Abstract
A cascade 6-endo-dig cyclization reaction was developed for the switchable synthesis of halogen and non-halogen-functionalized pyrazolo[3,4-b]pyridines from 5-aminopyrazoles and alkynyl aldehydes via C≡C bond activation with silver, iodine, or NBS. In addition to its wide substrate scope, the reaction showed good functional group tolerance as well as excellent regional selectivity. This new protocol manipulated three natural products, and the arylation, alkynylation, alkenylation, and selenization of iodine-functionalized products. These reactions demonstrated the potential applications of this new method.
1. Introduction
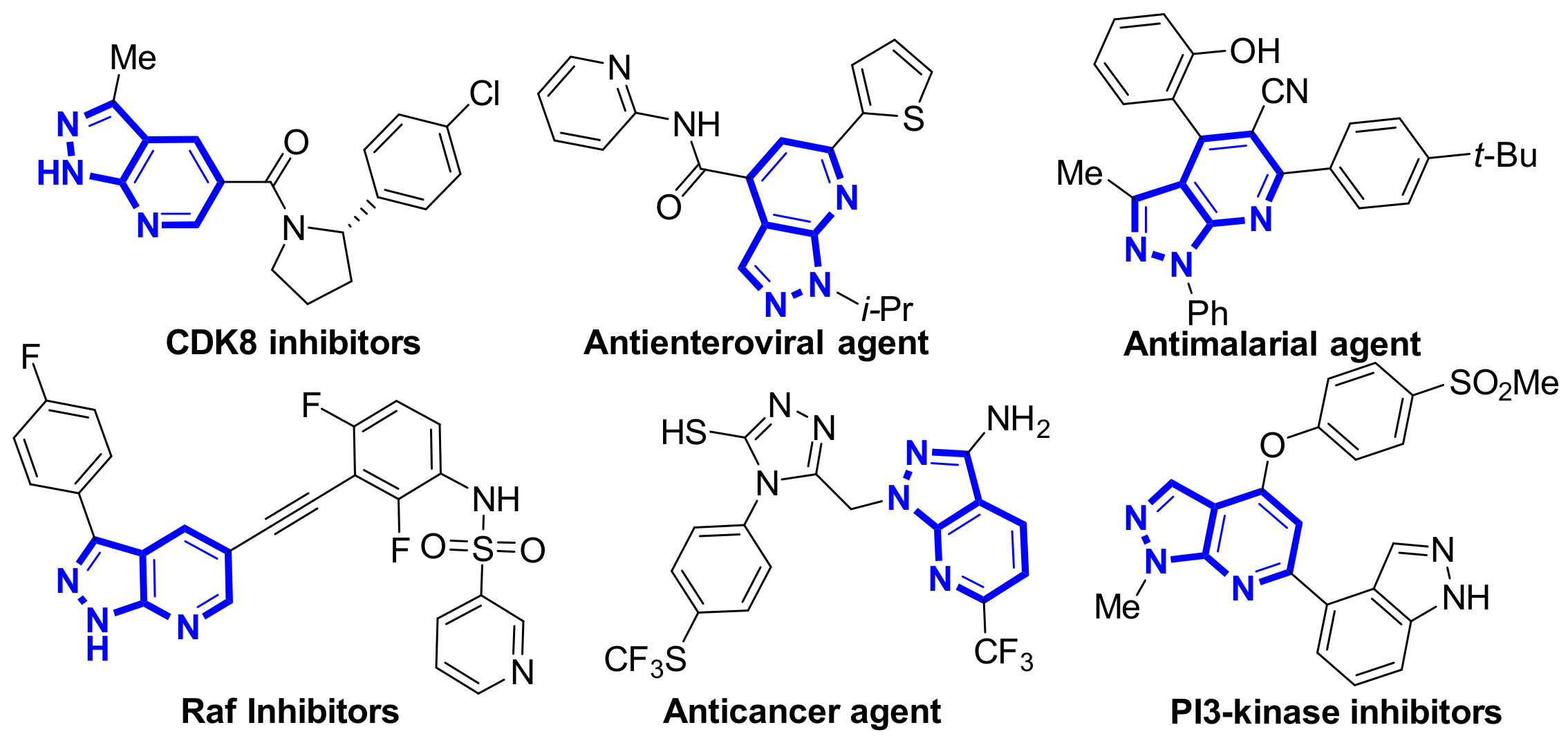
A series of natural products and biologically active molecules contain pyrazolo[3,4-b]pyridine as a key structural motif [1,2]. Several of these compounds are effective antienterovirals, antimalarials, anticancer agents, and kinase inhibitors (Figure 1) [3,4,5]. This has inspired the development of efficient methods to construct these compounds and has become a hot topic in modern organic synthesis.
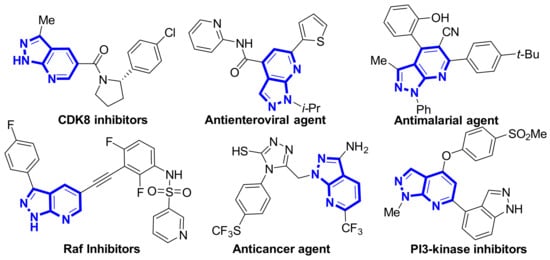
Figure 1.
Some biologically active pyrazolo[3,4-b]pyridine derivatives.
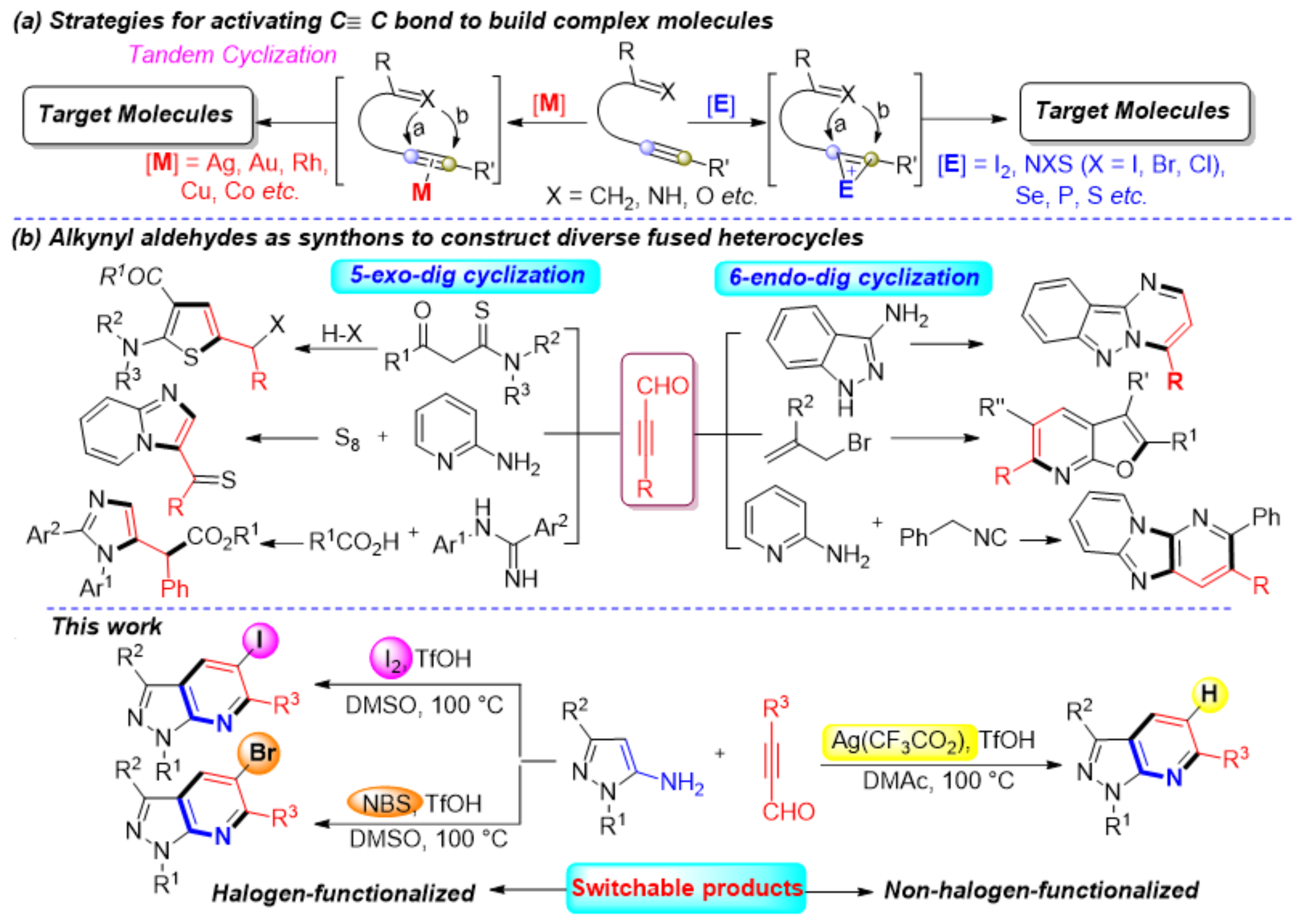
Recently, catalytic carbon-carbon bond activation has emerged as a useful tool to build complex molecules rapidly and efficiently [6,7,8,9]. There are versatile intermediates involved in these reactions, which could be trapped in situ by a second molecule that triggers subsequent tandem reactions [10,11,12,13,14,15]. The nucleophilic/electrophilic addition reactions of alkynes are well-known and provide a convenient way to synthesize functionalized molecules [16,17,18,19,20]. The high reactivity, good selectivity, excellent functional-group tolerance, and mild reaction conditions of these reactions have inspired significant research over the past few decades. Generally, this process forms highly active intermediates using transition metals, such as Ag, Au, Rh, Cu, and Co [21,22,23,24,25,26,27], or electrophiles like I2, NXS (X = I, Br, Cl), Se, S, and P (Scheme 1a) [28,29,30,31,32,33,34]. Each reagent type sees significant use in the development of synthetic methodologies and applications to prepare bioactive compounds or complex naturally occurring skeletons. However, to our knowledge, among those strategies, a direct and efficient protocol for the selective synthesis of polysubstituted and functionalized fused heterocycles, such as halogen-functionalized pyrazolo[3,4-b]pyridine frameworks, by C≡C bond activation has seldom been described. Thus, developing convenient and sustainable synthetic methods to build these high-value compounds merits attention.
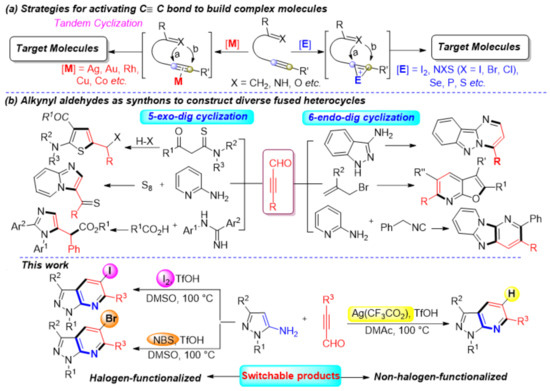
Scheme 1.
Strategies for the synthesis of diverse molecules via activating C≡C bond.
As a kind of synthetic block with bifunctional groups (C≡C and carbonyl), alkynyl aldehydes are essential synthons with rich and unexpected chemical properties [35,36,37,38]. Tandem cyclization reactions using alkynyl aldehydes as synthons yields a variety of heterocycles. Generally, tandem cyclization occurs in one of two ways, 5-exo-dig or 6-endo-dig cyclizations. For example, some efficient synthesis strategies have been reported for the synthesis of multi-substituted thiazoles, imidazo[1,2-a]pyridines, and imidazoles by using alkynyl aldehydes as synthons via 5-exo-dig cyclization [39,40,41]. The 6-endo-dig cyclization of alkynyl aldehydes is an alternative method to construct complex fused ring systems (Scheme 1b) [42,43,44]. These protocols typically use simple starting materials, with good functional tolerance and high yields. Inspired by these achievements, we sought to selectively activate the C≡C bond by changing the reaction conditions to obtain a series of compounds with a pyrazolo[3,4-b]pyridine structure core.
2. Results and Discussion
To evaluate our idea, we chose 3-methyl-1-phenyl-1H-pyrazol-5-amine (1a) and 3-phenylpropiolaldehyde (2a) as the model substrates for the optimization of the conditions. Through optimization of the catalyst, additive, solvent and temperature, the optimal reaction conditions can be summarized as follows: 1a (0.2 mmol) and 2a (0.2 mmol) in DMAc (1.5 mL) with Ag(CF3CO2) (10 mol%), TfOH (30 mol%), at 100 °C for 2 h (details appear in Supplementary Materials Tables S3–S6).
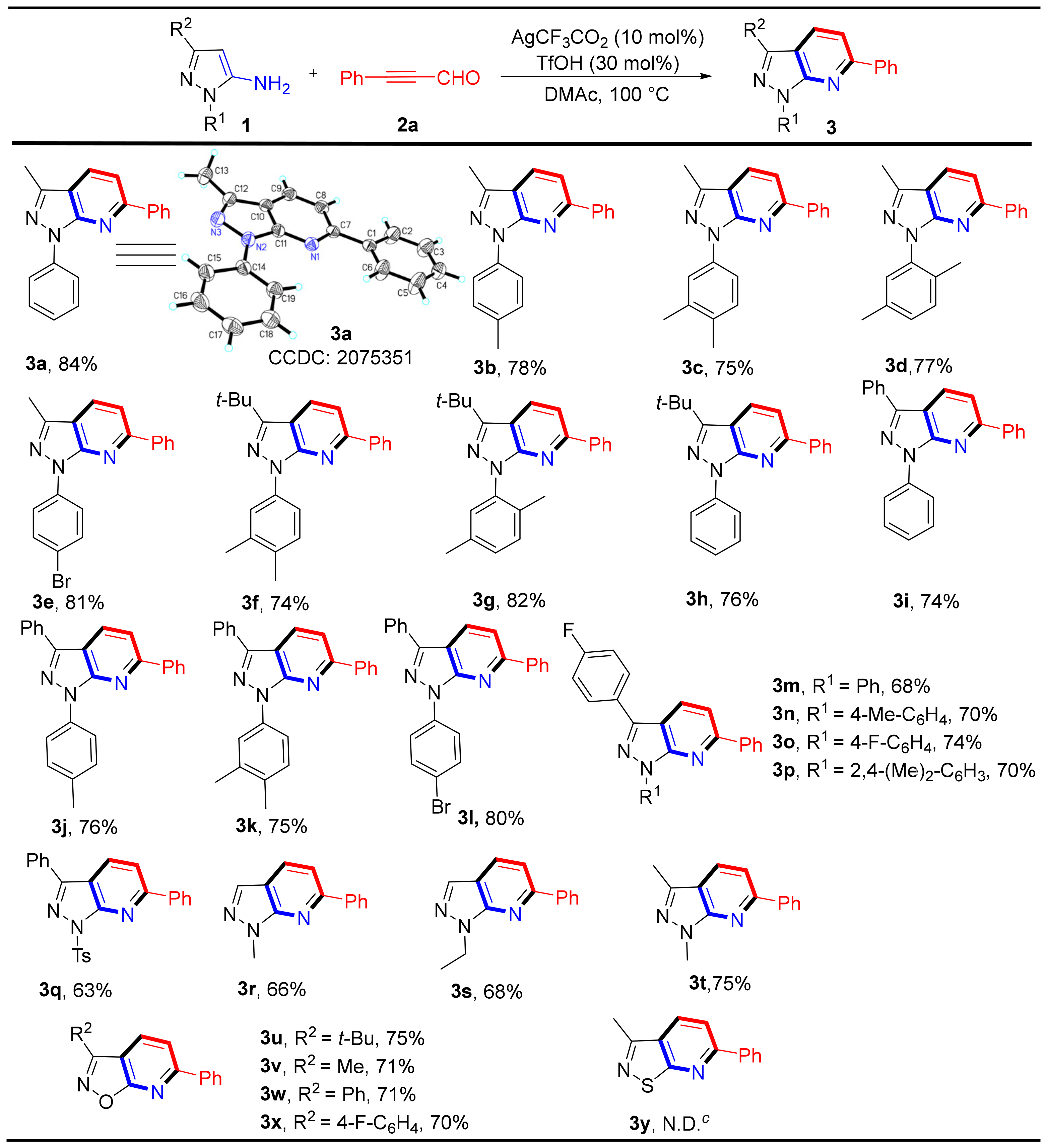
Having optimized the reaction conditions, we determined the versatility of this reaction. We examined a series of 5-aminopyrazole derivatives to test the generality of this method and evaluate the electronic influence of aromatic ring substitutions. As shown in Scheme 2, pyrazole rings bearing electron-donating groups (e.g., 3-Me, 3-(t-Bu), 1-Ph, 3-Ph) led to good yields (74–84%) of the corresponding products (3a–3d and 3f–3k). Notably, the structure of compound 3a was confirmed by X-ray single-crystal diffraction (Scheme 2). The substrates of aromatic rings attached to halogen atoms (e.g., 4-F, 4-Br) also led to their corresponding products (3e and 3l–3p) in yields between 68–81%. A strongly electron-deficient substrate was applied and afforded its product in 63% yield for the corresponding product 3q. Pyrazole rings only bearing alkyl groups were used as starting materials and yielded the expected products (3r–3t) in moderate to good yields (66–75%). Additionally, 5-aminoisoxazoles also readily reacted with 3-phenylpropiolaldehyde, yielding the desired products (3u–3x) in good yields (70–75%). However, 3-methylisothiazol-5-amine did not yield the desired product 3y.
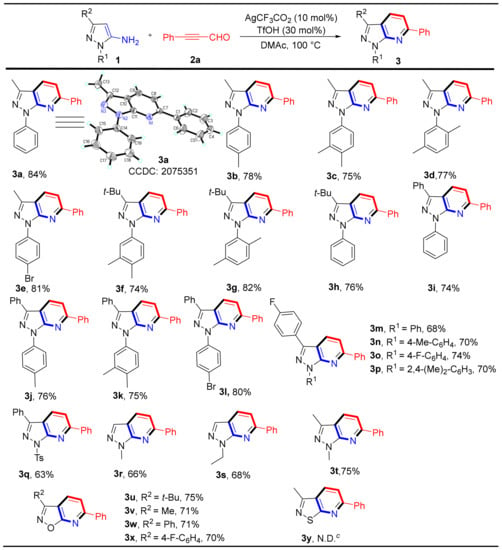
Scheme 2.
Substrate scope and Isolated yield of substituted 5-aminopyrazoles and derivatives. Reaction conditions: 1 (0.2 mmol), 2a (0.2 mmol), Ag(CF3CO2) (10 mol%), TfOH (30 mol%) in DMAc (1.5 mL) at 100 °C for 2 h. c N.D. = not detected.
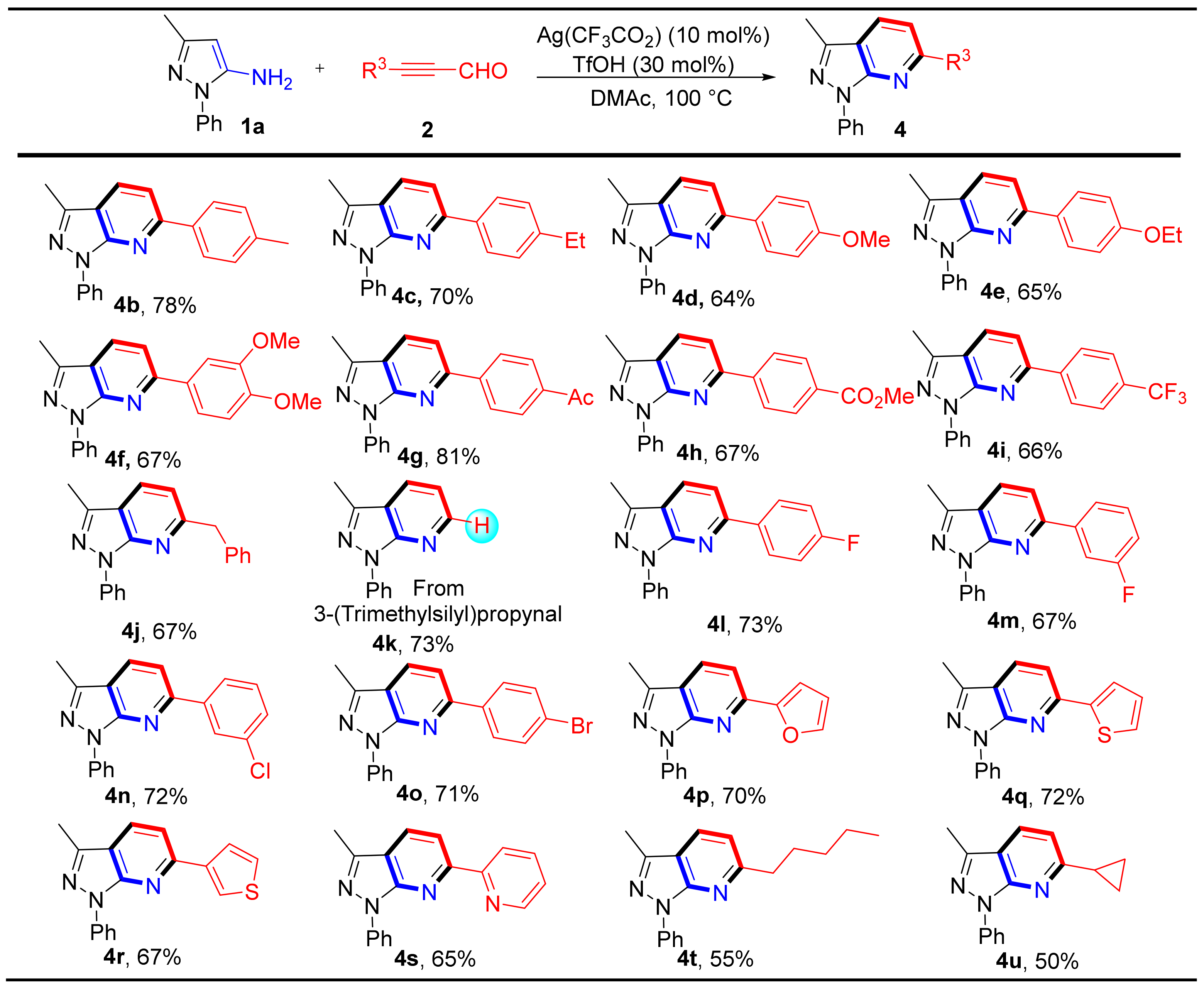
We next investigated the scope of alkynyl aldehydes derivatives for this reaction (Scheme 3). First, we examined 3-phenylpropiolaldehydes with phenyl rings containing electron-rich substituents (e.g., 4-Me, 4-Et, 4-OMe, 4-OEt, 3,4-(OMe)2). Annulation reactions occurred smoothly to deliver products (4b–4f) in 64–78% yields. For 3-phenylpropiolaldehyde containing electron-withdrawing groups (e.g., 4-Ac, 4-CO2Me, 4-CF3, 4-F, 3-F, 3-Cl, 4-Br), the reaction proceeded smoothly and afforded products (4g–4i and 4l–4o) in moderate to good yields (66–81%). In addition, when 4-phenylbut-2-ynal and 3-(trimethylsilyl)propiolaldehyde were used as starting materials, products 4j and 4k were obtained in good yields (67% and 73% respectively). It is worth noting that when using 3-(trimethylsilyl)propiolaldehyde, compound 4k was the product of the trimethylsilyl group removal. Furthermore, different heterocyclic aldehydes were also investigated including furan, thiophene, and pyridine to generate products 4p–4s in 65–72% yields. We were delighted to find that alkyl alkynyl aldehydes gave the corresponding products 4t-4u in moderate yields as well.
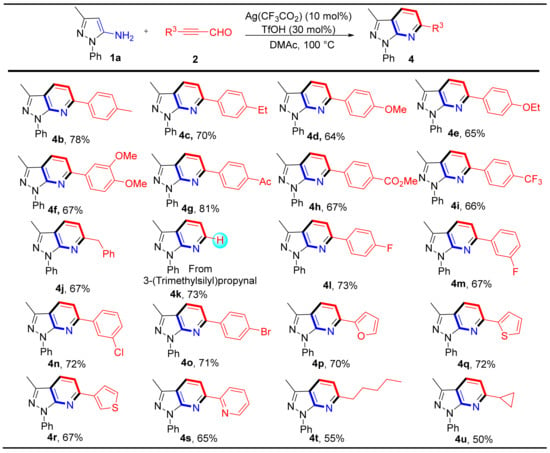
Scheme 3.
Substrate scope and Isolated yield of substituted alkynyl aldehydes and derivatives. Reaction conditions: 1a (0.2 mmol), 2 (0.2 mmol), Ag(CF3CO2) (10 mol%), TfOH (30 mol%) in DMAc (1.5 mL) at 100 °C for 2 h.
The iodinated product was detected when 1.0 equivalent of iodine was added to the reaction system (control experiment, Scheme 7d). We chose 1a and 2a as model substrates to investigate the optimal conditions to synthesize iodinated products (more details appear in Supplementary Materials Tables S7 and S8).
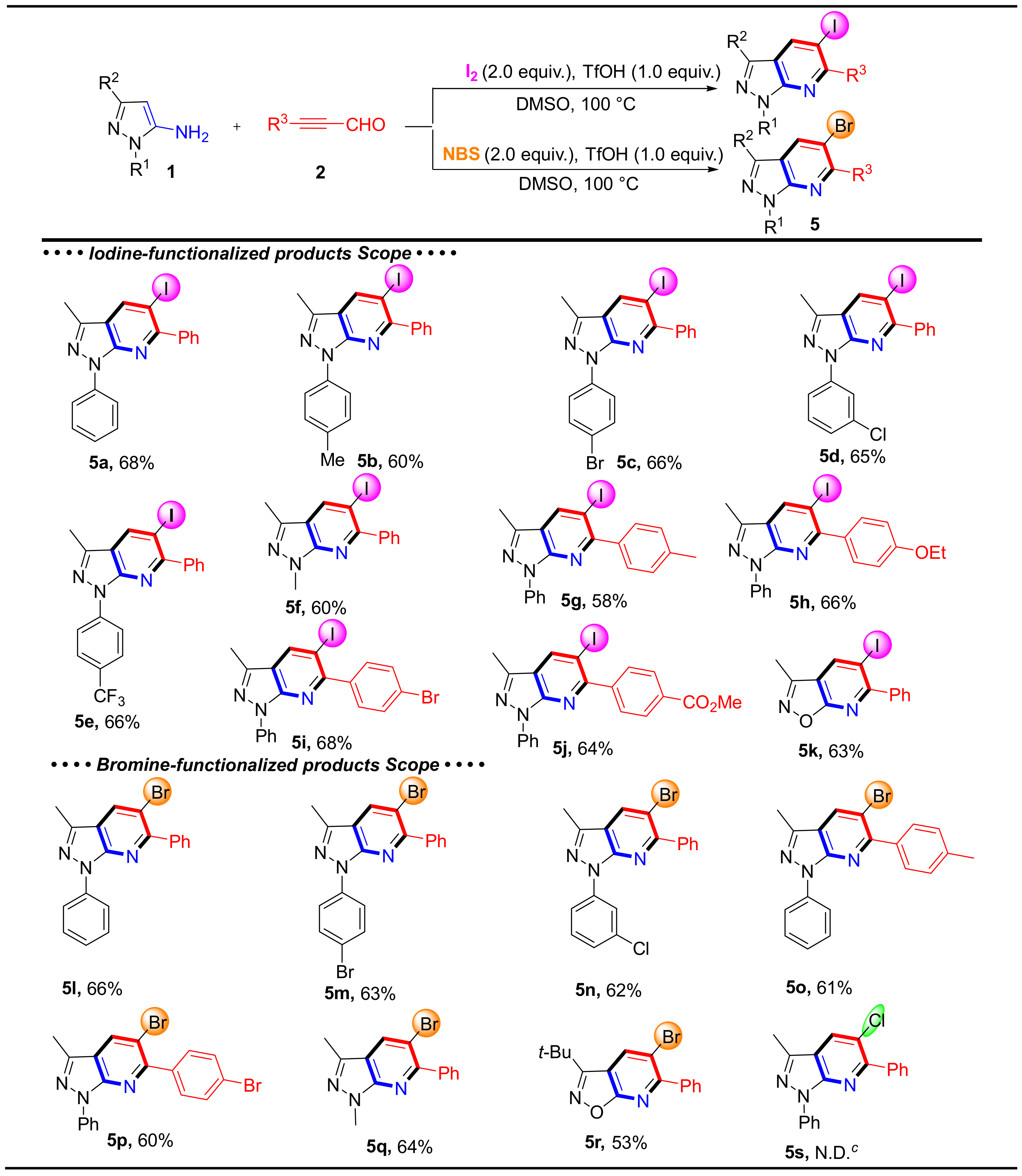
Next, various 5-aminopyrazoles and alkynyl aldehydes were tested to determine the scope of iodine-functionalized products (Scheme 4). These reactions produced the corresponding products 5a–5j 58–68% yields. Meanwhile, 3-methylisoxazol-5-amine tolerated the reaction conditions and reacted with 3-phenylpropiolaldehyde (2a) to generate 5k in moderate yield. When iodine was replaced by NBS, the expected compounds 5l–5r were obtained in moderated yields (53–66%). However, after many trials, the Cl-functionalized product 5s was not obtained.
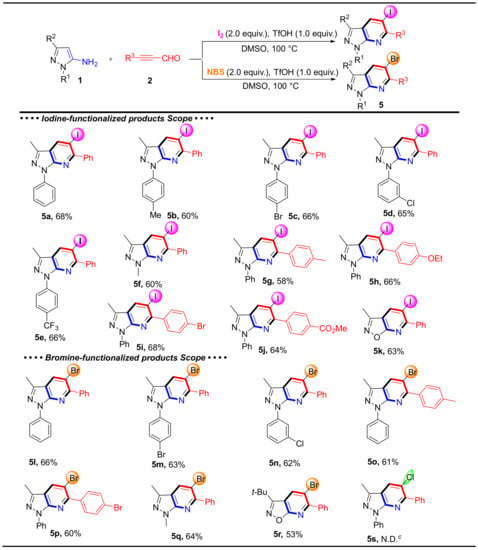
Scheme 4.
The substrate scope and Isolated yield of halogen-functionalized products. Reaction conditions: 1 (0.2 mmol), 2 (0.2 mmol), I2 or NBS (2.0 equiv.), TfOH (1.0 equiv.) in DMSO (2 mL) at 100 °C for 6 h. c N.D. = not detected.
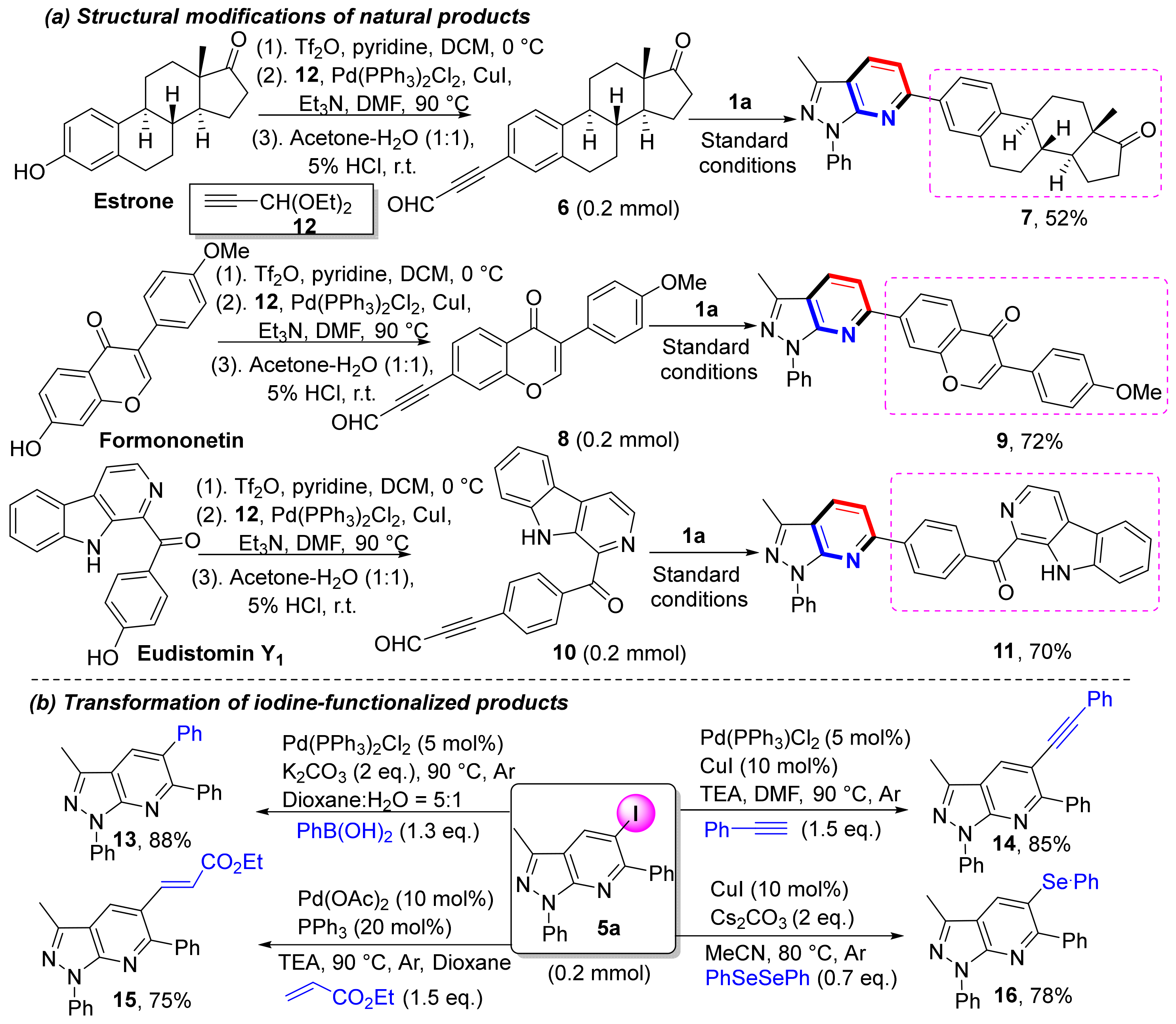
To demonstrate the applicability of this method, we modified natural products (Scheme 5a). For example, estrone, formononetin, and eudistomin Y1 are all biologically active natural products, and these compounds have phenolic hydroxyl groups that undergo conversion into trifluoromethane sulfonates. Those sulfonates undergo Sonogashira coupling and deketalization to afford alkynaldehyde intermediates (6, 8, and 10). By using these alkynaldehyde intermediates as substrates for this protocol, we successfully obtained three natural product functionalized pyrazolo[3,4-b]pyridines in moderate to good yields (7, 9, and 11). Because heteroaryl iodides are highly useful functional structures in synthetic organic chemistry, additional applications of iodine-functionalized products were conducted (Scheme 5b) [45]. A series of coupling reactions were examined to form iodine-functionalized products, including Suzuki, Sonogashira, and Heck couplings that yielded the expected products 13–15 in good yields. Furthermore, selenization of iodine-functionalized products afforded 16 in very good yield (78%).
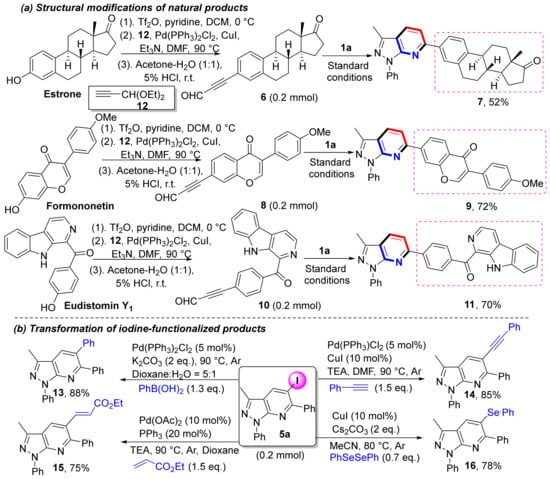
Scheme 5.
Strategies to synthesize diverse molecules via C≡C bond activation.
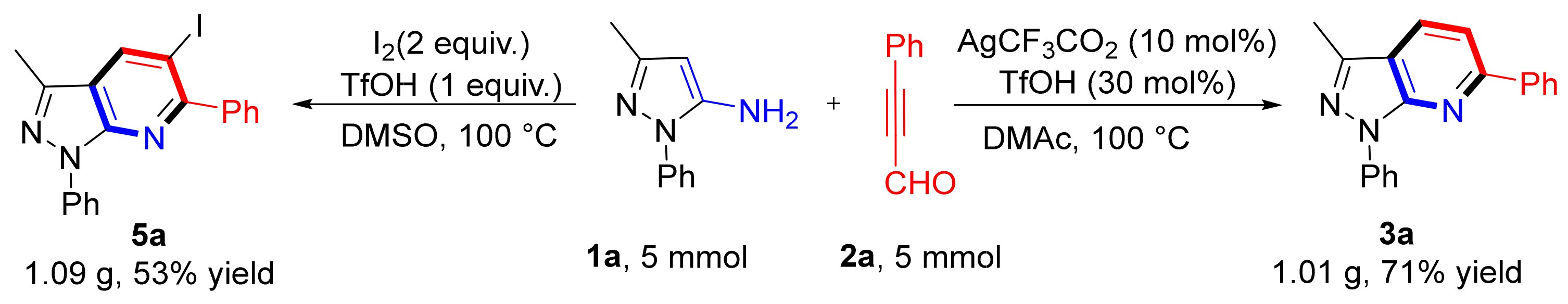
The reaction of 1a and 2a was scaled up to 5 mmol to illustrate the potential applications of this method; 3a and 5a formed 71% and 53% yields, respectively (Scheme 6). This promising result lays a good foundation for large-scale syntheses.
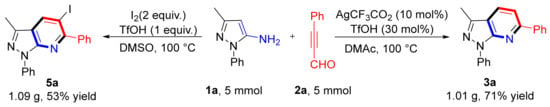
Scheme 6.
Scale-up reactions.
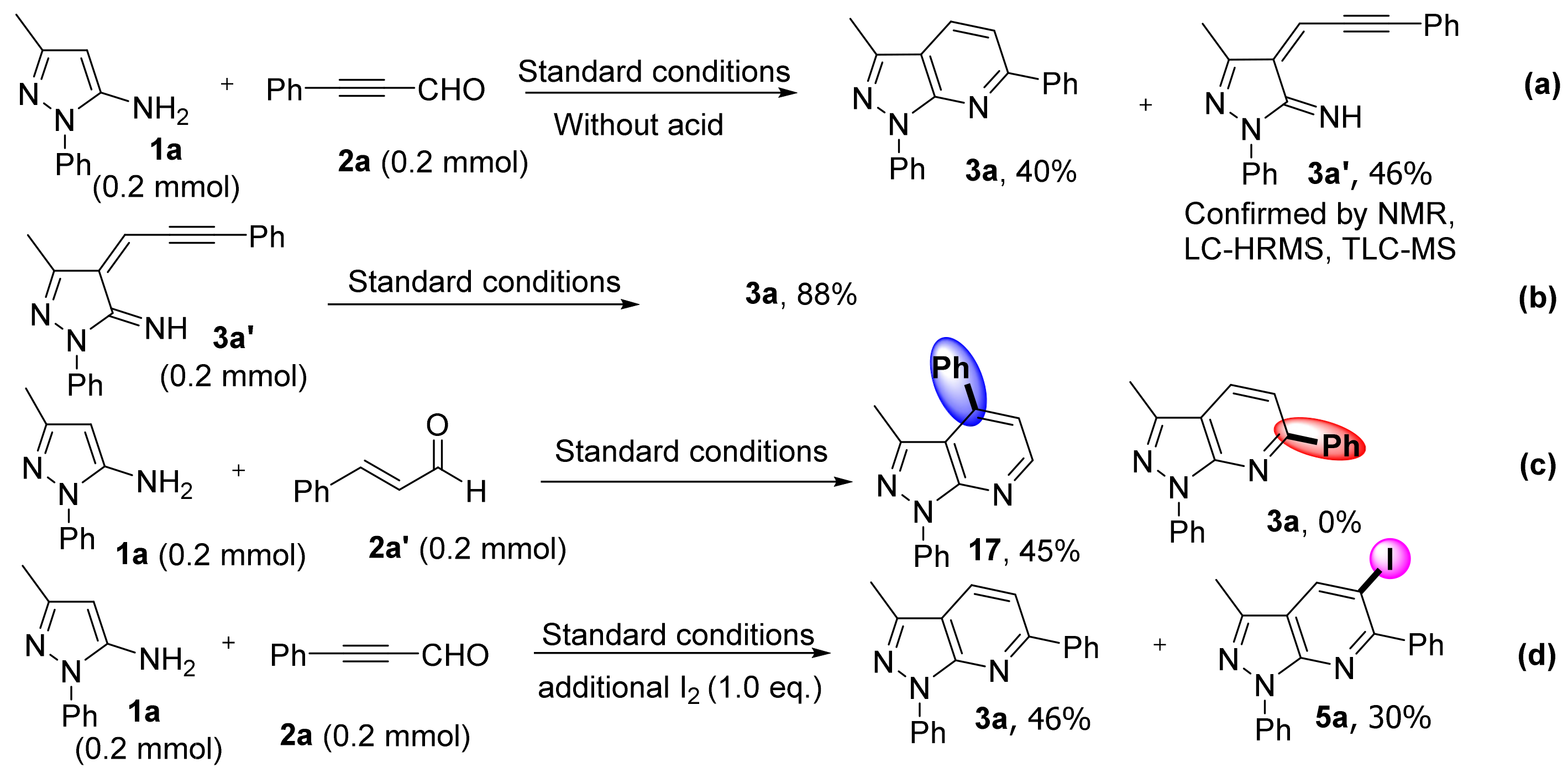
Relevant control experiments were conducted to probe the reaction mechanism for the formation of pyrazolo[3,4-b]pyridine frameworks. When the reaction of 1a with 2a was conducted without acid for 2 h, it afforded 3a and 3a′ in 40% and 46% yields, respectively, (Scheme 7a). Intermediate 3a′ was confirmed by TLC-MS(APCI), LC-HRMS, and NMR (the details can be seen in Supplementary Materials). In addition, intermediate 3a′ transform to 3a in 88% yield under standard conditions (Scheme 7b). These results suggested that 3a′ may serve as the intermediate in this reaction. To illustrate regioselectivity, we chose cinnamaldehyde (2a′) as a substrate to react with 1a under standard conditions. Compared with a standard simple, 3-methyl-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridine (17) was obtained in 45% yield, no product 3a was observed (Scheme 7c). These results confirmed the regioselectivity of this method, as it only afforded the C6 substituted pyrazolo[3,4-b]pyridine for alkynyl aldehydes substrates. Furthermore, when 1 eq. of iodine was added, non-iodinated and iodized products 3a and 5a were detected in 46% and 30% yields, respectively (Scheme 7d).
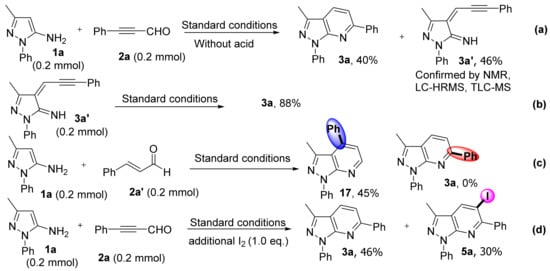
Scheme 7.
Control experiments. (a) Form intermediate 3a′. (b) Intermediate 3a′ transform to 3a under standard conditions. (c) Validation of regioselectivity experiments. (d) Add one equivalent of iodine under standard conditions.
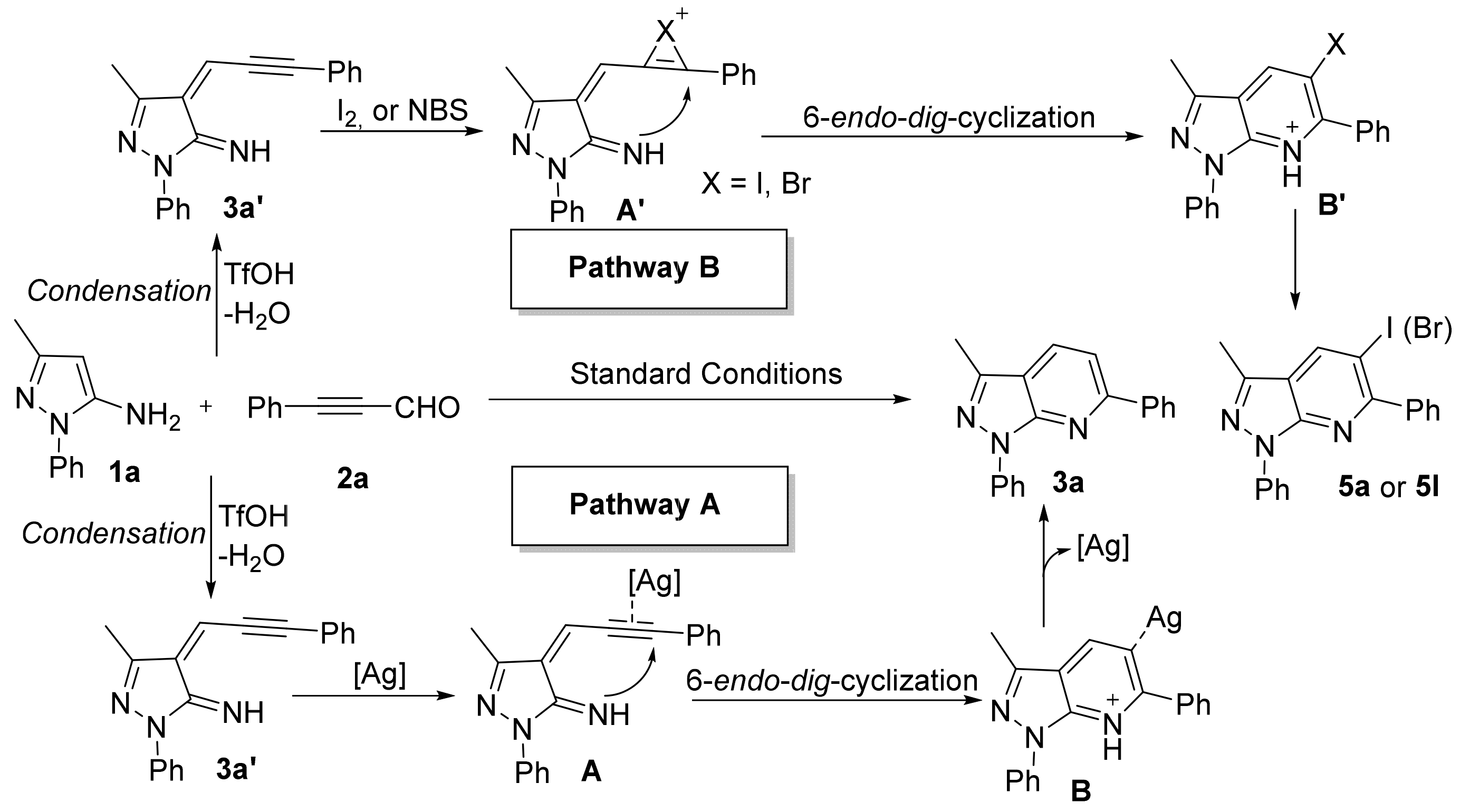
Considering the aforementioned control experiments and earlier works [46,47,48], a reaction mechanism is shown in Scheme 8 using 1a with 2a as a typical reaction. Initially, 3-methyl-1-phenyl-1H-pyrazol-5-amine (1a) undergoes condensation with 3-phenylpropiolaldehyde (2a) to form intermediate 3a′. Next, the silver salt coordinates to the alkyne of 3a′ to form intermediate A; this undergoes 6-endo-dig cyclization to form B. Finally, B undergoes demetallation to afford product 3a (Scheme 8, pathway A). Similarly, I2 or NBS adds to a triple bond that leads to intermediate A’, which undergoes 6-endo-dig cyclization to form B’, followed by acid loss from B’ to obtain 5a or 5l (Scheme 8, pathway B).
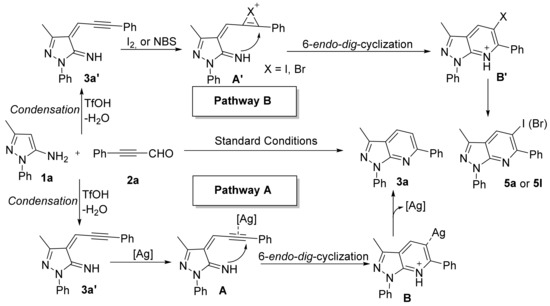
Scheme 8.
Plausible mechanistic pathway.
3. Materials and Methods
3.1. General Information
Aminopyrazoles and NBS were purchased from Shanghai Shaoyuan Co. Ltd. (Shanghai, China) 3-substituted propiolaldehyde and Ag(CF3CO2) were purchased from Leyan. Unless stated otherwise, all solvents and commercially available reagents were obtained from commercial suppliers and used without further purification. In addition, petroleum ether (b.p. 60–90 oC) was distilled prior to use for Column chromatography. Non-commercial starting materials were prepared as described below or according to literature procedures. TLC analysis was performed using pre-coated glass plates. Column chromatography was performed using silica gel (200–300 mesh). Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Advance 400 MHz spectrometer at ambient temperature using the non or partly deuterated solvent as internal standard (1H: δ 7.26 ppm and 13C{1H}: δ 77.0 ppm for CDCl3; 1H: δ 2.50 ppm and 13C{1H}: δ 40.0 ppm for DMSO-d6). Chemical shifts (δ) are reported in ppm, relative to the internal standard of tetramethylsilane (TMS). The coupling constants (J) are quoted in hertz (Hz). Resonances are described as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad) or combinations thereof. High resolution mass-spectrometric (HRMS) were obtained on an Apex-Ultra MS equipped with an electrospray source. Melting points were determined using SGW X-4 apparatus and not corrected. The X-ray diffraction data for the crystallized compound were collected on a Bruker Smart APEX CCD area detector diffractometer (graphite monochromator, Mo Kα radiation, λ = 0.71073 Å) at 296(2) K. All the heating procedures were conducted with an oil bath.
3.2. Synthetic Procedures
Typical Procedure (TP 1) for the Synthesis of 3 and 4 Taking 3a as an Example. A 25 mL pressure vial was charged with 1a (34.6 mg, 0.20 mmol, 1.0 equiv.), 2a (26 mg, 24.5 uL, 0.20 mmol, 1.0 equiv.), Ag(CF3CO2) (4.4 mg, 0.02 mmol, 10 mol%), TfOH (9 mg, 5.3 uL, 0.06 mmol, 30 mol%) and DMAc (1.5 mL).The vial was sealed and the reaction mixture was stirred at 100 °C for 2 h under air atmosphere (monitored by TLC). After the reaction was completed, and added 50 mL water to the mixture, then extracted with EtOAc 3 times (3 × 50 mL). The solution was dried over anhydrous Na2SO4, concentrated under reduced pressure, and dried under vacuum. The residue was purified by flash column chromatography by using ethyl acetate/petroleum ether mixture to obtain the corresponding product 3a.
Typical Procedure (TP 2) for the Synthesis of 5 Taking 5a as an Example. A 25 mL pressure vial was charged with 1a (34.6 mg, 0.20 mmol, 1.0 equiv.), 2a (26 mg, 24.5 uL, 0.20 mmol, 1.0 equiv.), I2 (101.5 mg, 0.40 mmol, 2.0 equiv.), TfOH (30 mg, 17.6 uL, 0.20 mmol, 1.0 equiv.) and DMSO (2.0 mL). The vial was sealed, and the reaction mixture was stirred at 100 °C for 6 h under air atmosphere (monitored by TLC). After the reaction was completed, and added 50 mL water to the mixture, then extracted with EtOAc 3 times (3 × 50 mL). The extract was washed with 10% Na2S2O3 solution, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography by using ethyl acetate/petroleum ether mixture to obtain the corresponding product 5a.
General Procedure for Synthesis of 13. K2CO3 (0.4 mmol, 2.0 equiv.), phenylboronic acid (0.26 mmol, 1.3 equiv.) and PdCl2(PPh3)2 (5 mol%) were added to a solution of 5a (0.2 mmol, 1.0 equiv.) in a 5:1 solvent mixture of dioxane and water. The reaction mixture was heated to 90 °C and stirred at this temperature until complete consumption of 5a was observed (monitored by TLC). After cooling to room temperature, the mixture was diluted with a mixture of EA and water and the aqueous layer was extracted with EtOAc (3 × 50 mL). dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography by using ethyl acetate/petroleum ether mixture to obtain the desired product 13 in 88% yield.
General Procedure for Synthesis of 14. 5a (0.2 mmol, 1 equiv.), PdCl2(PPh3)2 (5 mol%), CuI (10 mol%) and phenylacetylene (0.3 mmol, 1.5 equiv.) were added to a 25 mL Schlenk flask with a stir bar under an Ar atmosphere. Then DMF (2 mL) and TEA (1 mL) were added sequentially. The reaction mixture was then stirred at 90 °C. Afterwards 15 mL of water were added, and the reaction mixture was extracted with EtOAc (3 × 50 mL). The combined organic fractions were washed with brine and dried over Na2SO4. After filtration, the solvent was removed under reduced pressure. The residue was purified by flash column chromatography by using ethyl acetate/petroleum ether mixture to obtain the desired product 14 in 85% yield.
General Procedure for Synthesis of 15. 5a (0.2 mmol, 1 equiv.), Pd(OAc)2 (10 mol%), PPh3 (20 mol%) and ethyl acrylate (0.3 mmol, 1.5 equiv.) were added to a 25 mL Schlenk flask with a stir bar under an Ar atmosphere. Then dioxane (2 mL) and TEA (1 mL) were added sequentially. The reaction mixture was then stirred at 90 °C. Afterwards 50 mL of water were added, and the reaction mixture was extracted with EtOAc (3 × 50 mL). The combined organic fractions were washed with brine and dried over Na2SO4. After filtration, the solvent was removed under reduced pressure. The residue was purified by flash column chromatography by using ethyl acetate/petroleum ether mixture to obtain the desired product 15 in 75% yield.
General Procedure for Synthesis of 16. Adapting a literature procedure [49], A 25 mL Schlenk flask with a stir bar was charged with 5a (0.2 mmol, 1.0 equiv.), diphenyl diselenide (0.14 mmol, 0.7 equiv.) CuI (0.02 mmol, 10 mol%) and Cs2CO3 (0.4 mmol, 2.0 equiv.) in MeCN (2.0 mL). The vial was sealed and the resulting mixture was stirred at 80 °C for 24 h under an Ar atmosphere. After the reaction completed, and added 50 mL water to the mixture, then extracted with EtOAc 3 times (3 × 50 mL). The extract was washed with brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography by using ethyl acetate/petroleum ether mixture to obtain the desired product 16 in 78% yield.
4. Conclusions
In summary, a cascade 6-endo-dig cyclization reaction was developed for the switchable synthesis of halogen and non-halogen-functionalized pyrazolo[3,4-b]pyridines from 5-aminopyrazoles and alkynyl aldehydes. This method afforded diversified pyrazolo[3,4-b]pyridine frameworks via C≡C bond activation with silver, iodine, or NBS. The protocol was characterized by a wide substrate scope, good functional group tolerance, and excellent regional selectivity. The structural modification of estrone, formononetin, and eudistomin Y1 provided new ideas for syntheses of drug molecules. Iodine functionalization allowed several additional transformations, including arylation, alkenylation, alkynylation, and selenization to fabricate useful molecules.
5. Patents
A patent (Yantai University, CN 112300157, and 2021 A) has been derived from this manuscript. The patent is entitled Novel pyrazolopyridine compound with antitumor activity and preparation method thereof.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27196381/s1, Characterization data for product 3, 4, 5, 6–11, and 13–17, include 1H- and 13C-NMR spectroscopies are available online. CCDC 2075351 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033. References [50,51,52,53,54,55,56] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Y.-P.Z.; methodology, Y.-P.Z.; investigation, X.-Y.M. and Y.-J.H.; data curation, F.-R.L. and D.S.; writing—original draft preparation, X.-Y.M., Y.-J.H. and Y.-Y.S.; writing—review and editing, Y.-P.Z.; visualization, F.-R.L.; supervision, A.-X.W. and Y.-P.Z.; project administration, Y.-P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors also thank Talent Induction Program for Youth Innovation Teams in Colleges and Universities of Shandong Province. This work was supported by Science and Technology Innovation Development Plan of Yantai (2020MSGY114) and Yantai “Double Hundred Plan”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors also thank Talent Induction Program for Youth Innovation Teams in Colleges and Universities of Shandong Province. Xue-Han Li, Le-Yang Guan, Yu-Ting Han, Meng-Jiao Lei and Jia-Xin Chen are thanked for purification of some compounds.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3, 4, and 5 are available from the authors.
References
- Orlikova, B.; Chaouni, W.; Schumacher, M.; Aadil, M.; Diederich, M.; Kirsch, G. Synthesis and bioactivity of novel amino-pyrazolopyridines. Eur. J. Med. Chem. 2014, 85, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kitamura, N.; Musharrafieh, R.; Wang, J. Discovery of Potent and Broad-Spectrum Pyrazolopyridine-Containing Antivirals against Enteroviruses D68, A71, and Coxsackievirus B3 by Targeting the Viral 2C Protein. J. Med. Chem. 2021, 64, 8755–8774. [Google Scholar] [CrossRef] [PubMed]
- Nagender, P.; Naresh Kumar, R.; Malla Reddy, G.; Krishna Swaroop, D.; Poornachandra, Y.; Ganesh Kumar, C.; Narsaiah, B. Synthesis of novel hydrazone and azole functionalized pyrazolo[3,4-b]pyridine derivatives as promising anticancer agents. Bioorganic Med. Chem. Lett. 2016, 26, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Czodrowski, P.; Mallinger, A.; Wienke, D.; Esdar, C.; Pöschke, O.; Busch, M.; Rohdich, F.; Eccles, S.A.; Ortiz-Ruiz, M.J.; Schneider, R.; et al. Structure-Based Optimization of Potent, Selective, and Orally Bioavailable CDK8 Inhibitors Discovered by High-Throughput Screening. J. Med. Chem. 2016, 59, 9337–9349. [Google Scholar] [CrossRef]
- Eagon, S.; Hammill, J.T.; Sigal, M.; Ahn, K.J.; Tryhorn, J.E.; Koch, G.; Belanger, B.; Chaplan, C.A.; Loop, L.; Kashtanova, A.S.; et al. Synthesis and Structure–Activity Relationship of Dual-Stage Antimalarial Pyrazolo[3,4-b]pyridines. J. Med. Chem. 2020, 63, 11902–11919. [Google Scholar] [CrossRef]
- Haydl, A.M.; Breit, B.; Liang, T.; Krische, M.J. Alkynes as Electrophilic or Nucleophilic Allylmetal Precursors in Transition-Metal Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11312–11325. [Google Scholar] [CrossRef]
- Ru, G.; Zhang, T.; Zhang, M.; Jiang, X.; Wan, Z.; Zhu, X.; Shen, W.; Gao, G. Recent progress towards the transition-metal-catalyzed Nazarov cyclization of alkynes via metal carbenes. Org. Biomol. Chem. 2021, 19, 5274–5283. [Google Scholar] [CrossRef]
- Yao, T.; Xia, T.; Yan, W.; Xu, H.; Zhang, F.; Xiao, Y.; Zhang, J.; Liu, L. Copper-Catalyzed Chemodivergent Cyclization of N-(ortho-alkynyl)aryl-Pyrrole and Indoles. Org. Lett. 2020, 22, 4511–4516. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, F.; Zhang, J.; Liu, L. Palladium-Catalyzed Intermolecular Heck-Type Dearomative [4 + 2] Annulation of 2H-Isoindole Derivatives with Internal Alkynes. Org. Lett. 2020, 22, 5063–5067. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, S. A Strategy to Obtain o-Naphthoquinone Methides: Ag(I)-Catalyzed Cyclization of Enynones for the Synthesis of Benzo[h]chromanes and Naphthopyryliums. Org. Lett. 2019, 21, 1488–1492. [Google Scholar] [CrossRef]
- Wu, F.; Cheng, T.; Zhu, S. Construction of Partially Protected Nonsymmetrical Biaryldiols via Semipinacol Rearrangement of o-NQM Derived from Enynones. Org. Lett. 2021, 23, 71–75. [Google Scholar] [CrossRef]
- Rong, M.; Qin, T.; Zi, W. Rhenium-Catalyzed Intramolecular Carboalkoxylation and Carboamination of Alkynes for the Synthesis of C3-Substituted Benzofurans and Indoles. Org. Lett. 2019, 21, 5421–5425. [Google Scholar] [CrossRef]
- Rondla, N.R.; Levi, S.M.; Ryss, J.M.; Vanden Berg, R.A.; Douglas, C.J. Palladium-Catalyzed C-CN Activation for Intramolecular Cyanoesterification of Alkynes. Org. Lett. 2011, 13, 1940–1943. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Wang, H.; Guo, D.; Ye, D.; Xu, Y.; Jiang, H.; Liu, H. Silver-catalyzed intramolecular hydroamination of alkynes in aqueous media: Efficient and regioselective synthesis for fused benzimidazoles. Green. Chem. 2011, 13, 397–405. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Gao, J.; Zhang, H.; Yang, K.; Li, J.; Yan, X.; Li, Y.; Zhu, Y. I2-Mediated [3 + 2] annulation of methyl-azaarenes with alkyl 2-isocyanoacetates or amino acid ester hydrochlorides: Selective synthesis of iodine-functionalized and non-iodine-functionalized fused imidazoles. Org. Chem. Front. 2022, 9, 1403–1409. [Google Scholar] [CrossRef]
- Godoi, B.; Schumacher, R.F.; Zeni, G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011, 111, 2937–2980. [Google Scholar] [CrossRef]
- Fang, G.; Bi, X. Silver-catalysed reactions of alkynes: Recent advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef]
- Costello, J.P.; Ferreira, E.M. Regioselectivity Influences in Platinum-Catalyzed Intramolecular Alkyne O-H and N-H Additions. Org. Lett. 2019, 21, 9934–9939. [Google Scholar] [CrossRef]
- Chen, L.; Chen, K.; Zhu, S. Transition-Metal-Catalyzed Intramolecular Nucleophilic Addition of Carbonyl Groups to Alkynes. Chem 2018, 4, 1208–1262. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Zhang, J.; Liu, L. Gold-catalyzed intermolecular [4+1] spiroannulation via site-selective aromatic C(sp2)–H functionalization and dearomatization of phenol derivatives. Chem. Commun. 2020, 56, 8202–8205. [Google Scholar] [CrossRef]
- Ma, J.; Chen, K.; Fu, H.; Zhang, L.; Wu, W.; Jiang, H.; Zhu, S. Dual Catalysis: Proton/Metal-Catalyzed Tandem Benzofuran Annulation/Carbene Transfer Reaction. Org. Lett. 2016, 18, 1322–1325. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, G.; Shen, C.; He, W.; Ye, L. Synthesis of fused isoquinolines via gold-catalyzed tandem alkyne amination/intramolecular O-H insertion. Org. Chem. Front. 2016, 3, 491–495. [Google Scholar] [CrossRef]
- Zhou, M.; Song, R.; Wang, C.; Li, J. Synthesis of Azepine Derivatives by Silver-Catalyzed [5+2] Cycloaddition of γ-Amino Ketones with Alkynes. Angew. Chem. Int. Ed. 2013, 52, 10805–10808. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, L.; Zhu, S. 1,4-Addition of o-naphthoquinone methides induced by silver-catalyzed cyclization of enynones: An approach to unsymmetrical triarylmethanes and benzo[f]chromenes. Org. Chem. Front. 2020, 7, 3387–3392. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Xu, D.; Li, M.; Wei, W.; Liang, Y. Silver Trifluoromethanesulfonate-Catalyzed Annulation of Propargylic Alcohols with 3-Methyleneisoindolin-1-one. J. Org. Chem. 2020, 85, 2626–2634. [Google Scholar] [CrossRef]
- Niu, Y.; Yan, Z.; Gao, G.; Wang, H.; Shu, X.; Ji, K.; Liang, Y. Synthesis of Isoquinoline Derivatives via Ag- Catalyzed Cyclization of 2-Alkynyl Benzyl Azides. J. Org. Chem. 2009, 74, 2893–2896. [Google Scholar] [CrossRef]
- Huo, Z.; Gridnev, I.D.; Yamamoto, Y. A Method for the Synthesis of Substituted Quinolines via Electrophilic Cyclization of 1-Azido-2-(2-propynyl)benzene. J. Org. Chem. 2010, 75, 1266–1270. [Google Scholar] [CrossRef]
- Ouyang, H.; Tang, R.; Zhong, P.; Zhang, X.; Li, J. CuI/I2-Promoted Electrophilic Tandem Cyclization of 2-Ethynylbenzaldehydes with ortho-Benzenediamines: Synthesis of Iodoisoquinoline-Fused Benzimidazoles. J. Org. Chem. 2011, 76, 223–228. [Google Scholar] [CrossRef]
- Liu, L.; Chen, D.; Yao, J.; Zong, Q.; Wang, J.; Zhou, H. CuX-Activated N-Halosuccinimide: Synthesis of 3-Haloquinolines via Electrophilic Cyclization of Alkynyl Imines. J. Org. Chem. 2017, 82, 4625–4630. [Google Scholar] [CrossRef]
- Unoh, Y.; Hirano, K.; Miura, M. Metal-Free Electrophilic Phosphination/Cyclization of Alkynes. J. Am. Chem. Soc. 2017, 139, 6106–6109. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.C.; Hernández, J.G.; Bolm, C. Synthesis of 3-Iodobenzofurans by Electrophilic Cyclization under Solventless Conditions in a Ball Mill. Eur. J. Org. Chem. 2018, 2018, 2458–2461. [Google Scholar] [CrossRef]
- Zhou, J.; Li, W.; Zheng, H.; Pei, Y.; Liu, X.; Cao, H. Visible Light-Induced Cascade Cyclization of 3-Aminoindazoles, Ynals, and Chalcogens: Access to Chalcogen-Containing Pyrimido [1,2-b]-indazoles. Org. Lett. 2021, 23, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, F.; Yao, T.; Liu, X.; Liu, Y.; Liu, L. Dearomative Iodocyclization of N-(o-Alkynyl)aryl Isoindole. J. Org. Chem. 2022, 87, 7531–7535. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Liao, J.; Huang, J.; Qiu, H.; Chen, Q.; Chen, Y. Transition Metal-Mediated C=O and C=C Bond-Forming Reactions: A Regioselective Strategy for the Synthesis of Imidazo [1,2-a]pyridines and Imidazo [1,2-a]pyrazines. J. Org. Chem. 2014, 79, 11209–11214. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Zhao, L.; Cen, J.; Lin, J.; Zhu, Q.; Fu, M. One-Pot Regiospecific Synthesis of Imidazo[1,2-a]pyridines: A Novel, Metal-Free, Three-Component Reaction for the Formation of C–N, C–O, and C–S Bonds. Org. Lett. 2014, 16, 146–149. [Google Scholar] [CrossRef]
- Yang, D.; Yu, Y.; Wu, Y.; Feng, H.; Li, X.; Cao, H. One-Pot Regiospecific Synthesis of Indolizines: A Solvent-Free, Metal-Free, Three-Component Reaction of 2-(Pyridin-2-yl)acetates, Ynals, and Alcohols or Thiols. Org. Lett. 2018, 20, 2477–2480. [Google Scholar] [CrossRef]
- Tber, Z.; Hiebel, M.A.; El Hakmaoui, A.; Akssira, M.; Guillaumet, G.; Berteina-Raboin, S. Metal Free Formation of Various 3-Iodo-1H-pyrrolo [3′,2′:4,5]imidazo-[1,2-a]pyridines and [1,2-b]Pyridazines and Their Further Functionalization. J. Org. Chem. 2015, 80, 6564–6573. [Google Scholar]
- Luo, X.; Ge, L.; An, X.; Jin, J.; Wang, Y.; Sun, P.; Deng, W. Regioselective Metal-Free One-Pot Synthesis of Functionalized 2-Aminothiophene Derivatives. J. Org. Chem. 2015, 80, 4611–4617. [Google Scholar] [CrossRef]
- Wang, C.; Lai, J.; Chen, C.; Li, X.; Cao, H. Ag-Catalyzed Tandem Three-Component Reaction toward the Synthesis of Multisubstituted Imidazoles. J. Org. Chem. 2017, 82, 13740–13745. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, P.; Xu, F.; Qiu, R.; Tan, Q.; Long, L.; Ye, M. Lewis Acid-Catalyzed Intermolecular Annulation: Three-Component Reaction toward Imidazo[1,2-a]pyridine Thiones. J. Org. Chem. 2019, 84, 9369–9377. [Google Scholar] [CrossRef]
- Li, Z.; Ling, F.; Cheng, D.; Ma, C. Pd-Catalyzed Branching Cyclizations of Enediyne-Imides toward Furo[2,3-b]pyridines. Org. Lett. 2014, 16, 1822–1825. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Wang, J.; Song, G.; Tang, D.; Yao, F.; Lin, H.; Yan, W.; Li, H.; Xu, Z.; et al. Diversity-Oriented Synthesis of Imidazo-Dipyridines with Anticancer Activity via the Groebke–Blackburn–Bienaymé and TBAB-Mediated Cascade Reaction in One Pot. J. Org. Chem. 2019, 84, 12632–12638. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Lin, J.; Zhang, Z.; Wu, S.; He, Q.; Cao, H. Controllable Site-Selective Construction of 2- and 4-Substituted Pyrimido[1,2-b]indazole from 3-Aminoindazoles and Ynals. J. Org. Chem. 2021, 86, 9107–9116. [Google Scholar] [CrossRef]
- Rayadurgam, J.; Sana, S.; Sasikumar, M.; Gu, Q. Palladium catalyzed C–C and C–N bond forming reactions: An update on the synthesis of pharmaceuticals from 2015–2020. Org. Chem. Front. 2021, 8, 384–414. [Google Scholar] [CrossRef]
- Ding, Q.; Wu, J. Lewis Acid- and Organocatalyst-Cocatalyzed Multicomponent Reactions of 2-Alkynylbenzaldehydes, Amines, and Ketones. Org. Lett. 2007, 9, 4959–4962. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J. Efficient Generation of Biologically Active H-Pyrazolo[5,1-a]isoquinolines via Multicomponent Reaction. Org. Lett. 2010, 12, 4856–4859. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Chen, L.; Fu, J.; Zhu, J. Controllable synthesis of 3-iodo-2H-quinolizin-2-ones and 1,3-diiodo-2H-quinolizin-2-ones via electrophilic cyclization of azacyclic ynones. Chem Commun. 2019, 55, 12607–12610. [Google Scholar] [CrossRef]
- Dandapat, A.; Korupalli, C.; Prasad, D.J.C.; Singh, R.; Sekar, G. An Efficient Copper(I) Iodide Catalyzed Synthesis of Diaryl Selenides through CAr-Se Bond Formation Using Solvent Acetonitrile as Ligand. Synthesis 2011, 2011, 2297–2302. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Q.; Dong, Z.; Xu, J.; Yang, Z. Rhodium-Catalyzed 1,1-Hydroacylation of Thioacyl Carbenes with Alkynyl Aldehydes and Subsequent Cyclization. Org. Lett. 2019, 21, 3594–3599. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, M.; Cai, Q.; Jia, F.; Wu, A. A Cascade Coupling Strategy for One-Pot Total Synthesis of β-Carboline and Isoquinoline-Containing Natural Products and Derivatives. Chem. Eur. J. 2013, 19, 10132–10137. [Google Scholar] [CrossRef]
- Zheng, A.; Zhang, W.; Pan, J. One-Pot and Convenient Conversion of 5-Azidopyrazole-4-carboxaldehyde to Pyrazolo[3,4-b]pyridines. Synth. Commun. 2006, 36, 1549–1556. [Google Scholar] [CrossRef]
- Shekarrao, K.; Kaishap, P.P.; Saddanapu, V.; Addlagatta, A.; Gogoi, S.; Boruah, R.C. Microwave-assisted palladium mediated efficient synthesis of pyrazolo[3,4-b]pyridines, pyrazolo[3,4-b]quinolines, pyrazolo[1,5-a]pyrimidines and pyrazolo[1,5-a]quinazolines. RSC Adv. 2014, 4, 24001–24006. [Google Scholar] [CrossRef]
- Hamama, W.S.; Ibrahim, M.E.; Zoorob, H.H. Synthesis and Biological Evaluation of Some Novel Isoxazole Derivatives. J. Heterocycl. Chem. 2017, 54, 341–346. [Google Scholar] [CrossRef]
- Iaroshenko, V.O.; Mkrtchyan, S.; Gevorgyan, A.; Miliutina, M.; Villinger, A.; Volochnyuk, D.; Sosnovskikh, V.Y.; Langer, P. 2,3-Unsubstituted chromones and their enaminone precursors as versatile reagents for the synthesis of fused pyridines. Org. Biomol. Chem. 2012, 10, 890–894. [Google Scholar] [CrossRef]
- Qiu, R.; Qiao, S.; Peng, B.; Long, J.; Yin, G. A mild method for the synthesis of bis-pyrazolo[3,4-b:4′,3′-e]pyridine derivatives. Tetrahedron Lett. 2018, 59, 3884–3888. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).