121.15 | 7.61 |
| 5-Geranyloxy-7-methoxycoumarin | 7380-39-4 | 328.90 | 193.10 | 137.05 | 149.10 | 12.25 | |
| Herniarin | 7-Methoxycoumarin | 531-59-9 | 176.90 | 121.05 | 78.10 | 77.10 | 6.58 |
| Toncarine | 6-Methylcoumarin | 92-48-8 | 160.90 | 105.05 | 76.95 | 115.05 | 7.18 |
| Linear furanocoumarins | |||||||
| Xanthotoxin | 8-methoxypsoralen | 298-81-7 | 216.90 | 89.05 | 174.10 | 202.10 | 7.74 |
| Bergamottin | 5-geranyloxypsoralen | 7380-40-7 | 339.00 | 203.00 | 147.05 | 91.15 | 12.09 |
| Oxypeucedanin | 5-(2l,3l-epoxyisopentyloxy)psoralen | 26091-73-6 | 286.90 | 202.90 | 147.20 | 91.20 | 8.34 |
| Biacangelicol or Byakangelicol | 5-methoxy-8-(2l,3l-epoxyisopentyloxy) psoralen | 26091-79-2 | 317.00 | 233.05 | 231.10 | 218.10 | 8.21 |
| Psoralen | 66-97-7 | 186.90 | 131.10 | 77.10 | 115.10 | 6.98 | |
| Isopimpinellin | 482-27-9 | 246.90 | 216.95 | 232.05 | 189.05 | 7.57 | |
| Bergapten | 484-20-8 | 216.90 | 202.10 | 174.10 | 89.05 | 7.75 | |
| Imperatorin | 8-isopentenyloxypsoralen | 482-44-0 | 202.90 | 91.15 | 91.15 | 65.10 | 12.09 |
| Trioxsalen | 3902-71-4 | 229.00 | 115.15 | 142.20 | 128.10 | 9.62 | |
| 6′,7′-Epoxybergamottin | 206978-14-5 | 354.90 | 203.10 | 153.15 | 147.10 | 10.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosoky, N.S.; Satyal, P.; Setzer, W.N. Authentication of Citrus spp. Cold-Pressed Essential Oils by Their Oxygenated Heterocyclic Components. Molecules 2022, 27, 6277. https://doi.org/10.3390/molecules27196277
Dosoky NS, Satyal P, Setzer WN. Authentication of Citrus spp. Cold-Pressed Essential Oils by Their Oxygenated Heterocyclic Components. Molecules. 2022; 27(19):6277. https://doi.org/10.3390/molecules27196277
Chicago/Turabian StyleDosoky, Noura S., Prabodh Satyal, and William N. Setzer. 2022. "Authentication of Citrus spp. Cold-Pressed Essential Oils by Their Oxygenated Heterocyclic Components" Molecules 27, no. 19: 6277. https://doi.org/10.3390/molecules27196277
APA StyleDosoky, N. S., Satyal, P., & Setzer, W. N. (2022). Authentication of Citrus spp. Cold-Pressed Essential Oils by Their Oxygenated Heterocyclic Components. Molecules, 27(19), 6277. https://doi.org/10.3390/molecules27196277










