Monitoring Compositional Changes in Black Soldier Fly Larvae (BSFL) Sourced from Different Waste Stream Diets Using Attenuated Total Reflectance Mid Infrared Spectroscopy and Chemometrics
Abstract
1. Introduction
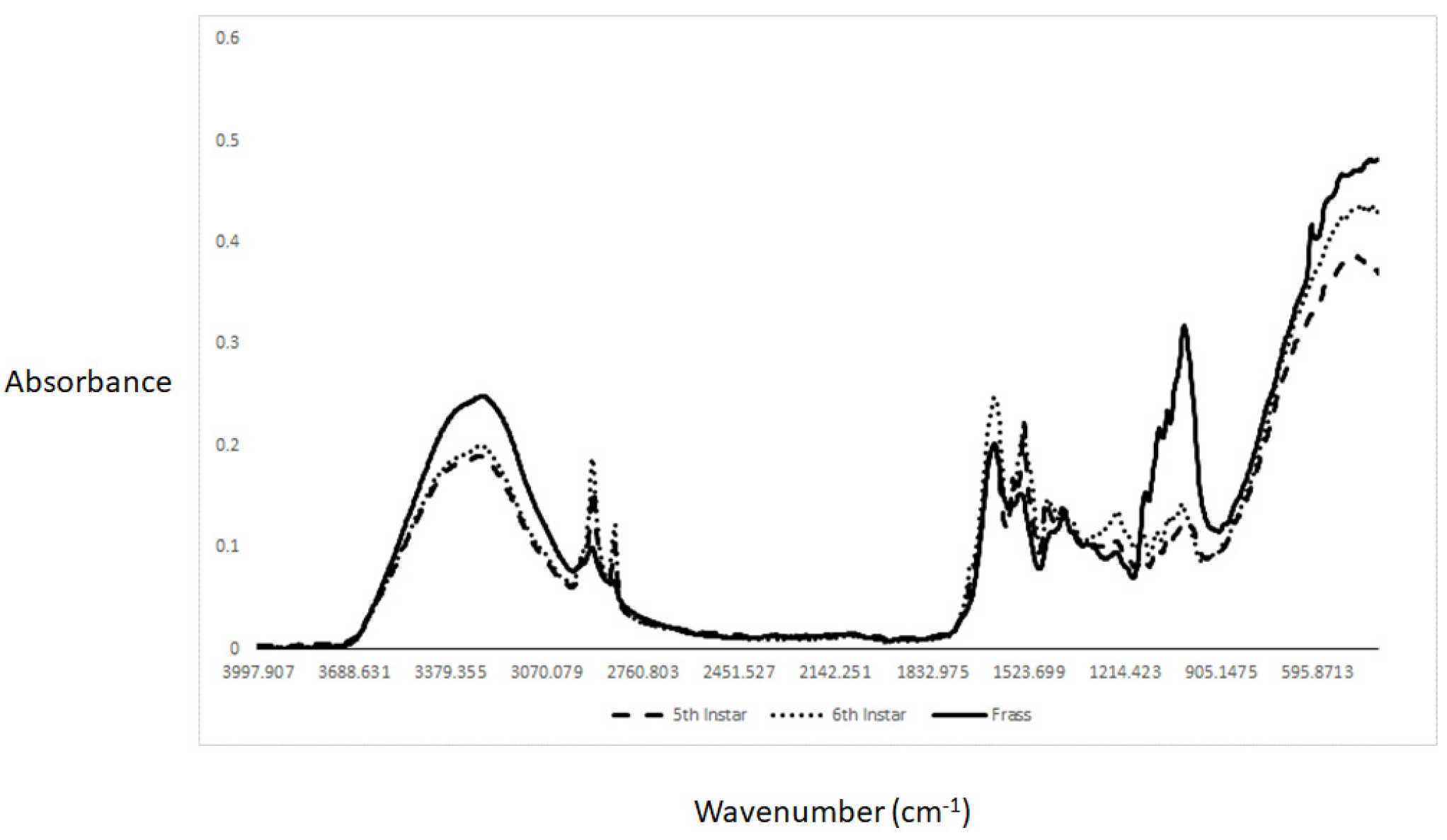
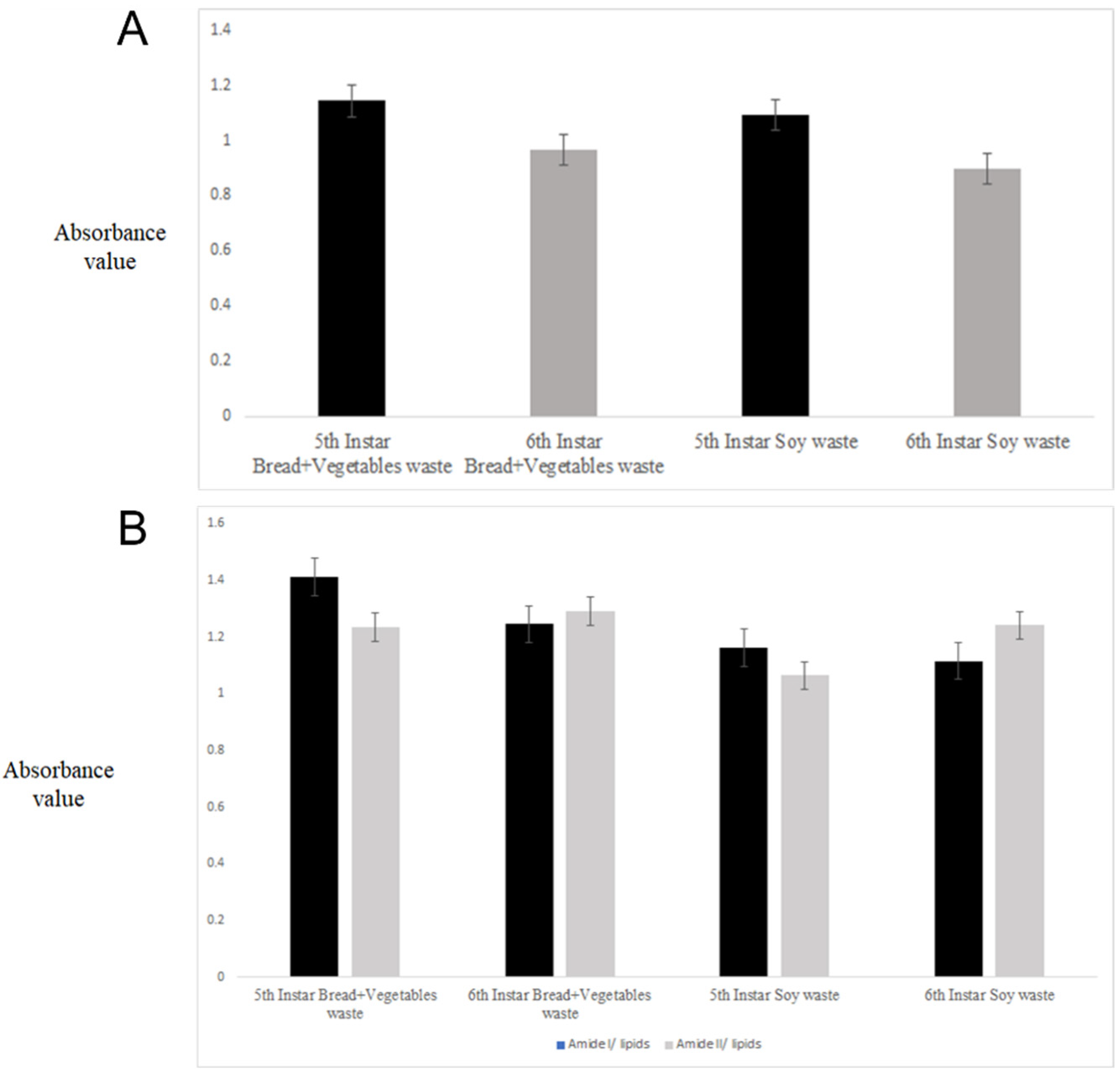
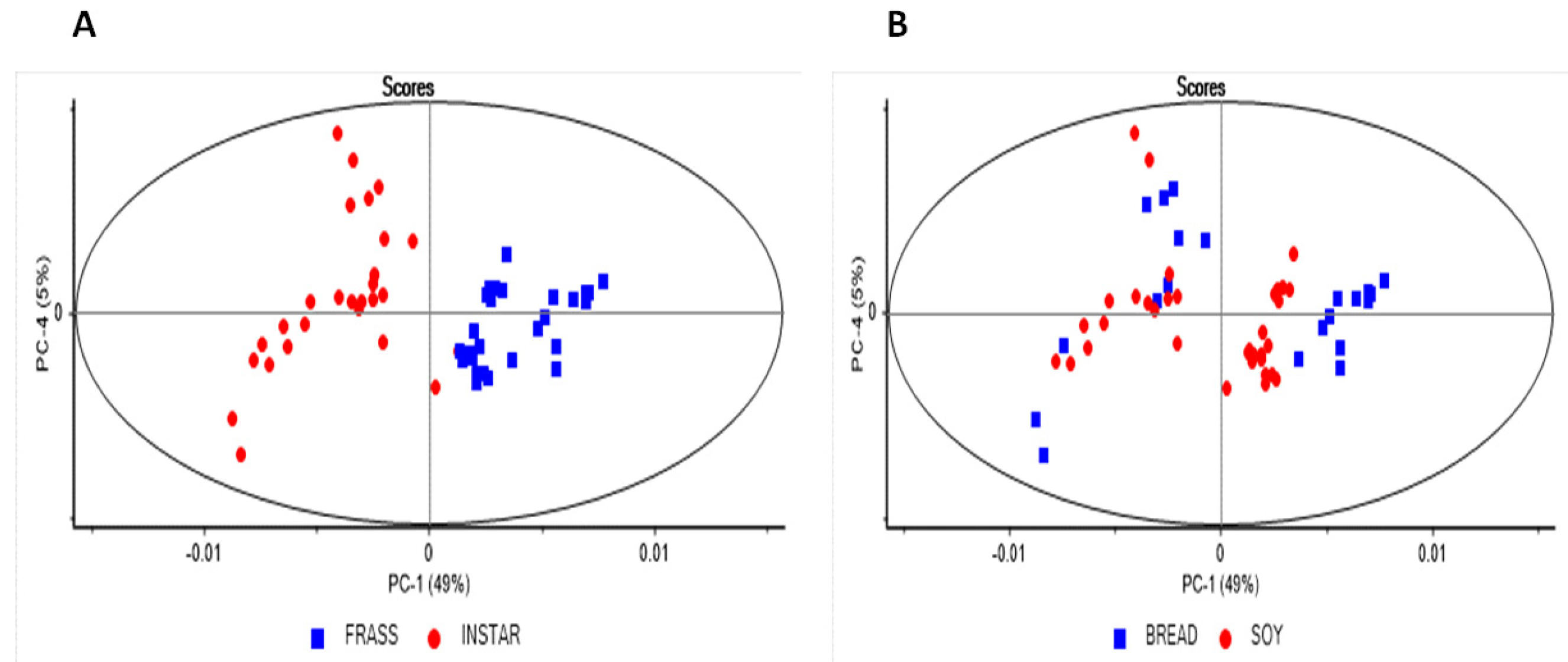
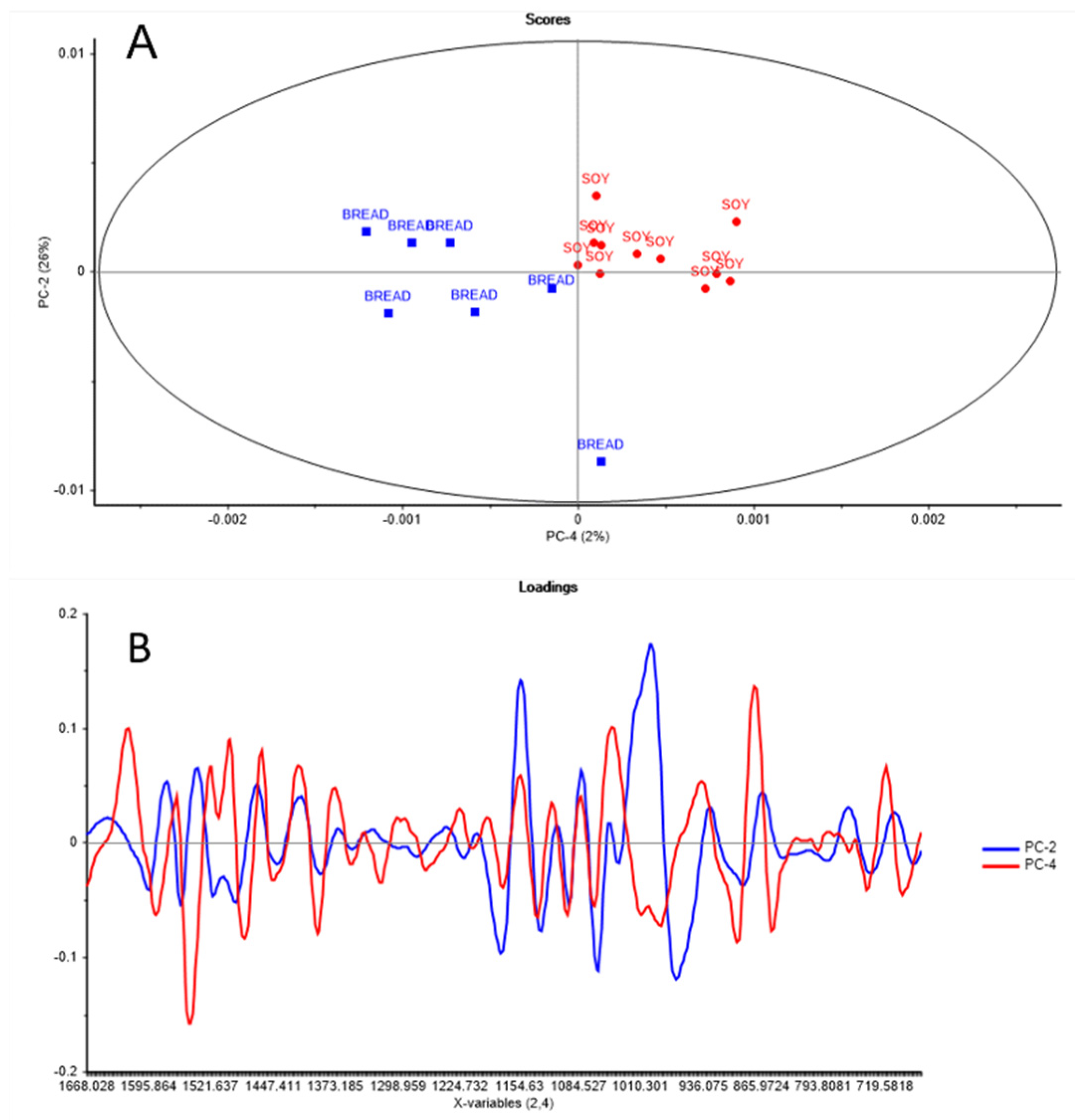
2. Results and Discussion
3. Material and Methods
3.1. Experimental Protocol, Samples, and Sampling
3.2. Mid-Infrared Spectra Collection
3.3. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results. UN DESA/POP/2022/TR/NO. 3. 2022. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 13 September 2022).
- Hopkins, I.; Newman, L.P.; Gill, H.; Danaher, J. The Influence of Food Waste Rearing Substrates on Black Soldier Fly Larvae Protein Composition: A Systematic Review. Insects 2021, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Alagappan, S.; Rowland, D.; Barwell, R.; Cozzolino, D.; Mikkelsen, D.; Mantilla, S.M.O.; James, P.; Yarger, O.; Hoffman, L. Organic side streams (bioproducts) as substrate for black soldier fly (Hermetia illucens) intended as animal feed: Chemical safety issues. Anim. Prod. Sci. 2022. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiab, K.K.M.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef] [PubMed]
- Galassi, G.; Jucker, C.; Parma, P.; Lupi, D.; Crovetto, G.M.; Savoldelli, S.; Colombini, S. Impact of Agro-industrial Byproducts on Bioconversion, Chemical Composition, in vitro Digestibility, and Microbiota of the Black Soldier Fly (Diptera: Stratiomyidae) Larvae. J. Insect Sci. 2021, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Alagappan, S.; Rowland, D.; Barwell, R.; Mantilla, S.; Mikkelsen, D.; James, P.; Yarger, O.; Hoffman, L. Legislative landscape of black soldier fly (Hermetia illucens) as feed. J. Insects Food Feed 2021, 8, 343–355. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Mantilla, S.M.O.; Mikkelsen, D.; James, P.; Yarger, O.; Cozzolino, D. Near Infrared Spectroscopy as a Traceability Tool to Monitor Black Soldier Fly Larvae (Hermetia illucens) Intended as Animal Feed. Appl. Sci. 2022, 12, 8168. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; VanLaerhoven, S.L. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Èntomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Heuel, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrügg, C.; Gangnat, I.D.M.; Kreuzer, M.; Terranova, M. Black soldier fly larvae meal and fat can completely replace soybean cake and oil in diets for laying hens. Poult. Sci. 2021, 100, 101034. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia-Pacific Èntomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Wong, C.-Y.; Rosli, S.-S.; Uemura, Y.; Ho, Y.C.; Leejeerajumnean, A.; Kiatkittipong, W.; Cheng, C.-K.; Lam, M.-K.; Lim, J.-W. Potential protein and biodiesel sources from black soldier fly larvae: Insights of larval harvesting instar and fermented feeding medium. Energies 2019, 12, 1570. [Google Scholar] [CrossRef]
- Gligorescu, A.; Toft, S.; Hauggaard-Nielsen, H.; Axelsen, J.A.; Nielsen, S.A. Development, growth and metabolic rate of Hermetia illucens larvae. J. Appl. Entomol. 2019, 143, 875–881. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.; Pineda-Mejia, J.; Dicke, M.; van Loon, J.J.A. Performance of the Black Soldier Fly (Diptera: Stratiomyidae) on Vege1 Residue-Based Diets Formulated Based on Protein and Carbohydrate Contents. J. Econ. Entomol. 2018, 111, 2676–2683. [Google Scholar] [CrossRef]
- Menino, R.; Esteves, C.; Fareleira, P.; Castelo-Branco, A. Do mineral fertilizers have a deleterious effect on soil fertility?—Case report. Open Access J. Sci. 2022, 5, 12–14. [Google Scholar] [CrossRef]
- Cammack, J.A.; Tomberlin, J.K. The Impact of Diet Protein and Carbohydrate on Select Life-History Traits of The Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef]
- Haas, J.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy for Chemical Analysis. Annu. Rev. Anal. Chem. 2016, 9, 45–68. [Google Scholar] [CrossRef]
- Ozaki, Y. Infrared Spectroscopy—Mid-infrared, Near-infrared, and Far-infrared/Terahertz Spectroscopy. Anal. Sci. 2021, 37, 1193–1212. [Google Scholar] [CrossRef]
- Johnson, J.B.; Naiker, M. Mid-infrared spectroscopy for entomological purposes: A review. J. Asia-Pacific Èntomol. 2020, 23, 613–621. [Google Scholar] [CrossRef]
- Mellado-Carretero, J.; García-Gutiérrez, N.; Ferrando, M.; Güell, C.; García-Gonzalo, D.; De Lamo-Castellví, S. Rapid discrimination and classification of edible insect powders using ATR-FTIR spectroscopy combined with multivariate analysis. J. Insects Food Feed. 2020, 6, 141–148. [Google Scholar] [CrossRef]
- Strug, I.; Utzat, C.; Cappione, A.; Gutierrez, S.; Amara, R.; Lento, J.; Capito, F.; Skudas, R.; Chernokalskaya, E.; Nadler, T. Development of a Univariate Membrane-Based Mid-Infrared Method for Protein Quantitation and Total Lipid Content Analysis of Biological Samples. J. Anal. Methods Chem. 2014, 2014, 657079. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. et Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Downey, G.; Blecker, C. Mid-Infrared Spectroscopy Coupled with Chemometrics: A Tool for the Analysis of Intact Food Systems and the Exploration of Their Molecular Structure-Quality Relationships—A Review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef] [PubMed]
- Ushakova, N.A.; Brodskii, E.S.; Kovalenko, A.A.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 2016, 468, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Èntomol. Exp. et Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef]
- Peiris, K.; Drolet, B.; Cohnstaedt, L.; Dowell, F. Infrared Absorption Characteristics of Culicoides sonorensis in Relation to Insect Age. Am. J. Agric. Sci. Technol. 2014, 2, 49–61. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Feng, W.; Yang, D.; Rehman, R.U.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical structure of chitin in the developing stages of black soldier fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef]
- Ibitoye, B.E.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- da Silva, G.D.P.; Hesselberg, T. A Review of the Use of Black Soldier Fly Larvae, Hermetia illucens (Diptera: Stratiomyidae), to Compost Organic Waste in Tropical Regions. Neotropical Èntomol. 2019, 49, 151–162. [Google Scholar] [CrossRef]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Effect of devitalization techniques on the lipid, protein, antioxidant, and chitin fractions of black soldier fly (Hermetia illucens) larvae. Eur. Food Res. Technol. 2020, 246, 2549–2568. [Google Scholar] [CrossRef]
- Mantilla, S.M.O.; Alagappan, S.; Sultanbawa, Y.; Smyth, H.; Cozzolino, D. A Mid Infrared (MIR) Spectroscopy Study of the Composition of Edible Australian Green Ants (Oecophylla smaragdina)—A Qualitative Study. Food Anal. Methods 2020, 13, 1627–1633. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
| R2 | SECV | LV | |
|---|---|---|---|
| Fingerprint region (1800 to 500 cm−1) | 0.85 | 0.19 | 4 |
| Lipid region 3000 to 2500 cm−1 | 0.90 | 0.16 | 7 |
| Full MIR range (3000–500 cm−1) | 0.90 | 0.20 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, L.C.; Zhang, S.; Alagappan, S.; Wills, V.; Yarger, O.; Cozzolino, D. Monitoring Compositional Changes in Black Soldier Fly Larvae (BSFL) Sourced from Different Waste Stream Diets Using Attenuated Total Reflectance Mid Infrared Spectroscopy and Chemometrics. Molecules 2022, 27, 7500. https://doi.org/10.3390/molecules27217500
Hoffman LC, Zhang S, Alagappan S, Wills V, Yarger O, Cozzolino D. Monitoring Compositional Changes in Black Soldier Fly Larvae (BSFL) Sourced from Different Waste Stream Diets Using Attenuated Total Reflectance Mid Infrared Spectroscopy and Chemometrics. Molecules. 2022; 27(21):7500. https://doi.org/10.3390/molecules27217500
Chicago/Turabian StyleHoffman, Louwrens C., Shuxin Zhang, Shanmugam Alagappan, Volant Wills, Olympia Yarger, and Daniel Cozzolino. 2022. "Monitoring Compositional Changes in Black Soldier Fly Larvae (BSFL) Sourced from Different Waste Stream Diets Using Attenuated Total Reflectance Mid Infrared Spectroscopy and Chemometrics" Molecules 27, no. 21: 7500. https://doi.org/10.3390/molecules27217500
APA StyleHoffman, L. C., Zhang, S., Alagappan, S., Wills, V., Yarger, O., & Cozzolino, D. (2022). Monitoring Compositional Changes in Black Soldier Fly Larvae (BSFL) Sourced from Different Waste Stream Diets Using Attenuated Total Reflectance Mid Infrared Spectroscopy and Chemometrics. Molecules, 27(21), 7500. https://doi.org/10.3390/molecules27217500







